Anticancer Activity of Astaxanthin-Incorporated Chitosan Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Characterization of Astazanthin-Incorporated Chitosan (ChitoAST) Nanoparticles
2.2. Antioxidants of ChitoAST Nanoparticles
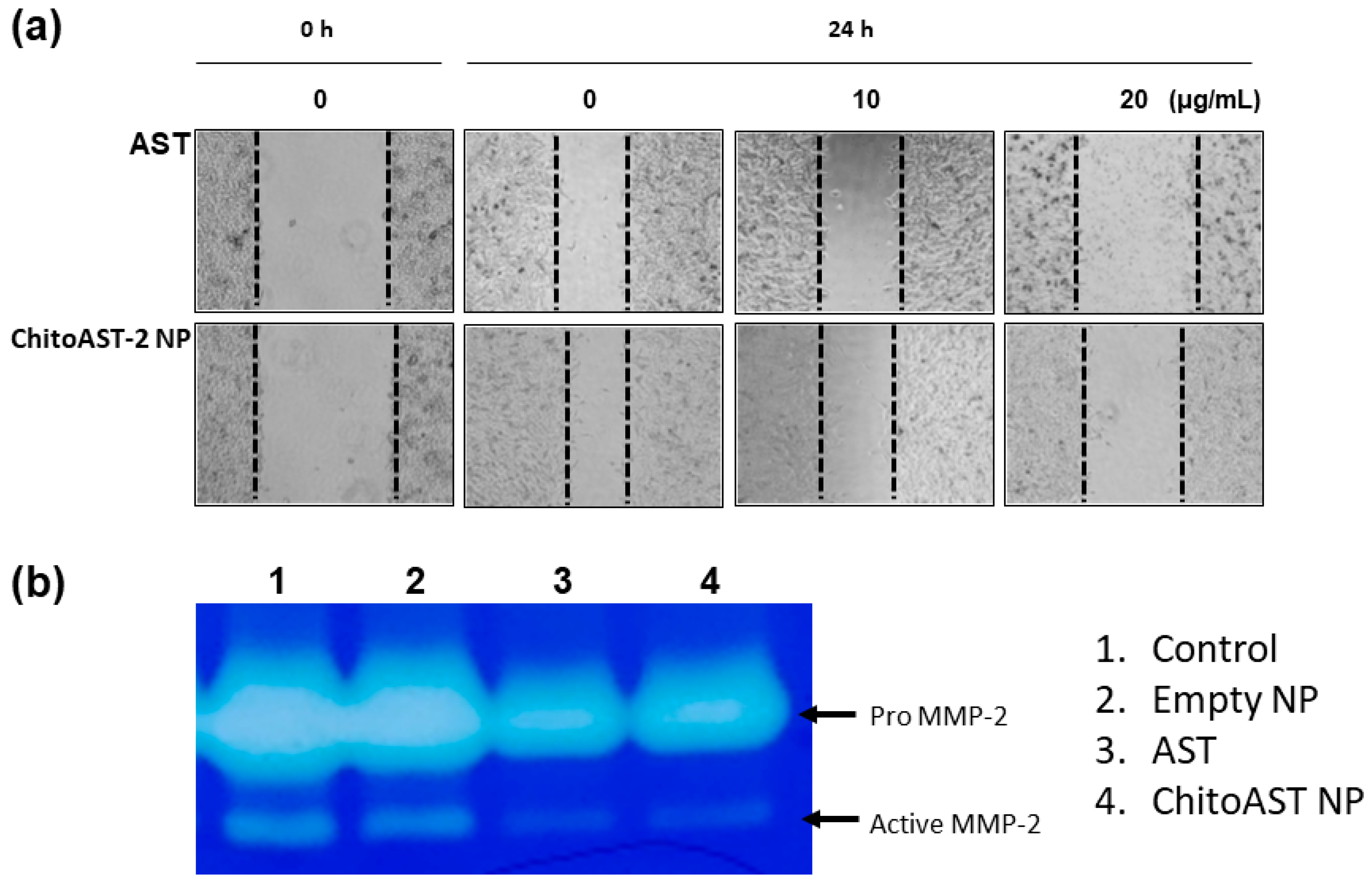
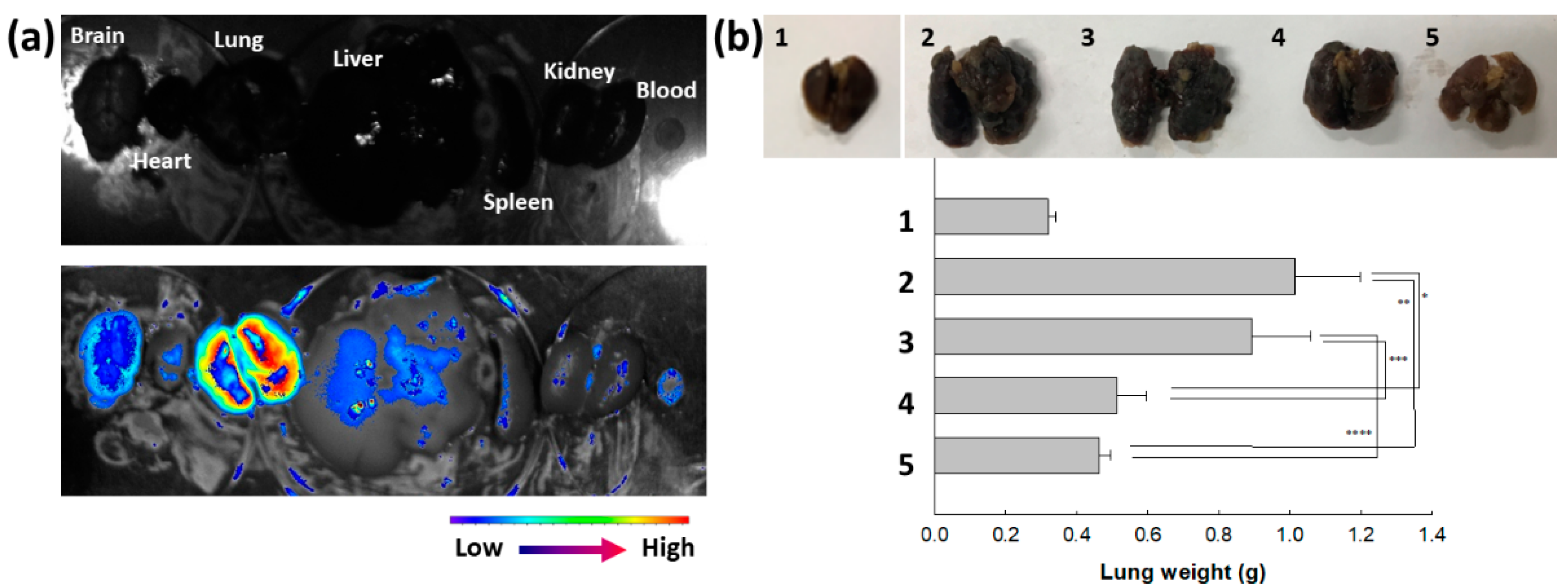
2.3. Anticancer Activity of ChitoAST Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparaton of AST-Incorporated Chitosan (ChitoAST) Nanoparticles
4.3. Preparation of Ce6-Incorporated ChitoAST Nanoparticles
4.4. Characterization of Nanoparticles
4.5. Cell Viability
4.6. ABTS Assay
4.7. UVB Irradiation Effect
4.8. Nitric Oxide (NO) Assay
4.9. Wound Healing Assay and Gelatin Zymography
4.10. Animal Pulmonary Metastasis Model and In Vivo Animal Tumor Imaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, K.; Ghosh, R.; Homechaudhuri, S.; Mitra, A. Biochemical composition of marine macroalgae from gangetic delta at the apex of bay of Bengal. Afr. J. Basic Appl. Sci. 2009, 1, 96–104. [Google Scholar]
- Cirino, P.; Brunet, C.; Ciaravolo, M.; Galasso, C.; Musco, L.; Vega Fernández, T.; Sansone, C.; Toscano, A. The sea urchin Arbacia lixula: A novel natural source of astaxanthin. Mar. Drugs 2017, 15, 187. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquacult. 2017, 10, 738–773. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, J.M.; Kim, S.; Yoon, M.J.; Park, K.S. Chemical transformation of astaxanthin from Haematococcus pluvialis improves its antioxidative and anti-inflammatory activities. ACS Omega 2020, 5, 19120–19130. [Google Scholar] [CrossRef]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of astaxanthin and their consequences for therapeutic application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Alam, R.; Łochyńska, M.; Stasiewicz, M. What do we know about antimicrobial activity of astaxanthin and fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 1999, 70, 185–189. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, J.W.; Kim, H. Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in Helicobacter pylori-infected gastric epithelial cells. Nutrients 2018, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Lin, K.C.; Lu, W.J.; Thomas, P.A.; Jayakumar, T.; Sheu, J.R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and IL-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int. J. Mol. Sci. 2015, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, N.; Shi, Y.; Zhou, B.; Sun, D.; Ma, B.; Xu, Z.; Yang, J.; Li, C. Astaxanthin protects dendritic cells from lipopolysaccharide-induced immune dysfunction. Mar. Drugs 2021, 19, 346. [Google Scholar] [CrossRef]
- Gao, J.; Yang, D.; Cao, R.; Pan, X.; Xia, J. Therapeutic mechanism of natural astaxanthin against renal clear cell carcinoma based on network pharmacology and bioinformatics. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 1763–1772. [Google Scholar] [PubMed]
- Ramamoorthy, K.; Raghunandhakumar, S.; Anand, R.S.; Paramasivam, A.; Kamaraj, S.; Nagaraj, S.; Ezhilarasan, D.; Lakshmi, T.; Dua, K.; Chellappan, D.K.; et al. Anticancer effects and lysosomal acidification in A549 cells by Astaxanthin from Haematococcus lacustris. Bioinformation 2020, 16, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Nakamura, S.; Maoka, T.; Yamada, T.; Imai, T.; Ohba, T.; Yako, T.; Hayashi, M.; Endo, K.; Saio, M.; et al. Antitumour effects of astaxanthin and adonixanthin on glioblastoma. Mar. Drugs 2020, 18, 474. [Google Scholar] [CrossRef] [PubMed]
- Kupcinskas, L.; Lafolie, P.; Lignell, A.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Trimarco, V.; Battistoni, A.; Tocci, G.; Coluccia, R.; Manzi, M.V.; Izzo, R.; Volpe, M. Single blind, multicentre, randomized, controlled trial testing the effects of a Novel Nutraceutical Compound on Plasma Lipid and Cardiovascular Risk Factors: Results of the Interim Analysis. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 850–857. [Google Scholar] [CrossRef]
- Lockwood, S.F.; O’Malley, S.; Mosher, G.L. Improved aqueous solubility of crystalline astaxanthin (3,3′-dihydroxy-beta, beta-carotene-4,4′-dione) by Captisol (sulfobutyl ether beta-cyclodextrin). J. Pharm. Sci. 2003, 92, 922–926. [Google Scholar] [CrossRef]
- Kim, S.; Cho, E.; Yoo, J.; In, M.J.; Chae, H.J. Solubility and storage stability of astaxanthin. Korean J. Biotechnol. Bioeng. 2008, 23, 546–550. [Google Scholar]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.Y.; Yagi, S.; Hirono-Hara, Y.; Kikukawa, H. A method of solubilizing and concentrating astaxanthin and other carotenoids. Mar. Drugs 2021, 19, 462. [Google Scholar] [CrossRef] [PubMed]
- Slonimskiy, Y.B.; Egorkin, N.A.; Friedrich, T.; Maksimov, E.G.; Sluchanko, N.N. Microalgal protein AstaP is a potent carotenoid solubilizer and delivery module with a broad carotenoid binding repertoire. FEBS J. 2021, 289, 999–1022. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, S.; Gref, R. Synthetic and bioinspired cage nanoparticles for drug delivery. Nanomedicine 2014, 9, 1545–1564. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Jeong, Y.I.; Choi, C.; Roh, S.H.; Kang, S.K.; Jang, M.K.; Nah, J.W. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int. J. Pharm. 2006, 319, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Jia, L. Nanoparticle formulation increases oral bioavailability of poorly soluble drugs: Approaches experimental evidences and theory. Curr. Nanosci. 2005, 1, 237–243. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Na, H.S.; Seo, D.H.; Kim, D.G.; Lee, H.C.; Jang, M.K.; Na, S.K.; Roh, S.H.; Kim, S.I.; Nah, J.W. Ciprofloxacin-encapsulated poly(DL-lactide-co-glycolide) nanoparticles and its antibacterial activity. Int. J. Pharm. 2008, 352, 317–323. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Kim, S.T.; Jin, S.G.; Ryu, H.H.; Jin, Y.H.; Jung, T.Y.; Kim, I.Y.; Jung, S. Cisplatin-incorporated hyaluronic acid nanoparticles based on ion-complex formation. J. Pharm. Sci. 2008, 97, 1268–1276. [Google Scholar] [CrossRef]
- Jafari, Z.; Bigham, A.; Sadeghi, S.; Dehdashti, S.M.; Rabiee, N.; Abedivash, A.; Bagherzadeh, M.; Nasseri, B.; Karimi-Maleh, H.; Sharifi, E.; et al. Nanotechnology-abetted astaxanthin formulations in multimodel therapeutic and biomedical applications. J. Med. Chem. 2022, 65, 2–36. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.S.; Sung, D.; Lim, J.M.; Choi, W.I. Potential antioxidant and wound healing effect of nano-liposol with high loading amount of astaxanthin. Int. J. Nanomed. 2020, 15, 9231–9240. [Google Scholar] [CrossRef]
- Kim, S.; Cho, E.; Yoo, J.; Cho, E.; Choi, S.J.; Son, M.S.; Lee, J.M.; In, M.J.; Kim, D.C.; Kim, J.H.; et al. b-CD-mediated encapsulation enhanced stability and solubility of astaxanthin. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 559–565. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Tie, S.; Li, J.; Su, W.; Tan, M. A smart cauliflower-like carrier for astaxanthin delivery to relieve colon inflammation. J. Control. Release 2022, 342, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Snipstad, S.; Westrøm, S.; Mørch, Y.; Afadzi, M.; Åslund, A.K.; de Lange Davies, C. Contact-mediated intracellular delivery of hydrophobic drugs from polymeric nanoparticles. Cancer Nanotechnol. 2014, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Kwak, T.W.; Lee, H.L.; Song, Y.H.; Kim, C.; Kim, J.; Seo, S.J.; Jeong, Y.I.; Kang, D.H. Vorinostat-eluting poly(DL-lactide-co-glycolide) nanofiber-coated stent for inhibition of cholangiocarcinoma cells. Int. J. Nanomed. 2017, 12, 7669–7680. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular mechanisms and genetics of oxidative stress in Alzheimer’s disease. J. Alzheimers Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Lee, R.; Margaritis, M.; Channon, K.M.; Antoniades, C. Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr. Med. Chem. 2012, 19, 2504–2520. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between oxidative stress, nutrition, and cancer initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Percário, S.; da Silva Barbosa, A.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; de Nazaré Araújo Moreira, T.; Dolabela, M.F. Oxidative stress in Parkinson’s disease: Potential benefits of antioxidant supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2360872. [Google Scholar] [CrossRef] [PubMed]
- Campolo, J.; De Maria, R.; Cozzi, L.; Parolini, M.; Bernardi, S.; Proserpio, P.; Nobili, L.; Gelosa, G.; Piccolo, I.; Agostoni, E.C.; et al. Antioxidant and inflammatory biomarkers for the identification of prodromal Parkinson’s disease. J. Neurol. Sci. 2016, 370, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sarada, R.; Shylaja, M.D.; Ravishankar, G.A. Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J. Food Sci. Technol. 2015, 52, 6703–6710. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Lin, C.W.; Yang, C.M.; Yang, C.H. Protective effect of astaxanthin on blue light light-emitting diode-induced retinal cell damage via free radical scavenging and activation of PI3K/Akt/Nrf2 pathway in 661W cell model. Mar. Drugs 2020, 18, 387. [Google Scholar] [CrossRef]
- Chung, B.Y.; Park, S.H.; Yun, S.Y.; Yu, D.S.; Lee, Y.B. Astaxanthin protects ultraviolet B-induced oxidative stress and apoptosis in human keratinocytes via intrinsic apoptotic pathway. Ann. Dermatol. 2022, 34, 125–131. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative effects of ascorbic acid and astaxanthin on ARPE-19 cells in an oxidative stress model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef]
- Krestinina, O.; Baburina, Y.; Krestinin, R. Mitochondrion as a target of astaxanthin therapy in heart failure. Int. J. Mol. Sci. 2021, 22, 7964. [Google Scholar] [CrossRef]
- Karimian, A.; Mir Mohammadrezaei, F.; Hajizadeh Moghadam, A.; Bahadori, M.H.; Ghorbani-Anarkooli, M.; Asadi, A.; Abdolmaleki, A. Effect of astaxanthin and melatonin on cell viability and DNA damage in human breast cancer cell lines. Acta Histochem. 2022, 124, 151832. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.; Gu, X.; Huang, X. The role of the reactive oxygen species scavenger agent, astaxanthin, in the protection of cisplatin-treated patients against hearing loss. Drug Des. Devel Ther. 2019, 13, 4291–4303. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Lin, Y.J.; Liu, W.; Lin, H.Y.; Chou, H.Y.; Thia, C.; Wu, J.H.; Chang, J.S.; Wen, Z.H.; Chang, J.J.; et al. Metabolic engineering probiotic yeast produces 3S, 3′S-astaxanthin to inhibit B16F10 metastasis. Food Chem. Toxicol. 2020, 135, 110993. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 15, RA32–RA40. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
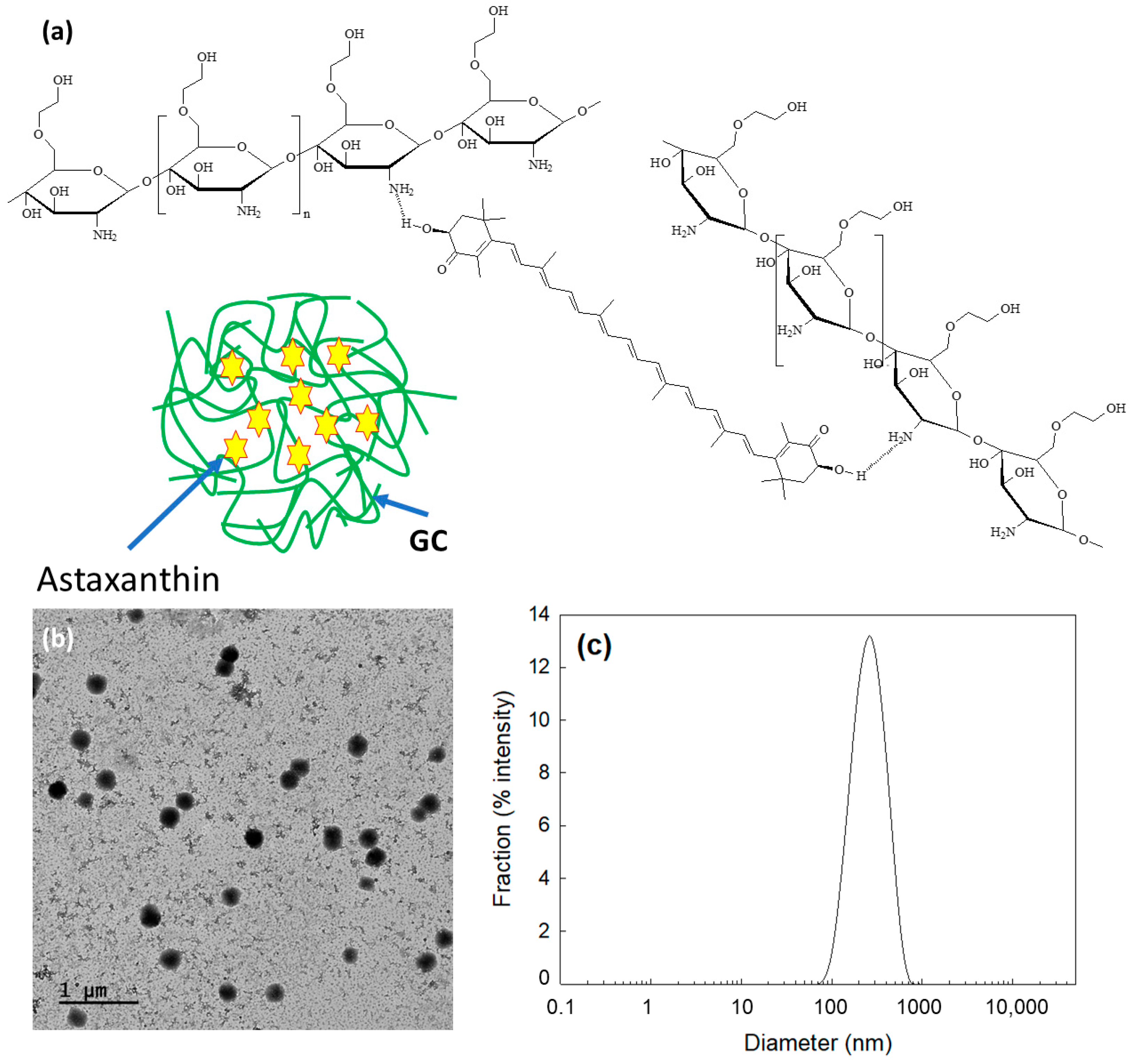
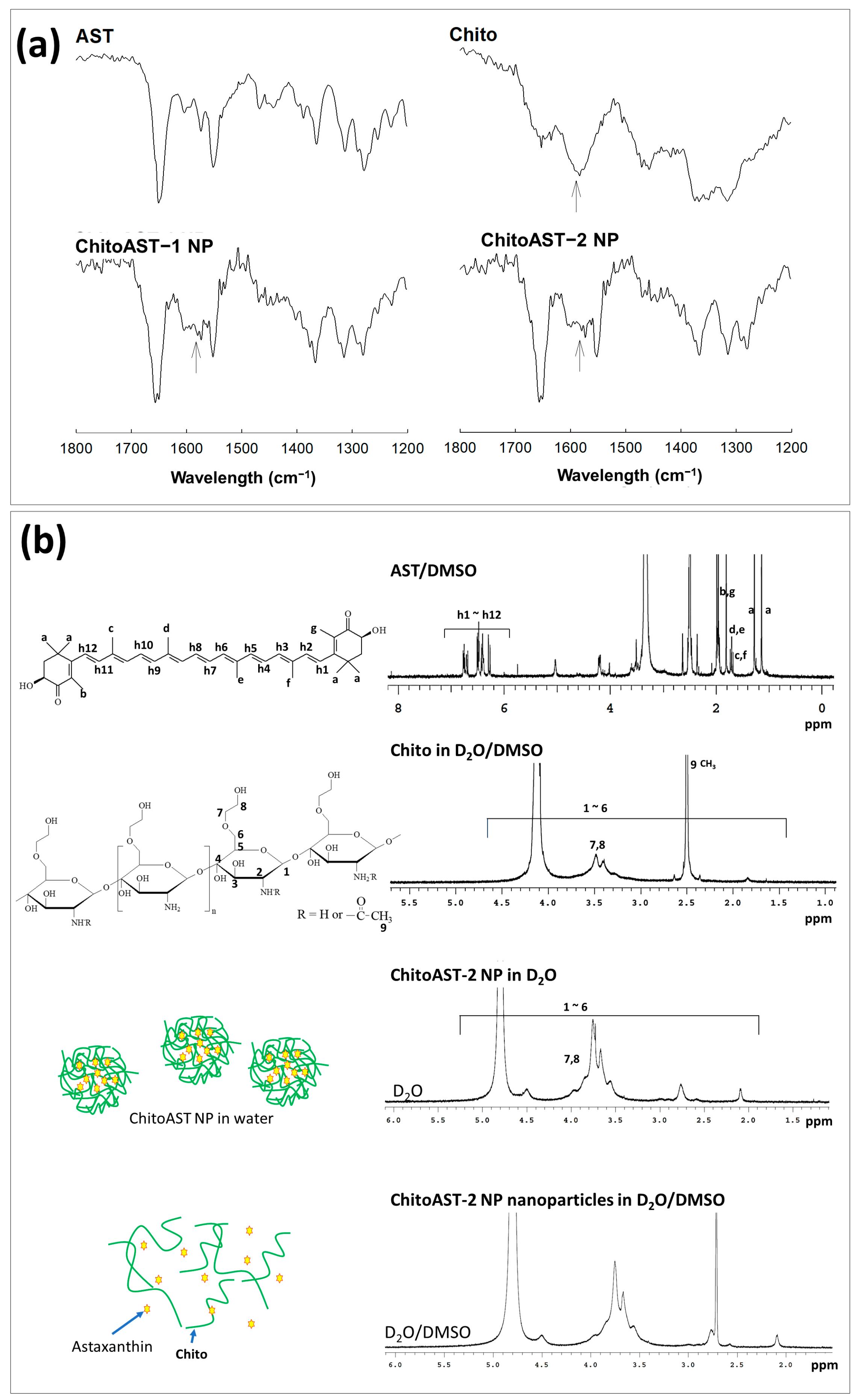
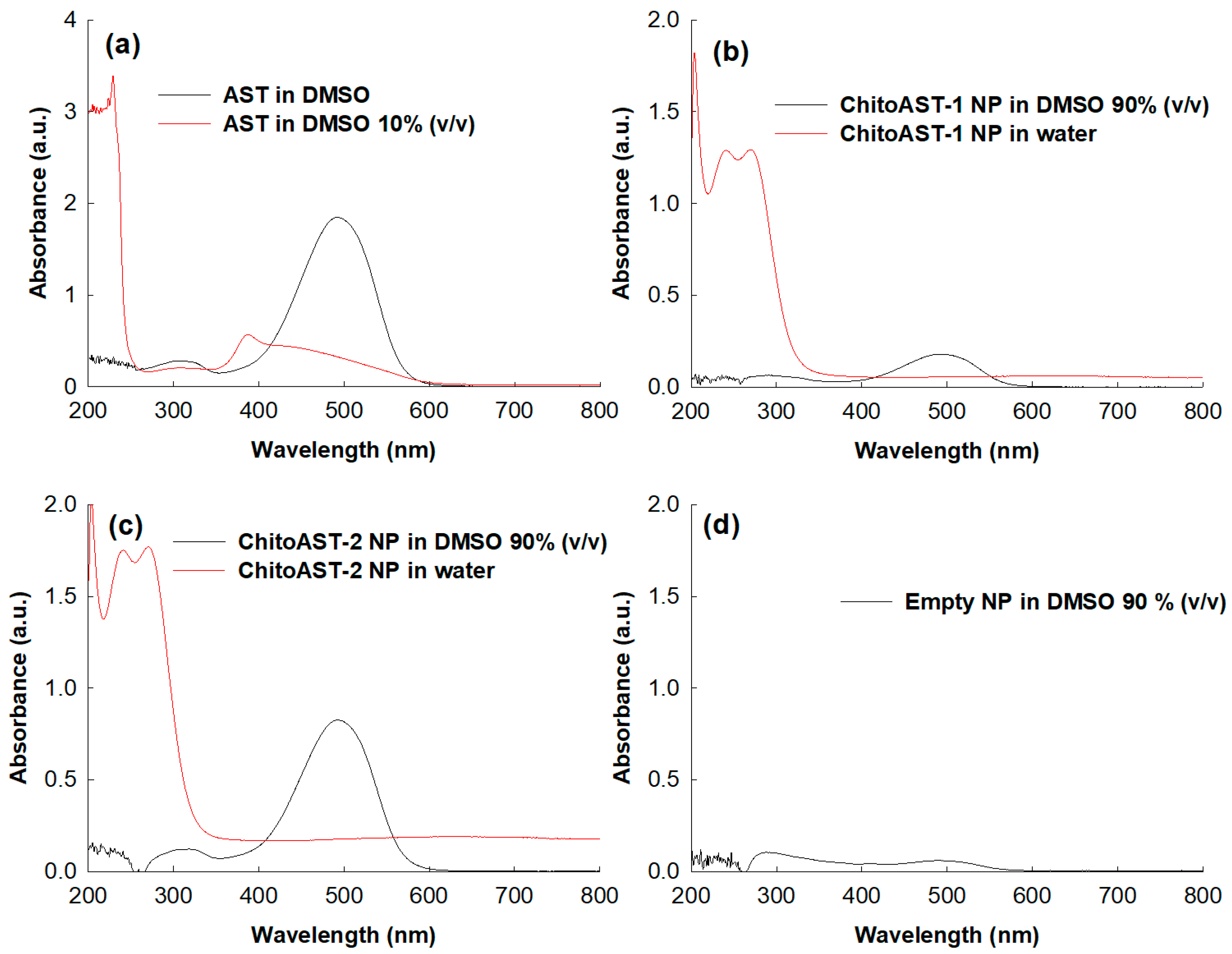


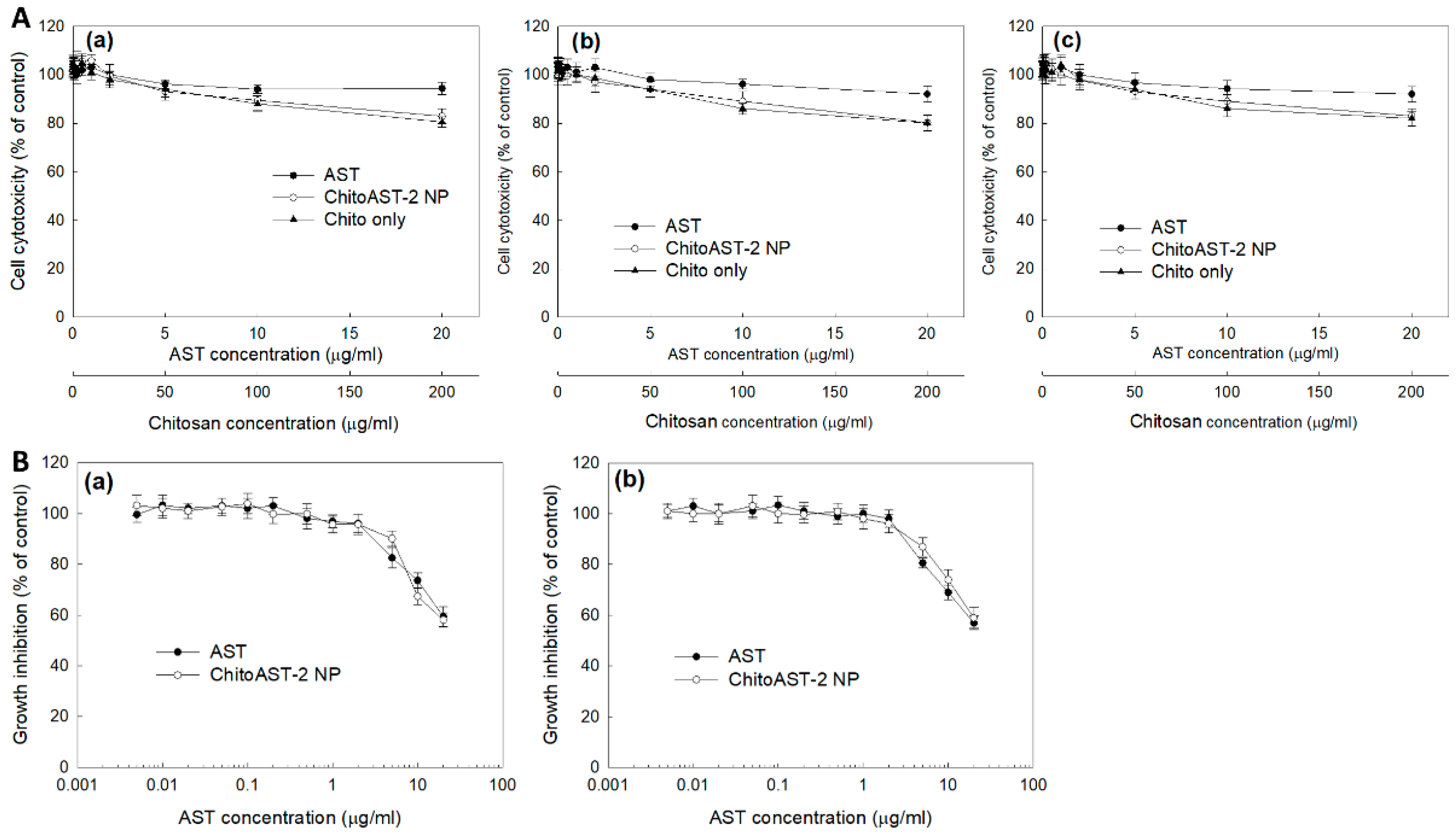
| Formulation | AST/GC (mg/mg) | Particle Size (nm) | Drug Contents a (%, w/w) | Loading Efficiency b (%, w/w) |
|---|---|---|---|---|
| Empty NP c | 0/100 | - | - | - |
| ChitoAST-1 | 1/100 | 380 ± 35 | 0.92 | 92.0 |
| ChitoAST-2 | 5/100 | 270 ± 23 | 4.3 | 86.4 |
| Sample | ABTS RC50 (µg/mL) a |
|---|---|
| AST | 11.8 |
| ChitoAST-2 NP | 29.3 |
| L-ascorbic acid | 4.1 |
| Trolox | 8.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, E.J.; Jeong, Y.-I.; Lee, K.-J.; Yu, Y.-B.; Ohk, S.-H.; Lee, S.-Y. Anticancer Activity of Astaxanthin-Incorporated Chitosan Nanoparticles. Molecules 2024, 29, 529. https://doi.org/10.3390/molecules29020529
Hwang EJ, Jeong Y-I, Lee K-J, Yu Y-B, Ohk S-H, Lee S-Y. Anticancer Activity of Astaxanthin-Incorporated Chitosan Nanoparticles. Molecules. 2024; 29(2):529. https://doi.org/10.3390/molecules29020529
Chicago/Turabian StyleHwang, Eun Ju, Young-IL Jeong, Kyong-Je Lee, Young-Bob Yu, Seung-Ho Ohk, and Sook-Young Lee. 2024. "Anticancer Activity of Astaxanthin-Incorporated Chitosan Nanoparticles" Molecules 29, no. 2: 529. https://doi.org/10.3390/molecules29020529





