Geopolymer-Based Materials for the Removal of Ibuprofen: A Preliminary Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Absorbing Materials
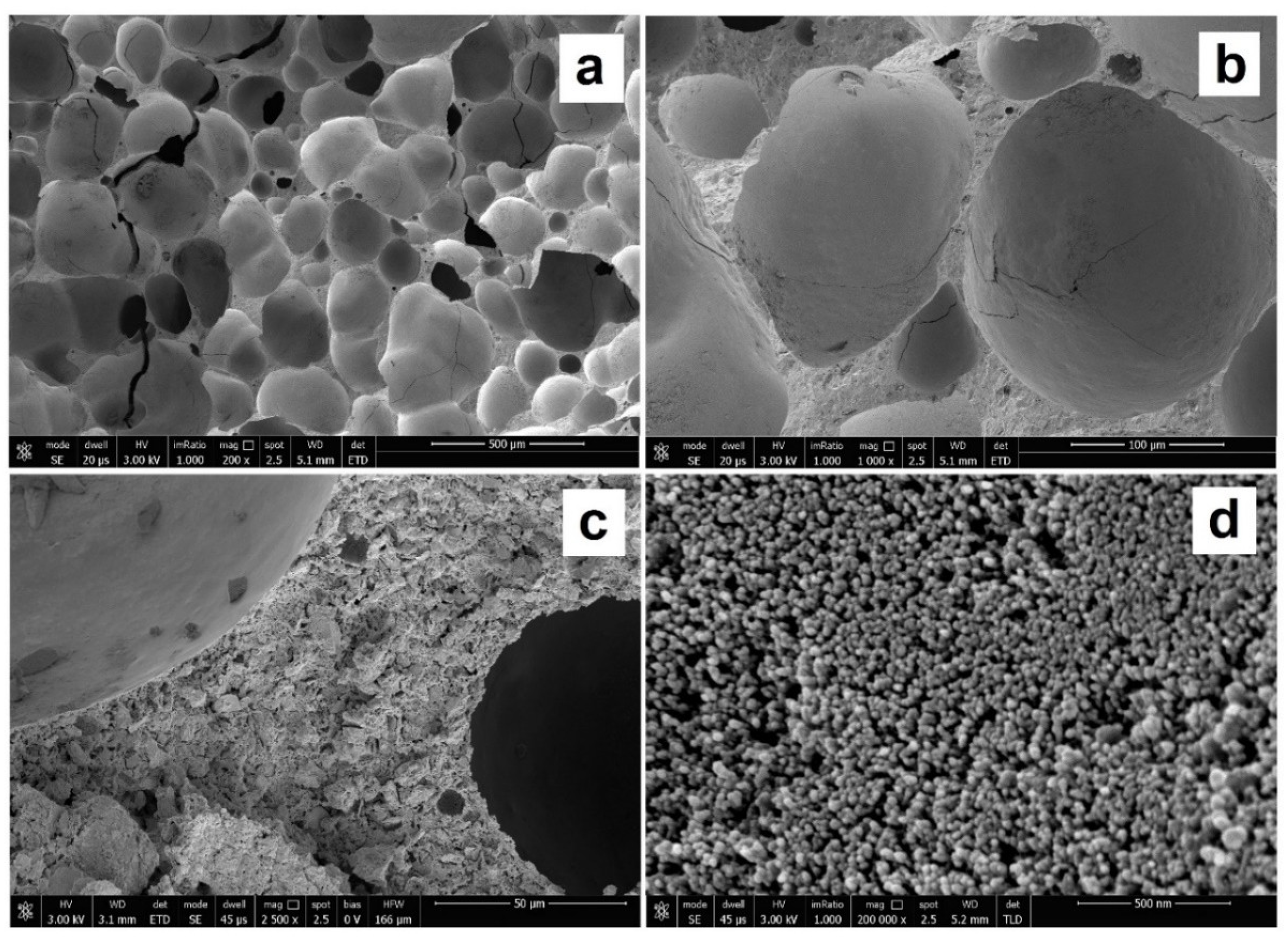
2.1.1. Preparation and Characterization of the Absorbing Foams
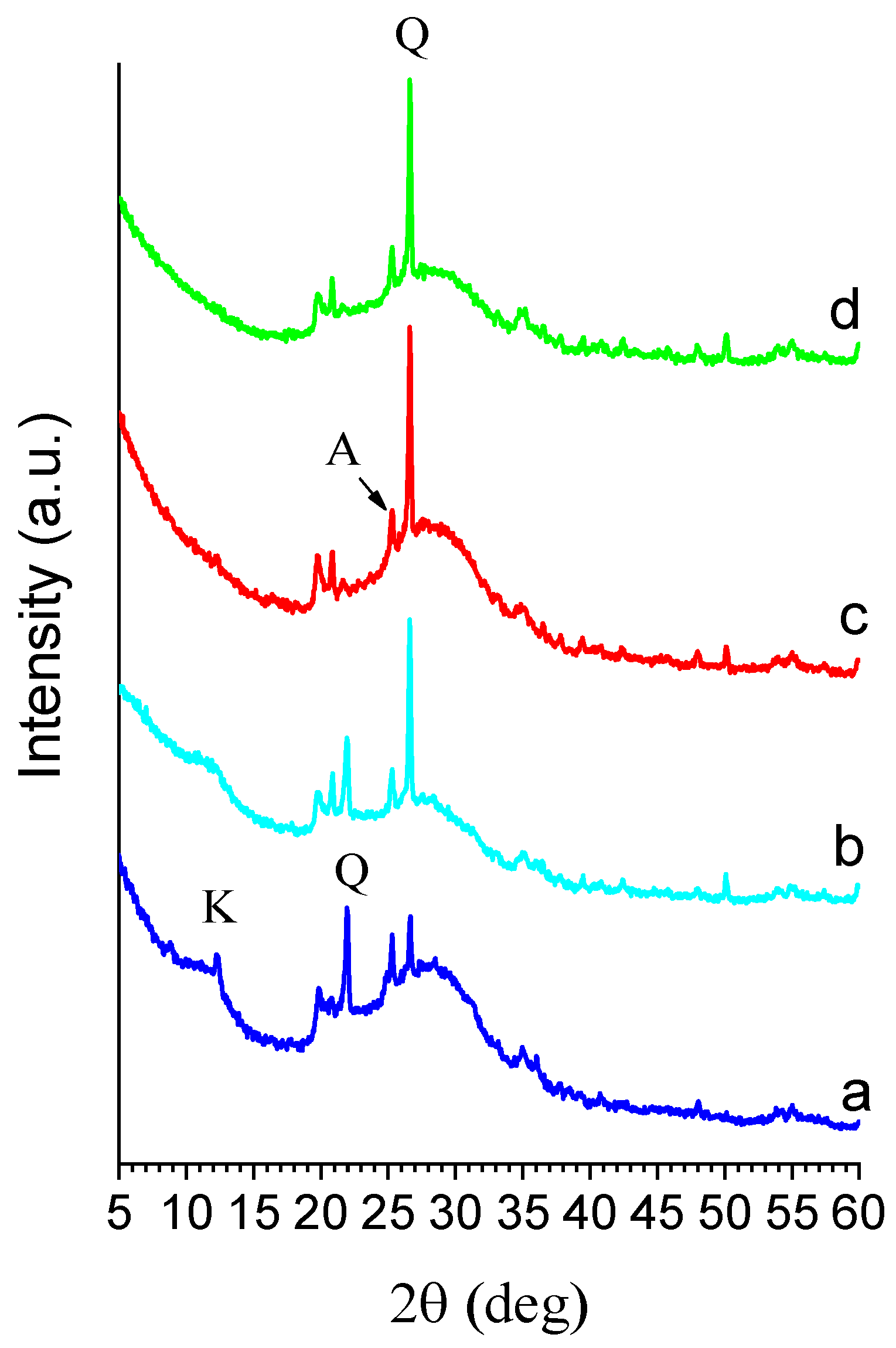
2.1.2. X-ray Diffraction
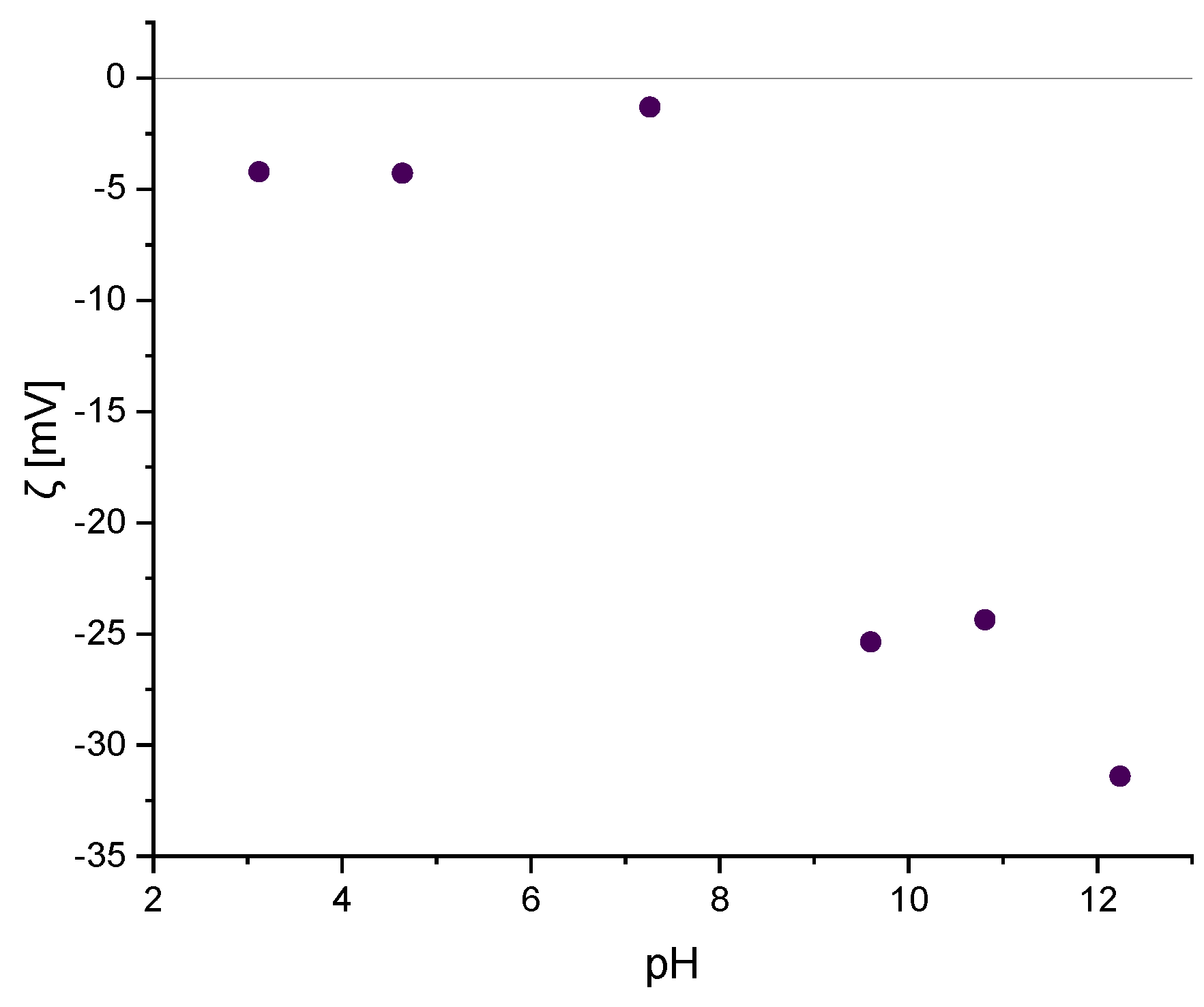
2.1.3. Zeta Potential Analysis
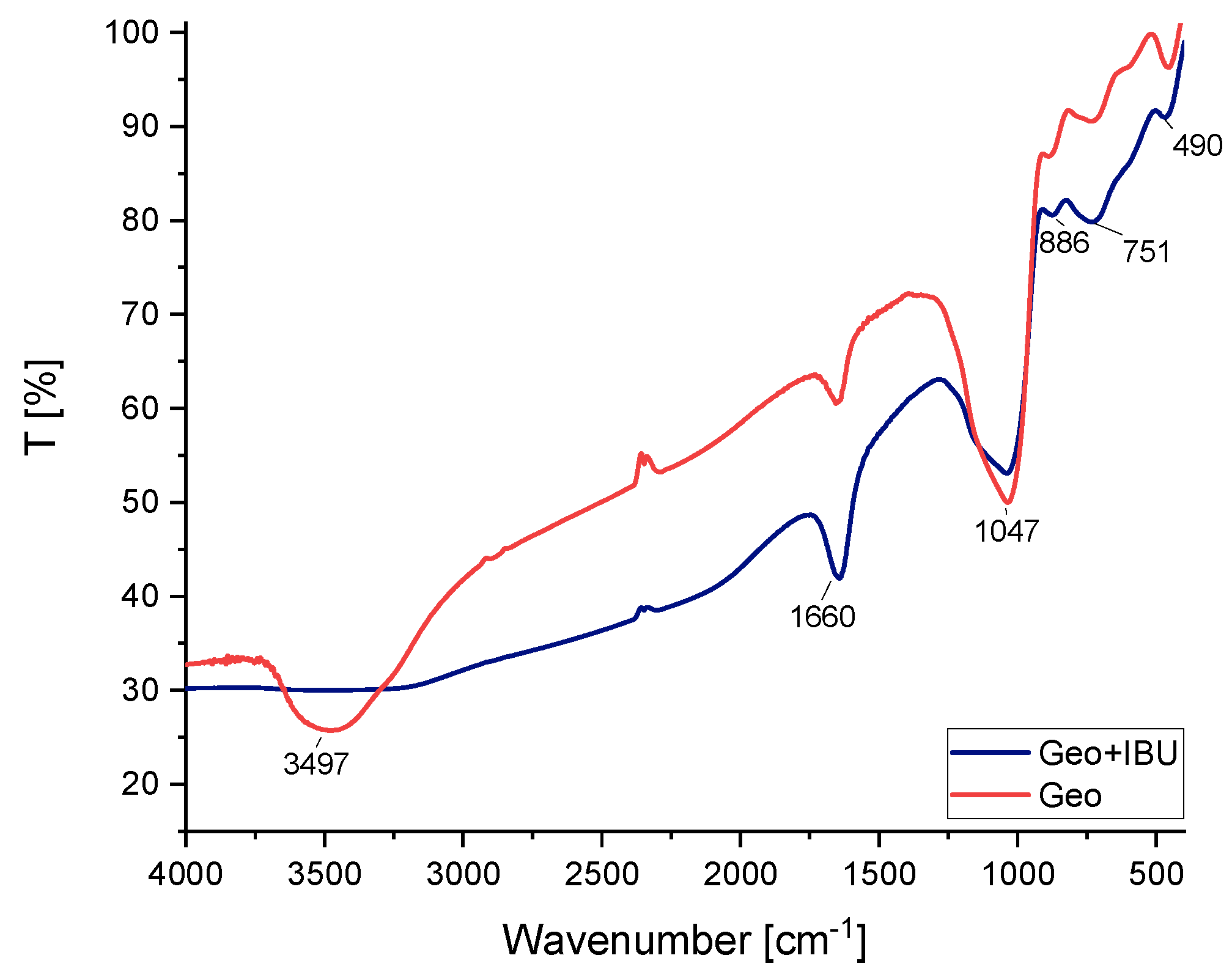
2.1.4. FT-IR Spectra
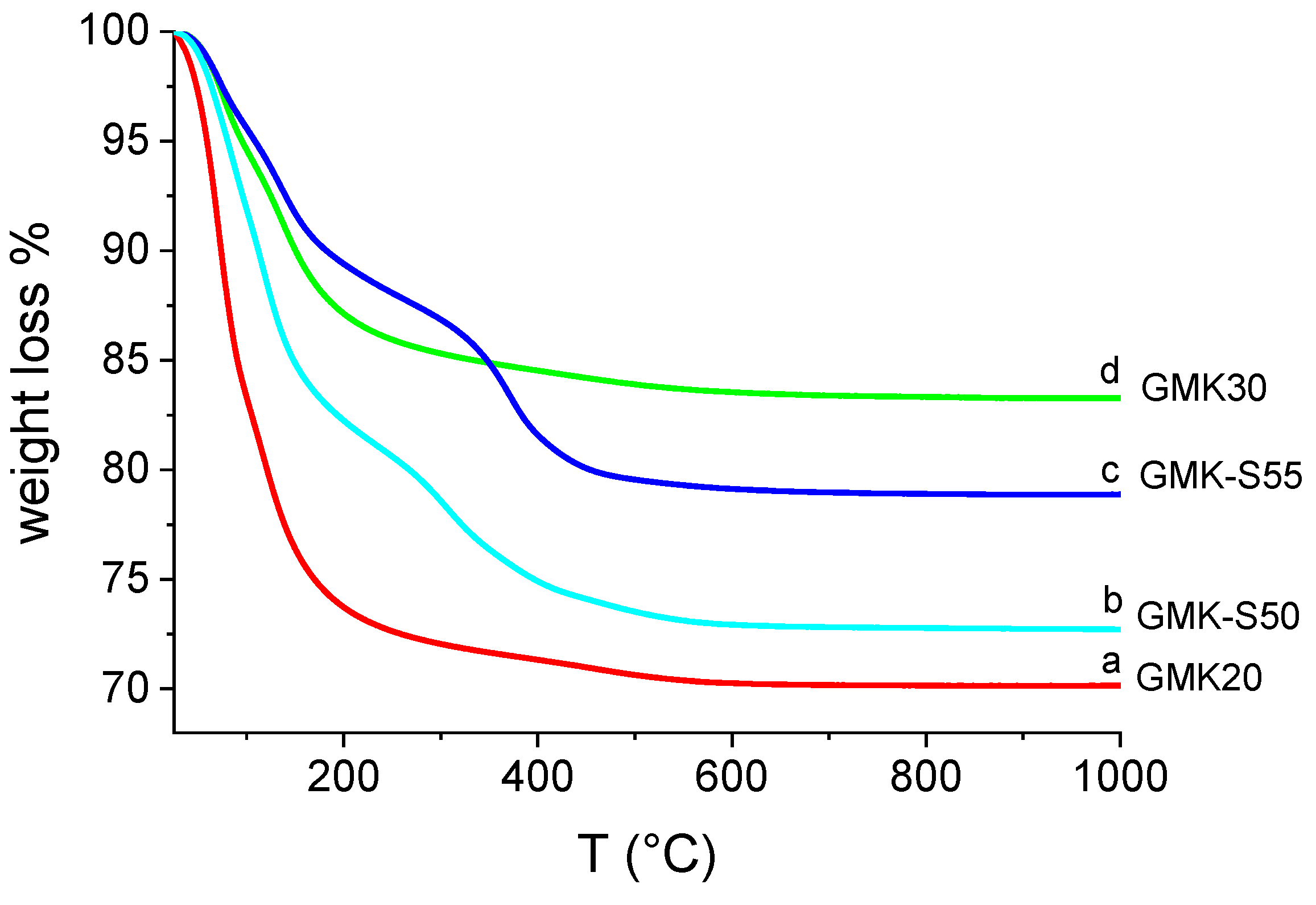
2.1.5. Thermogravimetric Analysis
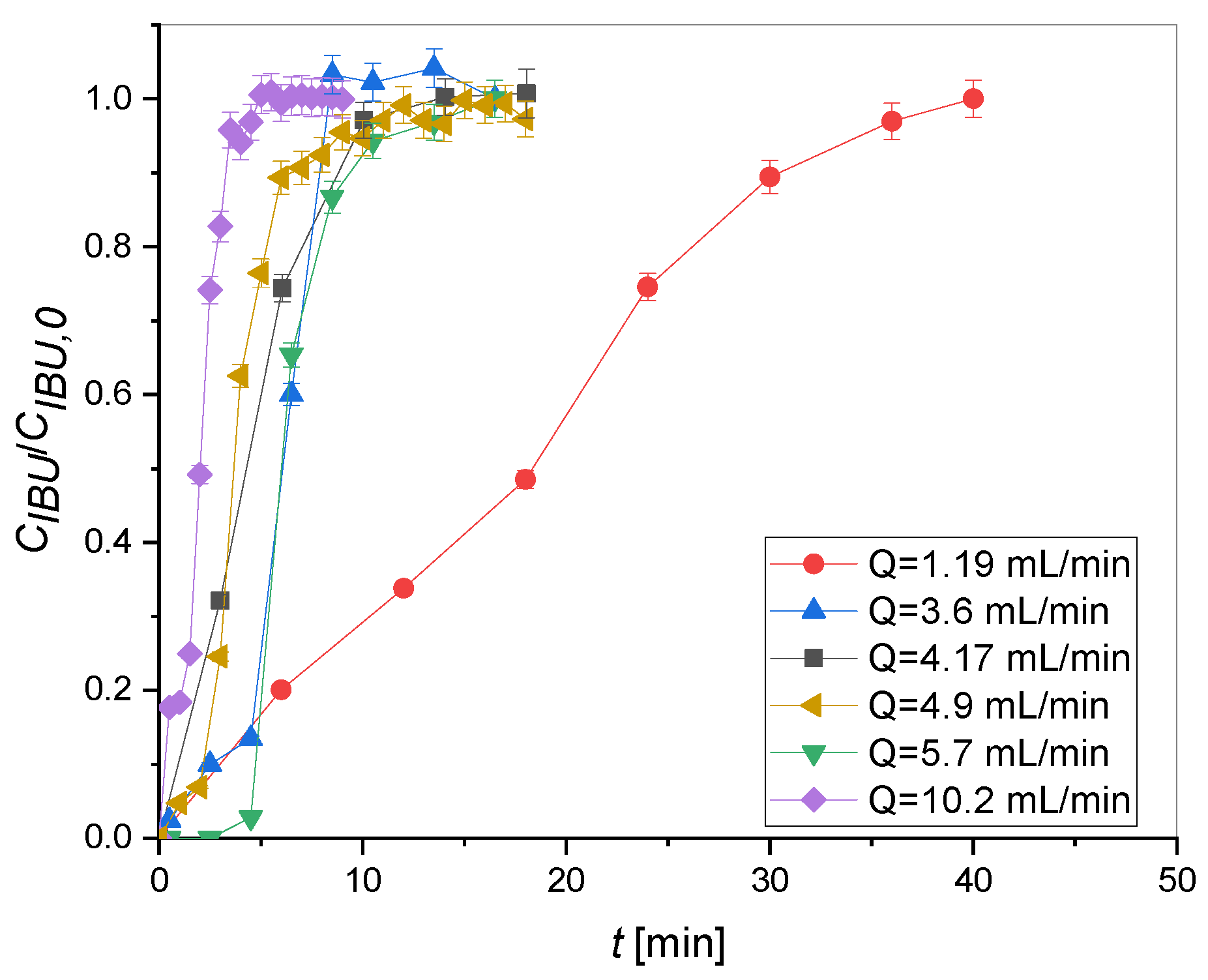
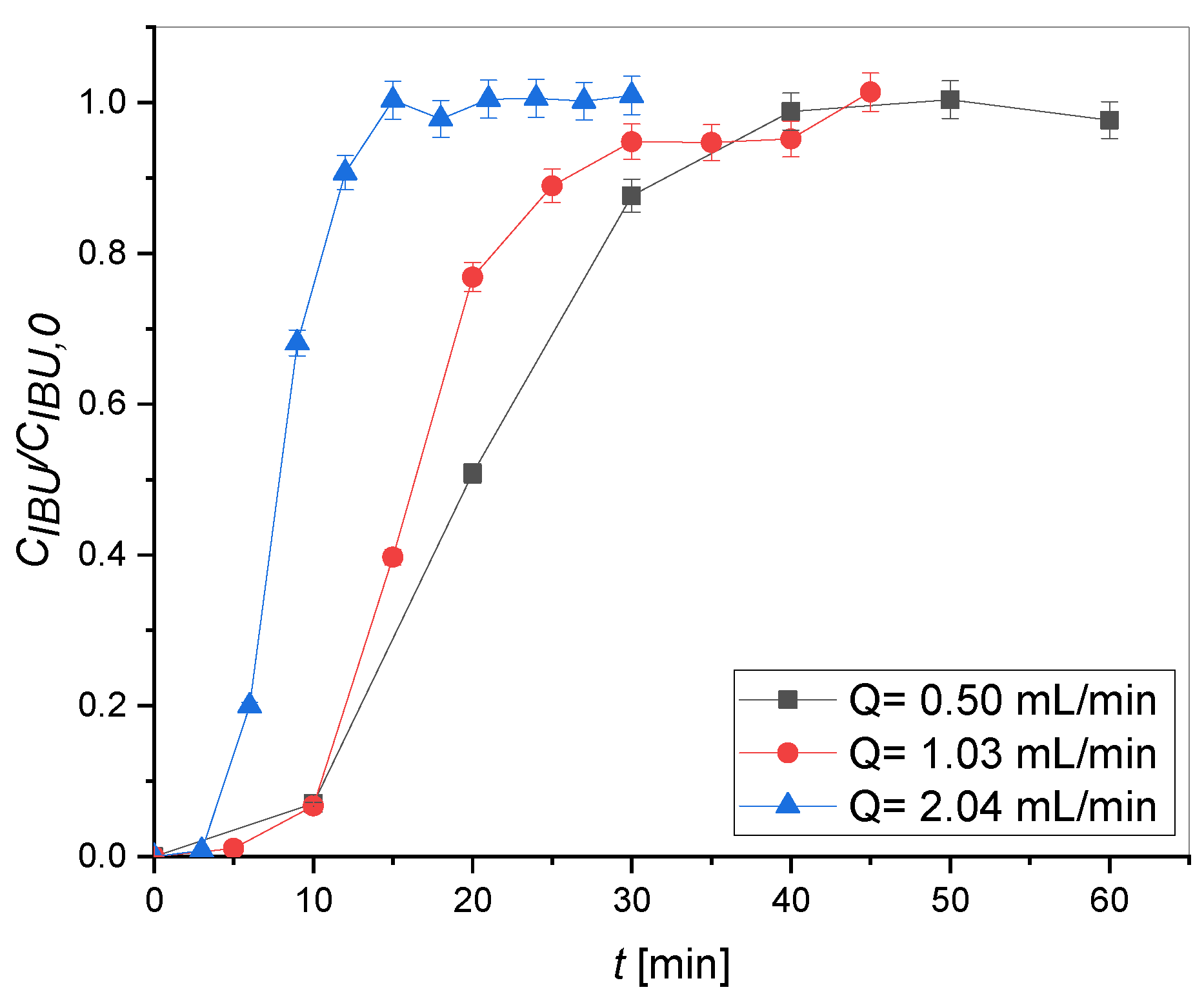
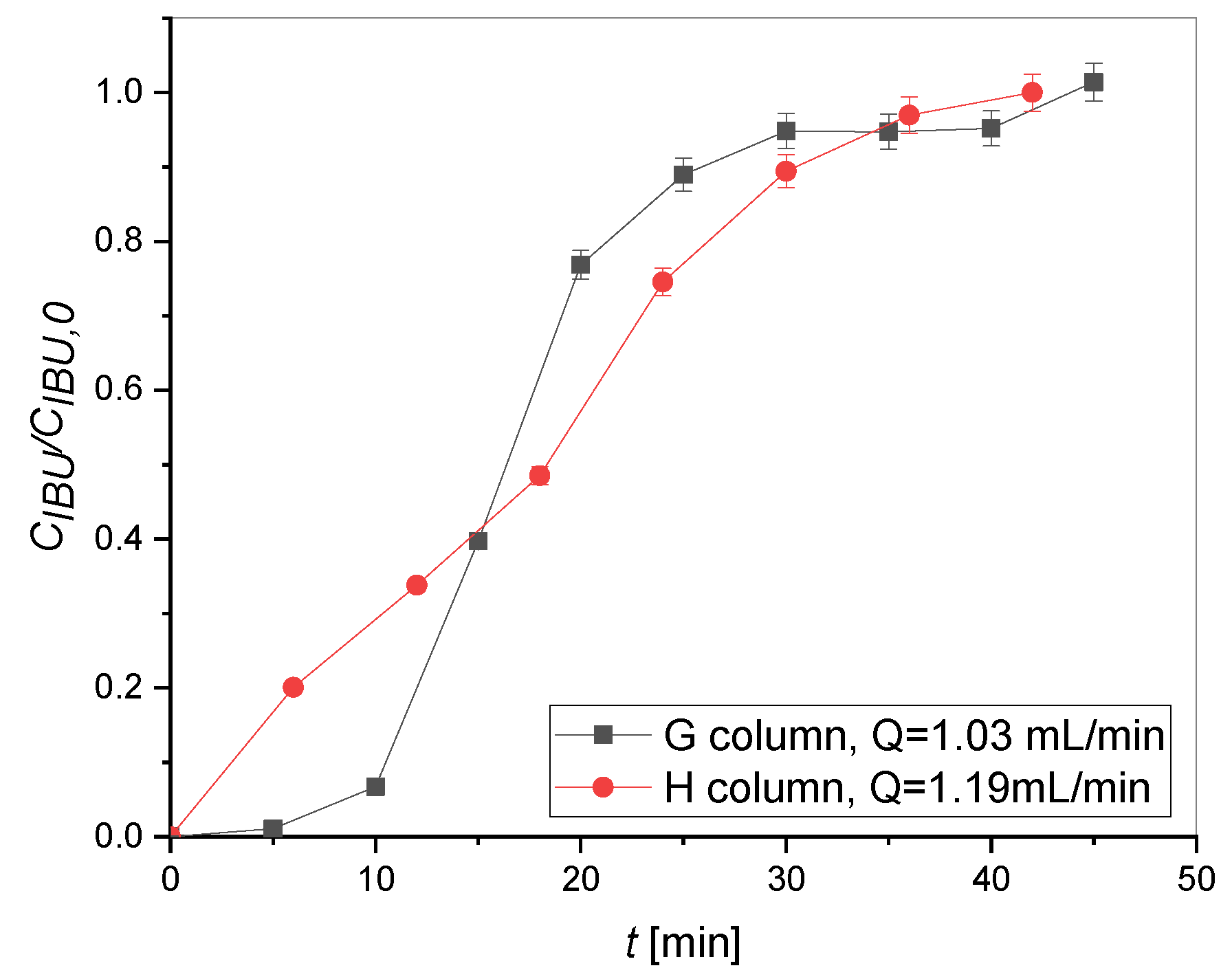
2.2. Adsorption Tests
2.3. Adsorption Mechanism
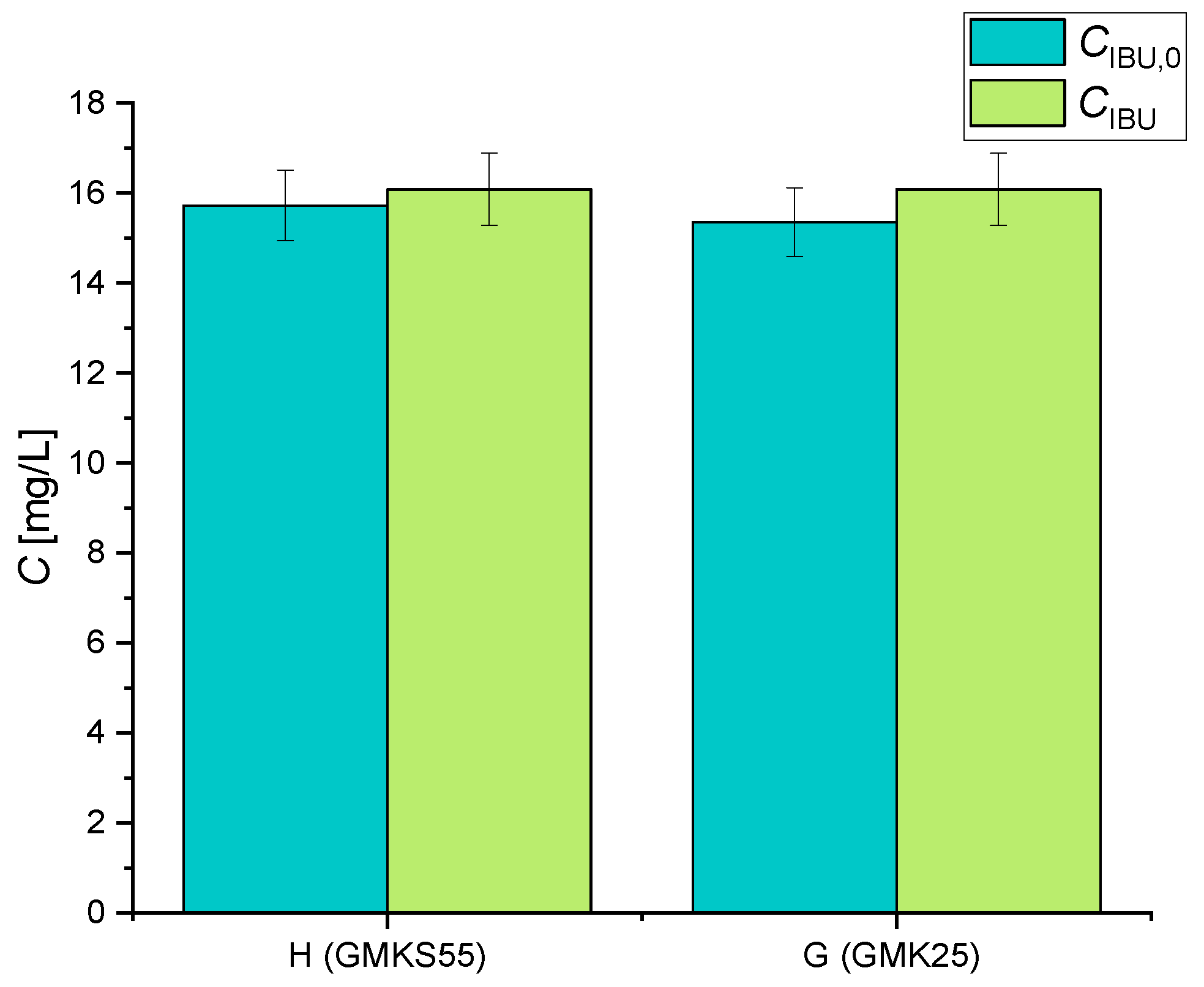
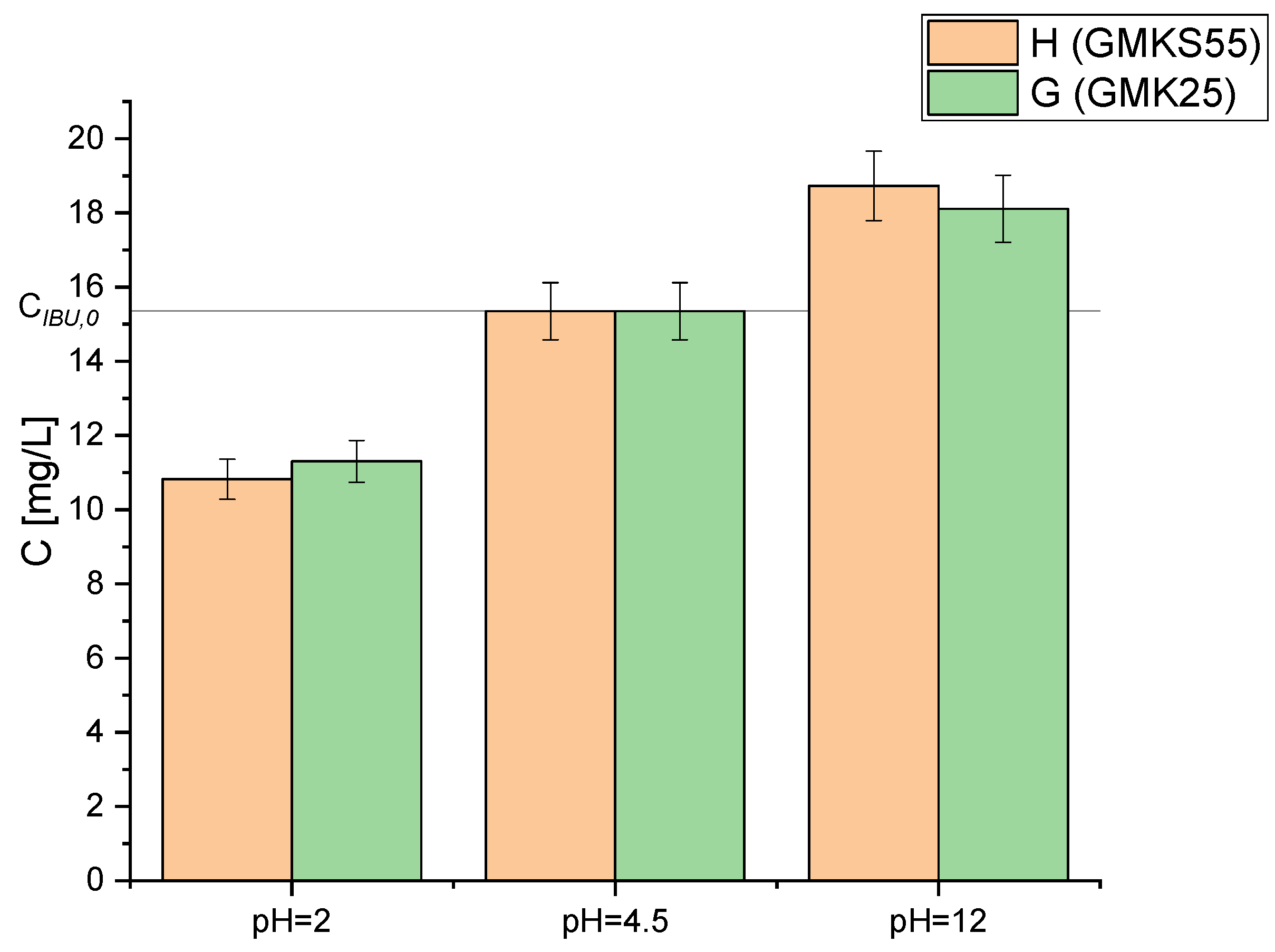
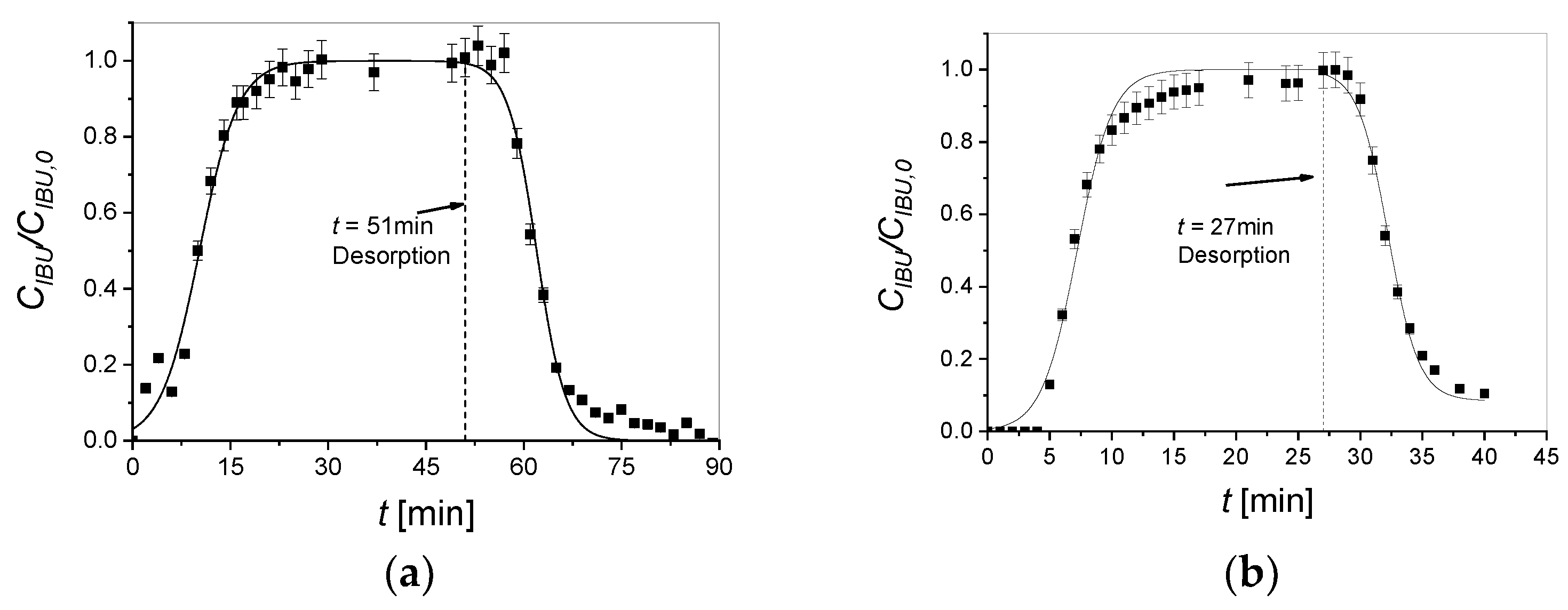
2.4. Adsorption/Desorption Tests
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Physical–Chemical Characterization of the Adsorbent
3.2.2. Geopolymer-Based Foams
Preparation of Neat Geopolymeric Mixture (GMK)
3.2.3. Preparation of Geopolymer-Based Hybrid Mixture (GMK-S)
3.2.4. Preparation of GMK and GMK-S Foams
3.2.5. Curing Treatments
3.2.6. Preparation of the Ibuprofen Solution
3.2.7. Adsorption Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Symbols
| CIBU [mg/L] | Ibuprofen concentration |
| CIBU,0 [mg/L] | Ibuprofen feed concentration |
| Q [mL/min] | Volumetric flow rate |
| QIBU [mg/g] | Mass of ibuprofen adsorbed per g of adsorbent solid |
| QIBU,tot [mg/g] | Mass of total ibuprofen adsorbed per g of adsorbent solid |
| qt [mg/g] | Mass of ibuprofen adsorbed per g of adsorbent solid |
| tE [min] | Final time of the breakthrough curve |
| V [L] | Liquid volume |
| wADS [g] | Adsorbent material mass present in the column |
| Y [%] | IBU removal percentage |
| Abbreviations | |
| CECs | Contaminants of emerging concern |
| IBU | Ibuprofen |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
References
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A Review of the Fate of Micropollutants in Wastewater Treatment Plants. WIREs Water 2015, 2, 457–487. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.-F.; Barjoveanu, G.; Fiore, S. Emerging Pollutants Removal through Advanced Drinking Water Treatment: A Review on Processes and Environmental Performances Assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an Emerging Organic Contaminant in Environment, Distribution and Remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Calamari, D.; Natangelo, M.; Fanelli, R. Presence of Therapeutic Drugs in the Environment. Lancet 2000, 355, 1789–1790. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Paździor, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef] [PubMed]
- Jan-Roblero, J.; Cruz-Maya, J.A. Ibuprofen: Toxicology and Biodegradation of an Emerging Contaminant. Molecules 2023, 28, 2097. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Igwegbe, C.A. Removal of Ibuprofen from Aqueous Media by Adsorption: A Comprehensive Review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef]
- PubChem Ibuprofen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3672 (accessed on 3 October 2023).
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Russo, V.; Trifuoggi, M.; Di Serio, M.; Tesser, R. Fluid-Solid Adsorption in Batch and Continuous Processing: A Review and Insights into Modeling. Chem. Eng. Technol. 2017, 40, 799–820. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.; Chouchene, K.; Wali, A.; Labille, J.; Roche, N.; Ksibi, M. Adsorption of Anti-Inflammatory and Analgesic Drugs Traces in Water on Clay Minerals. Chemosphere 2024, 353, 141469. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Oh, S.Y.; Park, H.S. Sorptive Removal of Ibuprofen from Water Using Selected Soil Minerals and Activated Carbon. Int. J. Environ. Sci. Technol. 2012, 9, 85–94. [Google Scholar] [CrossRef]
- Li, Z.; Gómez-Avilés, A.; Sellaoui, L.; Bedia, J.; Bonilla-Petriciolet, A.; Belver, C. Adsorption of Ibuprofen on Organo-Sepiolite and on Zeolite/Sepiolite Heterostructure: Synthesis, Characterization and Statistical Physics Modeling. Chem. Eng. J. 2019, 371, 868–875. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Le, H.T.N.; Do, T.S.; Pham, V.T.; Lam Tran, D.; Ho, V.T.T.; Tran, T.V.; Nguyen, D.C.; Nguyen, T.D.; Bach, L.G.; et al. Metal-Organic Framework MIL-53(Fe) as an Adsorbent for Ibuprofen Drug Removal from Aqueous Solutions: Response Surface Modeling and Optimization. J. Chem. 2019, 2019, e5602957. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Tirth, V.; Gnanamoorthy, G.; Gupta, N.; Algahtani, A.; Islam, S.; Choudhary, N.; Modi, S.; Jeon, B.-H. Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes. Materials 2021, 14, 6333. [Google Scholar] [CrossRef] [PubMed]
- Louisy, E.; Olivero, S.; Michelet, V.; Mija, A. On the Influence of the Cis/Trans Stereochemistry of Limonene Oxides toward the Synthesis of Biobased Thermosets by Crosslinking with Anhydrides. ACS Sustain. Chem. Eng. 2022, 10, 7169–7179. [Google Scholar] [CrossRef]
- Tahmasebi Yamchelou, M.; Law, D.; Brkljača, R.; Gunasekara, C.; Li, J.; Patnaikuni, I. Geopolymer Synthesis Using Low-Grade Clays. Constr. Build. Mater. 2021, 268, 121066. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-1-84569-638-2. [Google Scholar]
- Roviello, G.; Chianese, E.; Ferone, C.; Ricciotti, L.; Roviello, V.; Cioffi, R.; Tarallo, O. Hybrid Geopolymeric Foams for the Removal of Metallic Ions from Aqueous Waste Solutions. Materials 2019, 12, 4091. [Google Scholar] [CrossRef] [PubMed]
- Medri, V.; Papa, E.; Mor, M.; Vaccari, A.; Natali Murri, A.; Piotte, L.; Melandri, C.; Landi, E. Mechanical Strength and Cationic Dye Adsorption Ability of Metakaolin-Based Geopolymer Spheres. Appl. Clay Sci. 2020, 193, 105678. [Google Scholar] [CrossRef]
- Zhao, J.; Liebscher, M.; Michel, A.; Junger, D.; Trindade, A.C.C.; de Andrade Silva, F.; Mechtcherine, V. Development and Testing of Fast Curing, Mineral-Impregnated Carbon Fiber (MCF) Reinforcements Based on Metakaolin-Made Geopolymers. Cem. Concr. Compos. 2021, 116, 103898. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Monzó, M.; Vicent, M.; Barba, A.; Palomo, A. Alkaline Activation of Metakaolin–Fly Ash Mixtures: Obtain of Zeoceramics and Zeocements. Microporous Mesoporous Mater. 2008, 108, 41–49. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Messina, F.; Ferone, C.; Asprone, D.; Cioffi, R. Lightweight Geopolymer-Based Hybrid Materials. Compos. Part B Eng. 2017, 128, 225–237. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Molino, A.J.; Menna, C.; Ferone, C.; Asprone, D.; Cioffi, R.; Ferrandiz-Mas, V.; Russo, P.; Tarallo, O. Hybrid Fly Ash-Based Geopolymeric Foams: Microstructural, Thermal and Mechanical Properties. Materials 2020, 13, 2919. [Google Scholar] [CrossRef]
- Papa, E.; Landi, E.; Miccio, F.; Medri, V. K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test. Materials 2022, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, Structure and Properties of Hybrid Materials Based on Geopolymers and Polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Ricciotti, L.; Occhicone, A.; Petrillo, A.; Ferone, C.; Cioffi, R.; Roviello, G. Geopolymer-Based Hybrid Foams: Lightweight Materials from a Sustainable Production Process. J. Clean. Prod. 2020, 250, 119588. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khahro, S.H.; Low, A.; Ayoub, M. Optimization of Synthesis of Geopolymer Adsorbent for the Effective Removal of Anionic Surfactant from Aqueous Solution. J. Environ. Chem. Eng. 2021, 9, 104949. [Google Scholar] [CrossRef]
- Yasmin, P. 3D-Printed Geopolymers for Adsorption of Carbamazepine Removal. Master’s Thesis, Lappeenranta–Lahti University of Technology LUT, Lappeenranta, Finland, 2021. [Google Scholar]
- Abukhadra, M.R.; AlHammadi, A.A.; Khim, J.S.; Ajarem, J.S.; Allam, A.A.; Shaban, M.S. Enhanced Adsorption and Visible Light Photocatalytic Removal of 5-Fluorouracil Residuals Using Environmental NiO/Geopolymer Nanocomposite: Steric, Energetic, and Oxidation Studies. J. Environ. Chem. Eng. 2022, 10, 108569. [Google Scholar] [CrossRef]
- Ettahiri, Y.; Bouargane, B.; Fritah, K.; Akhsassi, B.; Pérez-Villarejo, L.; Aziz, A.; Bouna, L.; Benlhachemi, A.; Novais, R.M. A State-of-the-Art Review of Recent Advances in Porous Geopolymer: Applications in Adsorption of Inorganic and Organic Contaminants in Water. Constr. Build. Mater. 2023, 395, 132269. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Shu, L.; Feng, Y.; Lv, C.; Liu, H.; Xu, F.; Wang, Q.; Zhao, C.; Kong, Q. Safety Evaluation and Ibuprofen Removal via an Alternanthera Philoxeroides-Based Biochar. Environ. Sci. Pollut. Res. 2021, 28, 40568–40586. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative Performance of Geopolymers Made with Metakaolin and Fly Ash after Exposure to Elevated Temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; Van Deventer, J.S.J. The Effects of Temperature on the Local Structure of Metakaolin-Based Geopolymer Binder: A Neutron Pair Distribution Function Investigation. J. Am. Ceram. Soc. 2010, 93, 3486–3492. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical Evolution of Na-Geopolymer Derived from Metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, X.; Zhang, D.; Liu, Y.; Huang, G. Synthesis and Thermal Properties of Modified Room Temperature Vulcanized (RTV) Silicone Rubber Using Polyhedral Oligomeric Silsesquioxane (POSS) as a Cross Linking Agent. RSC Adv. 2014, 4, 41453–41460. [Google Scholar] [CrossRef]
- Selkälä, T.; Suopajärvi, T.; Sirviö, J.A.; Luukkonen, T.; Kinnunen, P.; de Carvalho, A.L.C.B.; Liimatainen, H. Surface Modification of Cured Inorganic Foams with Cationic Cellulose Nanocrystals and Their Use as Reactive Filter Media for Anionic Dye Removal. ACS Appl. Mater. Interfaces 2020, 12, 27745–27757. [Google Scholar] [CrossRef]
- Oh, S.; Shin, W.S.; Kim, H.T. Effects of pH, Dissolved Organic Matter, and Salinity on Ibuprofen Sorption on Sediment. Environ. Sci. Pollut. Res. 2016, 23, 22882–22889. [Google Scholar] [CrossRef]
- Tchadjie, L.N.; Ekolu, S.O. Enhancing the Reactivity of Aluminosilicate Materials toward Geopolymer Synthesis. J. Mater. Sci. 2018, 53, 4709–4733. [Google Scholar] [CrossRef]
- Gharzouni, A.; Ouamara, L.; Sobrados, I.; Rossignol, S. Alkali-Activated Materials from Different Aluminosilicate Sources: Effect of Aluminum and Calcium Availability. J. Non-Cryst. Solids 2018, 484, 14–25. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Dąbek, L.; Świątkowski, A. Comparative Study on the Adsorption Kinetics and Equilibrium of Common Water Contaminants onto Bentonite. Desalination Water Treat. 2020, 186, 373–381. [Google Scholar] [CrossRef]
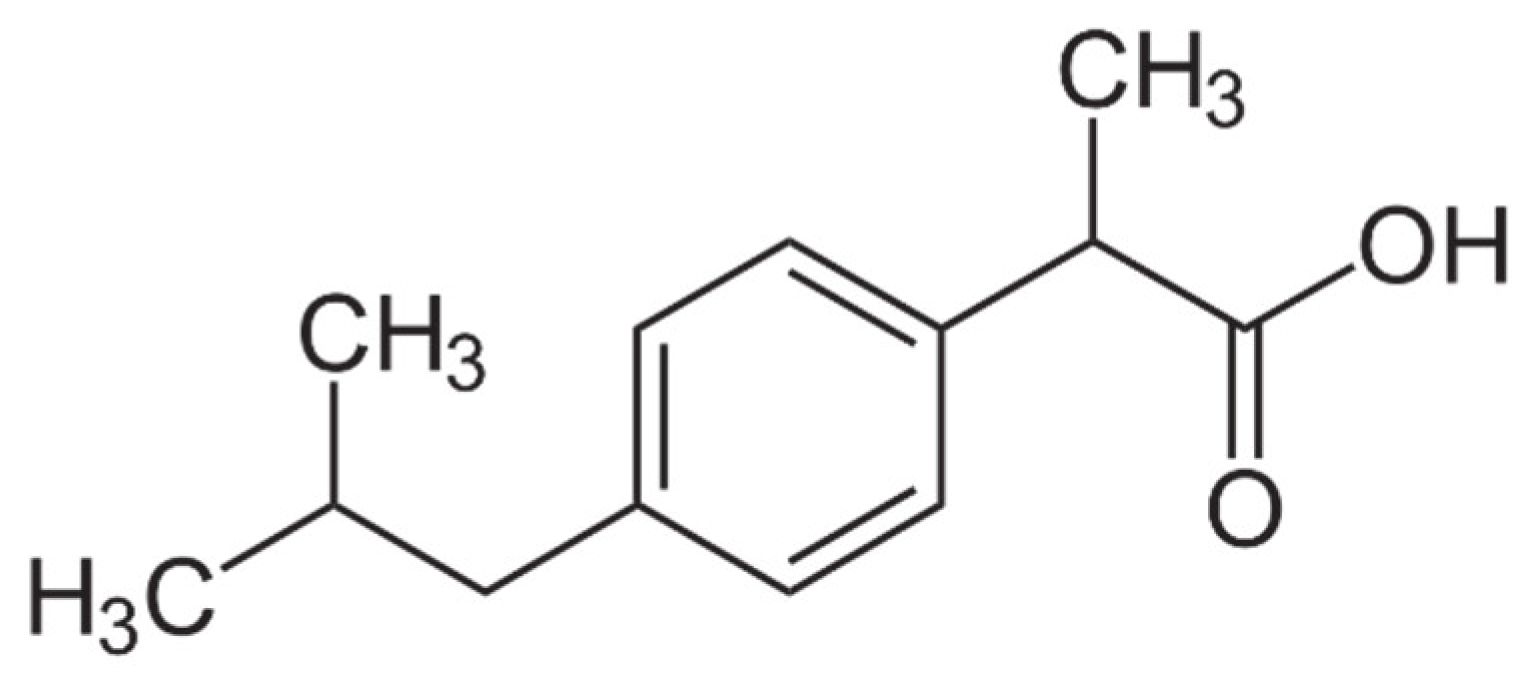



| Property | Values |
|---|---|
| IUPAC name | (±)-2-(4-Isobutylphenyl)propanoic acid |
| Molar mass | 206.28 g/mol |
| Solubility in water (at 25 °C) | 21 mg/L |
| pKa | 4.91 |
| Column | Q [mL/min] | Y [%] |
|---|---|---|
| H | 1.90 | 42.93 |
| 3.60 | 15.08 | |
| 4.17 | 91.54 | |
| 4.90 | 91.56 | |
| 5.70 | 92.06 | |
| 10.20 | 90.94 | |
| G | 0.50 | 35.10 |
| 1.03 | 29.55 | |
| 2.04 | 13.72 |
| Compound | Metakaolin | Sodium Silicate Solution |
|---|---|---|
| SiO2 | 52.90 | 27.40 |
| Al2O3 | 42.00 | - |
| Na2O | - | 8.15 |
| K2O | 0.90 | - |
| TiO2 | 1.95 | - |
| Fe2O3 | 1.75 | - |
| CaO | 0.25 | - |
| MgO | 0.25 | - |
| H2O | - | 64.45 |
| Sample | MK (g) | SS (g) | DMS (g) | Si (g, wt.%) | Apparent Density (g cm−3) |
|---|---|---|---|---|---|
| GMK015 | 50.0 | 70.0 | - | 0.018 (0.015) | 1.97 |
| GMK02 | 44.8 | 62.8 | - | 0.022 (0.02) | 1.98 |
| GMK03 | 99.9 | 140.2 | - | 0.072 (0.03) | 1.96 |
| GMK05 | 100.1 | 140.4 | - | 0.121 (0.05) | 1.96 |
| GMK10 | 202.2 | 186.9 | - | 0.381 (0.10) | 1.88 |
| GMK15 | 133.7 | 118.2 | - | 0.378 (0.15) | 1.85 |
| GMK20 | 153.8 | 135.8 | - | 0.580 (0.20) | 1.62 |
| GMK25 (G) | 176.0 | 155.2 | - | 0.830 (0.25) | 1.38 |
| GMK30 | 211.6 | 187.6 | - | 1.198 (0.30) | 1.42 |
| GMK-S03 | 45.3 | 63.4 | 12.0 | 0.032 (0.03) | 0.701 |
| GMK-S06 | 43.5 | 60.9 | 10.4 | 0.063 (0.06) | 0.508 |
| GMK-S30 | 73.4 | 65.2 | 15.6 | 0.418 (0.30) | 0.422 |
| GMK-S35 | 61.5 | 54.6 | 13.0 | 0.407 (0.35) | 0.495 |
| GMK-S50 | 115.4 | 102.5 | 24.5 | 1.211 (0.50) | 0.408 |
| GMK-S55 (H) | 119.0 | 105.6 | 25.2 | 1.374 (0.55) | 0.386 |
| Adsorbent | Adsorbent Amount [g] | Ibuprofen Concentration [mg/L] | Time [min] | pH |
|---|---|---|---|---|
| G (GMK25) | 0 | 15.35 | 0 | 4.5 |
| 0.104 | 15.78 | 10 | 4.5 | |
| 0.104 | 15.72 | 60 | 4.5 | |
| 0.104 | 16.08 | 120 | 4.5 | |
| 0 | 15.35 | 0 | 4.5 | |
| 0.008 | 11.30 | 1440 | 2 | |
| 0.008 | 18.11 | 1440 | 12 | |
| H (GMK-S55) | 0 | 15.27 | 0 | 4.5 |
| 0.112 | 16.45 | 10 | 4.5 | |
| 0.112 | 17.24 | 60 | 4.5 | |
| 0.112 | 16.08 | 120 | 4.5 | |
| 0 | 15.35 | 0 | 4.5 | |
| 0.008 | 10.82 | 1440 | 2 | |
| 0.008 | 18.73 | 1440 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparo, R.; Di Serio, M.; Roviello, G.; Ferone, C.; Trifuoggi, M.; Russo, V.; Tarallo, O. Geopolymer-Based Materials for the Removal of Ibuprofen: A Preliminary Study. Molecules 2024, 29, 2210. https://doi.org/10.3390/molecules29102210
Paparo R, Di Serio M, Roviello G, Ferone C, Trifuoggi M, Russo V, Tarallo O. Geopolymer-Based Materials for the Removal of Ibuprofen: A Preliminary Study. Molecules. 2024; 29(10):2210. https://doi.org/10.3390/molecules29102210
Chicago/Turabian StylePaparo, Rosanna, Martino Di Serio, Giuseppina Roviello, Claudio Ferone, Marco Trifuoggi, Vincenzo Russo, and Oreste Tarallo. 2024. "Geopolymer-Based Materials for the Removal of Ibuprofen: A Preliminary Study" Molecules 29, no. 10: 2210. https://doi.org/10.3390/molecules29102210









