2.1. Crystalline Structure
In
Figure 1a,b, the PXRD patterns of the synthesized MOFs (Eu
1-xLn
x)
2bdc
3·nH
2O (Ln = La, Gd) are shown. We found that mixed Eu-La and Eu-Gd terephthalates were isostructural to the Ln
2bdc
3·4H
2O crystalline phase (Ln = Ce − Yb) [
25] at the whole concentration range, and additional peaks were not observed. In the Ln
2bdc
3·4H
2O structure, the lanthanide (III) ions are bound to two water molecules and six terephthalate ions through oxygen atoms, and the Ln
3+ coordination number (CN) is equal to 8,
Figure 1c. The PXRD patterns of (Eu
1-xLu
x)
2bdc
3·nH
2O MOFs are shown in
Figure 2. Analysis of the data demonstrates that the structure of the compounds formed depends on the lutetium content. Thus, compounds with a concentration of lutetium (III) ions 90 at.% and less are isostructural to the Ln
2bdc
3·4H
2O crystalline phase. In contrast, when the concentration of lutetium is 95 at.% and more, the positions of the PXRD maxima do not correspond to any known Ln
2bdc
3·nH
2O structure, such as Eu
2bdc
3·4H
2O [
25], Tb
2bdc
3 [
25,
26], Yb
2bdc
3·6H
2O [
27], Lu
2bdc
3·10H
2O [
28], Sc
2bdc
3 [
29], Er
2bdc
3·3H
2O [
30].
To prove that compounds containing more than 95 at.% of lutetium have (Eu
1-xLu
x)
2bdc
3·nH
2O composition (containing some water molecules),samples were calcined at the temperature of 200 °C. The positions of the PXRD maxima of the obtained substances correspond to the anhydrous terephthalate structures (Eu
1-xLu
x)
2bdc
3, which are isostructural to Tb
2bdc
3 (
Figure 3). The formation of anhydrous terephthalate is the result of the loss of coordinated water molecules by (Eu
1-xLu
x)
2bdc
3·nH
2O, resulting in the formation of (Eu
1-xLu
x)
2bdc
3. Thermogravimetric analysis of Lu
2bdc
3·nH
2O in a temperature range of 30–230 °C allowed us to find the number of coordinated water molecules, n, in the synthesized microcrystalline lutetium terephthalate hydrate Lu
2bdc
3·nH
2O (
Figure 4a) under the assumption that this compound was formed in a single crystalline phase. The mass loss was observed at 60–150 °C. As previously reported [
19,
20], the mass loss in this temperature range can be attributed to the dehydration of the compounds, resulting in the formation of anhydrous terephthalate: Lu
2bdc
3·nH
2O → Lu
2bdc
3 + nH
2O. The average weight loss was found to be (5.31 ± 0.18)%, which corresponds to 2.62 ± 0.09 water molecules per unit formula of Lu
2bdc
3·nH
2O. The product of calcination was assigned to the anhydrous terephthalate based on the PXRD data showing that the calcination product, Lu
2bdc
3, is isostructural to Tb
2bdc
3,
Figure 3. Therefore, we proposed that the new unknown crystalline phase of obtained lutetium terephthalate hydrate has the composition of Lu
2bdc
3·2.5H
2O. This crystalline phase dominates at the lutetium content of 95 at.% and more in our samples. Thermogravimetric analysis of Eu
2bdc
3·4H
2O (
Figure 4b) confirmed the number of water molecules per unit formula of europium(III) terephthalate hydrate, where weight loss of 8.51% (5.31% ± 0.18) corresponds to 4.32 water molecules. Most probably, the thermogravimetric analysis gives overestimated values of the number of water molecules per unit formula of metal terephthalate because of the presence of a small amount of the absorbed water in the MOF pores.
Unit cell parameters were refined using UnitCell software [
31],
Table 1. This program can retrieve unit cell parameters from diffraction data using a least-squares method from the positions of the indexed diffraction maxima of PXRD patterns (Pawley method [
32]). For the compounds (Eu
1-xLa
x)
2bdc
3·4H
2O, the increase in La
3+ content leads to unit cell volumes increase due to a higher ionic radius of La
3+ ions (1.160 Å, the coordination number is eight) than the ionic radius of Eu
3+ ions (1.066 Å) [
33]. The ionic radius of the Gd
3+ ion (1.053 Å) is close to that of Eu
3+. Therefore, the unit cell parameters do not change significantly in the (Eu
1-xGd
x)
2bdc
3·4H
2O series. The Lu
3+ ion (ionic radius is 0.977 Å) is smaller than Eu
3+; therefore, substitution of Eu
3+ by Lu
3+ ion results in a decrease in the unit cell volumes in (Eu
1-xLu
x)
2bdc
3·4H
2O series with the lutetium (III) ions content of 90 at.% and less, where the MOF is formed as tetrahydrate, Ln
2bdc
3·4H
2O.
2.2. Particle Morphology
Scanning electron microscopy (SEM) was used to observe the shape and the size of the particles of the synthesized MOFs (Eu
1-xLn
x)
2bdc
3·nH
2O (Ln = La, Gd, Lu). The SEM images are shown in
Figure 5, where (Eu
1-xLa
x)
2bdc
3·nH
2O, (Eu
1-xGd
x)
2bdc
3·nH
2O, (Eu
1-xLu
x)
2bdc
3·nH
2O compounds are presented in the left, central, and right columns, respectively. The length and width of the particles obtained from the SEM images are given in
Table 2. In the (Eu
1-xLa
x)
2bdc
3·4H
2O and (Eu
1-xGd
x)
2bdc
3·4H
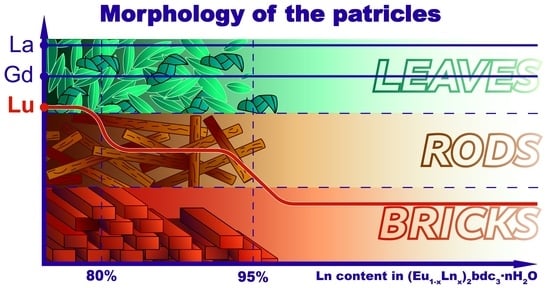
2O series, the particles have a similar oval plate shape, which can resemble one a petal or a leaf, as well as a similar size of about 6 × 2 μm. We noticed that the particles have different shapes depending on the content of lutetium ion in the (Eu
1-xLu
x)
2bdc
3·nH
2O series. Thus, at the Lu
3+ content below 90 at.%, the particles have the shape of rods and a size of about 4 × 0.8 μm. However, in (Eu
1-xLu
x)
2bdc
3·nH
2O MOFs with a concentration of Lu
3+ more than 90%, the particles are “brick”-shaped and are significantly larger, about 10 × 5 μm. At the Lu
3+ concentration of 90 and 95 at.%, the mixture of “bricks” and “rods” is observed. The shape difference probably results from the change in the crystalline structure at the Lu
3+ concentration of 95% among the (Eu
1-xLu
x)
2bdc
3·nH
2O series. Detailed analysis of the shape (Eu
1-xLa
x)
2bdc
3·4H
2O and (Eu
1-xGd
x)
2bdc
3·4H
2O microparticles revealed that leaf-shaped microparticles are made of rods, whereas the separate rods about of the same size are observed in the (Eu
1-xLu
x)
2bdc
3·4H
2O (x = 0.8, 0.9) samples. We propose that the reason might be in different dopant properties. Lu
3+ is the smallest ion among the trivalent lanthanide cations [
33]. Therefore, the Lu
3+ ion possesses the highest positive charge density among lanthanides. Therefore, we propose that the lutetium terephthalate surface has the highest surface charge density among the series. In the literature [
34], a higher surface charge density corresponds to a higher colloidal stability of the solution. We believe that small rod”-shaped particles initially form in the mixed terephthalates containing 80–90 at.% dopant. Therefore, Gd and La-doped particles coagulate, forming “leaf”-shaped particles, whereas Lu-doped particles are still colloidally stable and save their “rod” form if they correspond to the Ln
2bdc
3·4H
2O crystalline phase.
It is worth noting that the ultrasound-assisted method allowed us to obtain microparticles with a very small size variance (near 1–3 μm). This result suggests that using this method, it is possible to obtain particles with a certain morphology and size by changing the dopant content.
2.3. Luminescence Properties
Europium and terbium terephthalates demonstrate a pronounced antenna effect where terephthalate ion as a synthesizer or an “antenna” effectively absorbs UV radiation and transfers energy to a luminescent lanthanide ion followed by the metal-centered emission [
18]. Upon the excitation, the terephthalate ion is promoted into the S
n state, followed by the fast internal conversion to the S
1 state. Due to the heavy atom effect caused by the lanthanide atom, the S
1 state efficiently undergoes intersystem crossing to the T
1 triplet electronic excited state [
18]. The T
1 state of the terephthalate ion is close in energy to the
5D
1 energy level of the Eu
3+ ion. Therefore, an efficient energy transfer from sensitizer to luminescent lanthanide ion occurs. The
5D
1 level of the Eu
3+ then undergoes an internal conversion into the
5D
0 state, followed by the emission into the
7F
J (J = 0–4) lower-lying energy levels.
For the synthesized luminescent materials (Eu
1-xLn
x)
2bdc
3·nH
2O (Ln = La, Gd, Lu and x = 0, 0.80, 0.90, 0.95, 0.98), emission spectra were measured upon 310-nm excitation (
Figure 6 and
Figure 7). The emission spectra for the Gd and La-doped compounds are similar to the pure europium terephthalateemission spectrum. This observation agrees with the PXRD data showing that (Eu
1-xLn
x)
2bdc
3·nH
2O (Ln = La, Gd) compounds are isostructural to Eu
2bdc
3·4H
2O compound (
Figure 6). The emission spectra of Gd and La-doped terephthalates consist of the narrow bands corresponding to
5D
0-
7F
J (J = 0–4) transitions of Eu
3+:
5D
0-
7F
1 (588.2 and 591.6 nm),
5D
0-
7F
2 (614.6 nm), and
5D
0-
7F
4 (696.6 nm).
5D
0-
7F
0 and
5D
0-
7F
3 bands were not observed in the emission spectra due to their weak intensity.
The emission spectra of the (Eu
1-xLu
x)
2bdc
3·nH
2O compounds are shown in
Figure 7. This figure clearly shows the difference in the fine structure of the measured emission spectra for the Lu concentration of 0, 80, 90, 95, and 98 at.%. The first three emission spectra are identical to the spectrum of Eu
2bdc
3·4H
2O. This observation is consistent with PXRD data demonstrating that (Eu
1-xLu
x)
2bdc
3·nH
2O compounds (x = 0.80, 0.90) crystalize in Eu
2bdc
3·4H
2O phase without formation of any additional phase. The emission spectra of the (Eu
1-xLu
x)
2bdc
3·nH
2O compounds consist of narrow bands corresponding to
5D
0-
7F
1 (587.9, 591.3, and 591.8 nm),
5D
0-
7F
2 (614.2 nm), and
5D
0-
7F
4 (696.7 nm). On the contrary, the emission spectra of the (Eu
1-xLu
x)
2bdc
3·nH
2O (x = 0.95, 0.98) compounds are different from the spectrum of pure europium terephthalate. Such difference can be explained by the formation of another phase, namely Ln
2bdc
3·2.5H
2O, where the local symmetry of the europium (III) ion is different from that of Eu
2bdc
3·4H
2O. These spectra consist of narrow bands corresponding to
5D
0-
7F
1 (588.6, 591.6, and 593 nm),
5D
0-
7F
2 (613.7, 616.0, and 622.0 nm), and
5D
0-
7F
4 (698.2 nm).
The photoluminescence decay curves were measured under UV-excitation and monitored at 614 nm (
5D
0-
7F
2) for the (Eu
1-xLn
x)
2bdc
3·nH
2O (Ln = La, Gd, Lu and x = 0, 0.80, 0.90, 0.95, 0.98) compounds (
Figure 8 and
Figure 9). The photoluminescence decay curves of La and Gd doped terephthalates (
Figure 8) were accurately fitted by single exponential functions (Equation (1)), resulting in time constants
τ of about 0.43–0.47 ms for the Gd doped terephthalates and about 0.43–0.45 ms for the La-doped terephthalates (
Table 3). For the (Eu
1-xLu
x)
2bdc
3·nH
2O materials, the photoluminescence decay curves were fitted by the single exponential functions (Equation (1)) at the lutetium content less than 90 at.% resulting in time constants
τ of about 0.43–0.46 ms (
Figure 9). Meanwhile, at lutetium concentrations more than 90 at.% The photoluminescence decay curves can be fitted only using the double exponential functions (Equation (2)) with the time constants
τ1 of about 0.34–0.46 ms and
τ2 of about 1.5 ms. The two-exponential decay of the highly doped Eu-Lu terephthalates (Eu
1-xLu
x)
2bdc
3·nH
2O (x = 0.95, 0.98) indicates the two different coordination sites of the Eu
3+ ion. The fast component (0.3–0.5 ms) is close to that of the water-coordinated Eu
3+ ion in the Ln
2bdc
3·4H
2O structure, whereas the slow component (1.5–1.6 ms) corresponds to the Eu
3+ ion exclusively coordinated to carboxylic groups of terephthalate ions [
20] due to the absence of luminescence quenching of Eu
3+ by coordinated water molecules containing high-frequency OH vibrational modes.
For all synthesized materials, photoluminescence quantum yields (PLQYs) were measured (
Table 3). PLQYs for the Gd and La-doped terephthalates are found to be approximately equal. But for the (Eu
1-xLu
x)
2bdc
3·nH
2O materials, PLQYs grow with the Lu concentration increase and reach a maximum of 23% at the 95 at.% Lu. The significantly higher values of photoluminescence quantum yield in Eu-Lu terephthalates at high Lu content can be explained by the presence in these materials’ Eu
3+ coordination sites bound only to carboxylic groups of terephthalate ions [
20] and not to the water molecules, which quenches the Eu
3+ luminescence.
Due to the different nature of the f-f transitions of Eu
3+ ions, these ions can be used as structural probes [
35,
36]. Thus, the probability of hypersensitive
5D
0-
7F
2 forced electric dipole transition is strongly affected by the local environment of the europium ion, whereas the probability of
5D
0-
7F
1 magnetic dipole transition intensity is significantly less sensitive to changes in the Eu
3+ coordination sphere. The analysis of asymmetry ratio R
as, which is equal to the ratio of the integral intensity of these transitions (
5D
0-
7F
2)/(
5D
0-
7F
1), allows one to track the changes in the local environment of the europium (III) ions [
37,
38]. The higher values of the asymmetry ratio correspond to the greater deviation of the Eu
3+ environment from the centrosymmetric one [
39]. The asymmetry ratio for the synthesized compounds is given in
Table 4. The asymmetry ratio decreases with the increasing dopant concentration caused by the distortion of the crystal structure near the luminescent centers. The lutetium-doped materials have a minimal asymmetry ratio at the whole concentration range of the dopant, especially when the concentration of lutetium is 95 at.% and even more when the phase transition occurs. The reason for this effect is that the lutetium (III) ion has a smaller ionic radius than the gadolinium(III) and lanthanum(III) doping ions, and the environment of the luminescent center is not distorted. Lanthanum and gadolinium-doped terephthalates have nearly equal asymmetry ratios because of the almost identical structures of these materials.