Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process
Abstract
:1. Introduction
2. Results and Discussion
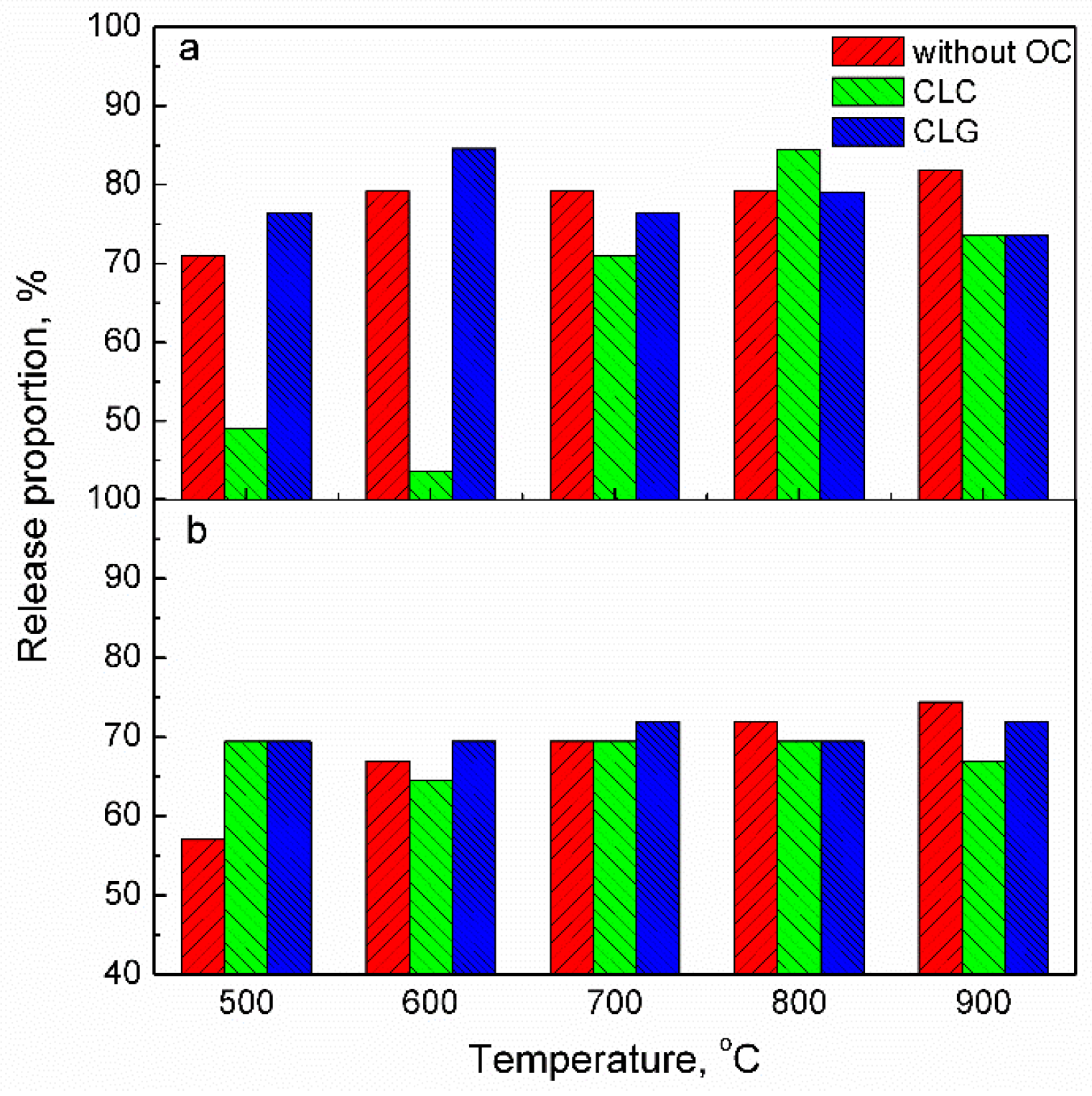
2.1. Effect of Oxygen Carrier on Release Behavior of Mercury in Coal
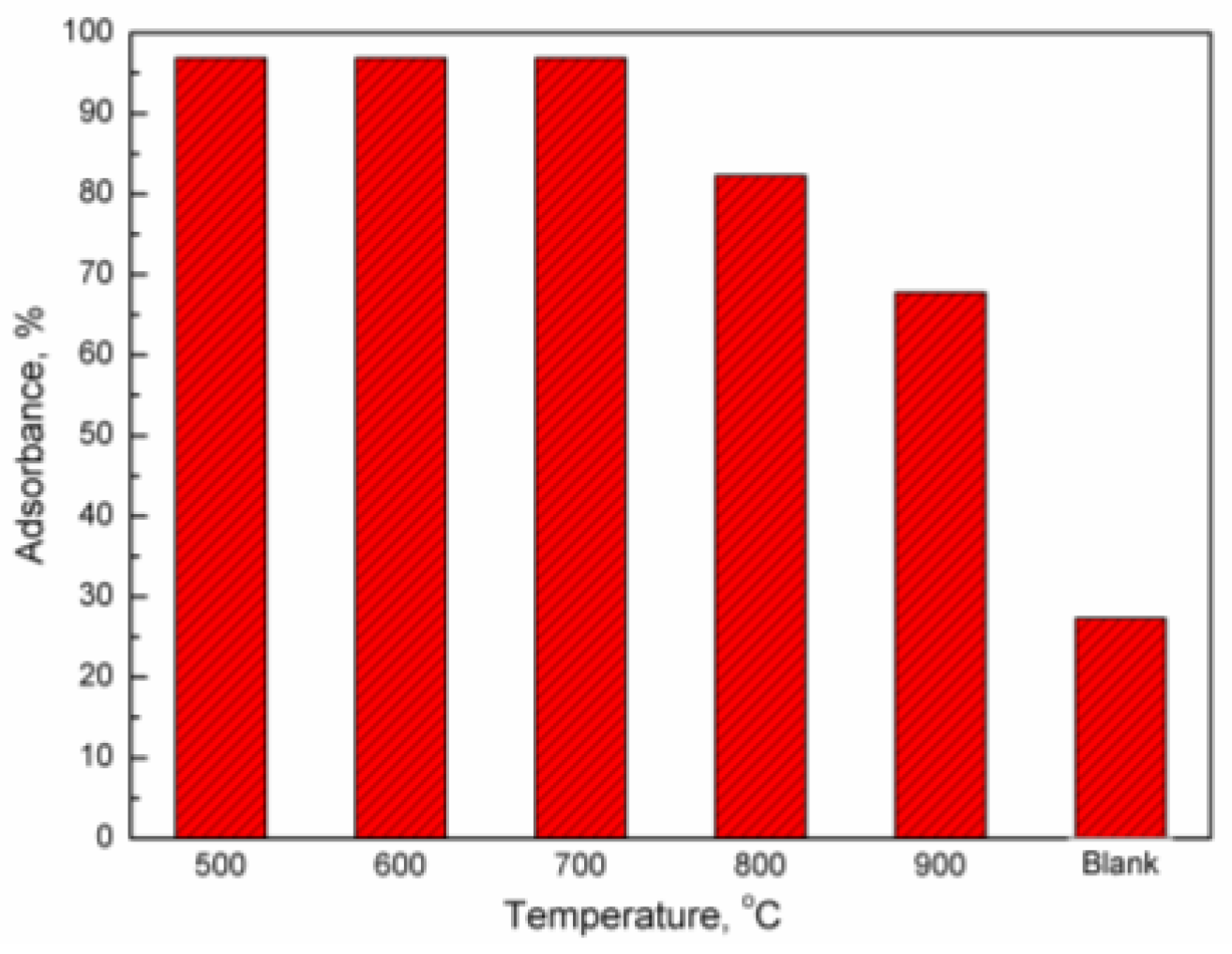
2.2. Adsorption of Mercury Vapor by Iron-Based Oxygen Carrier
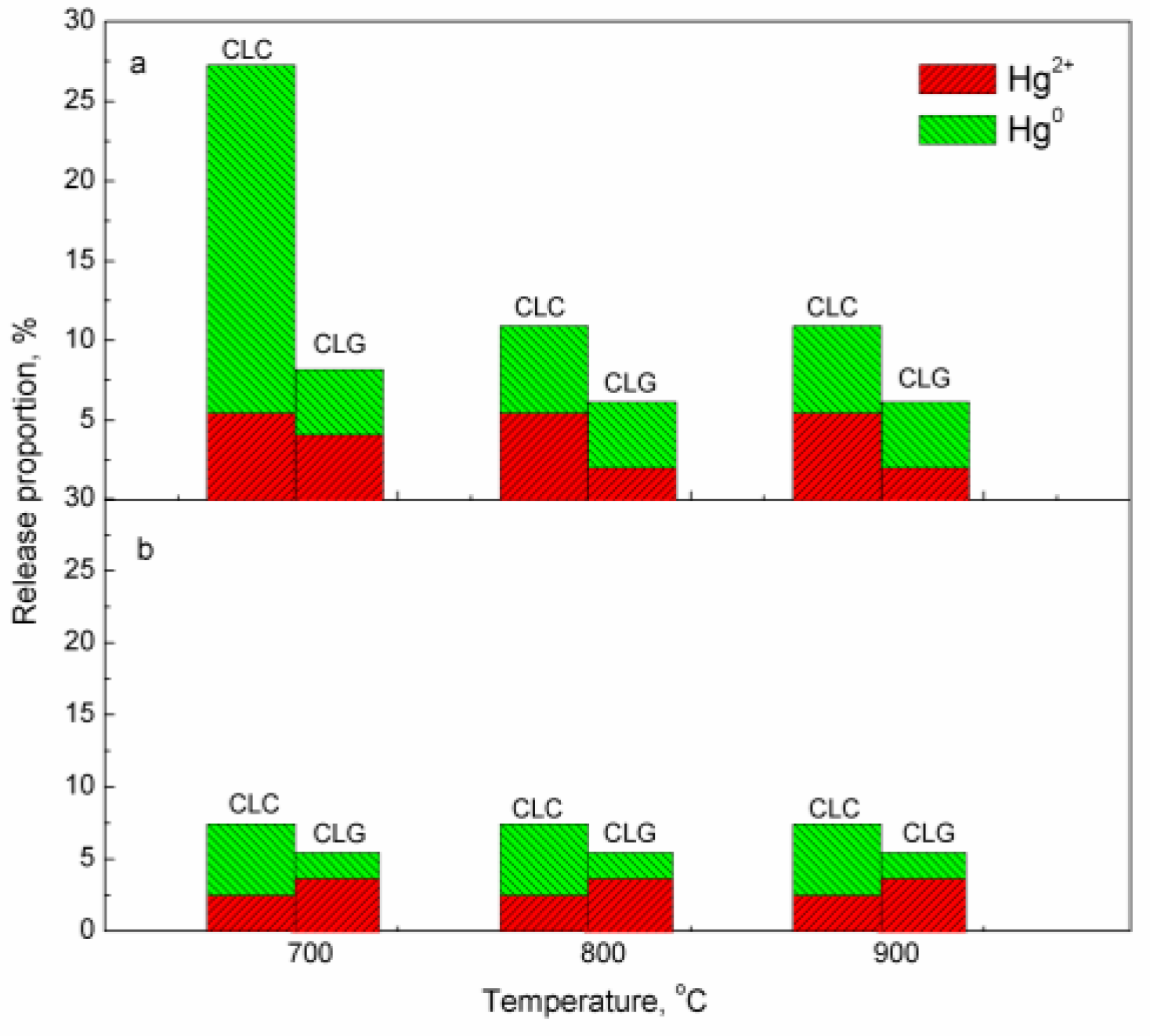
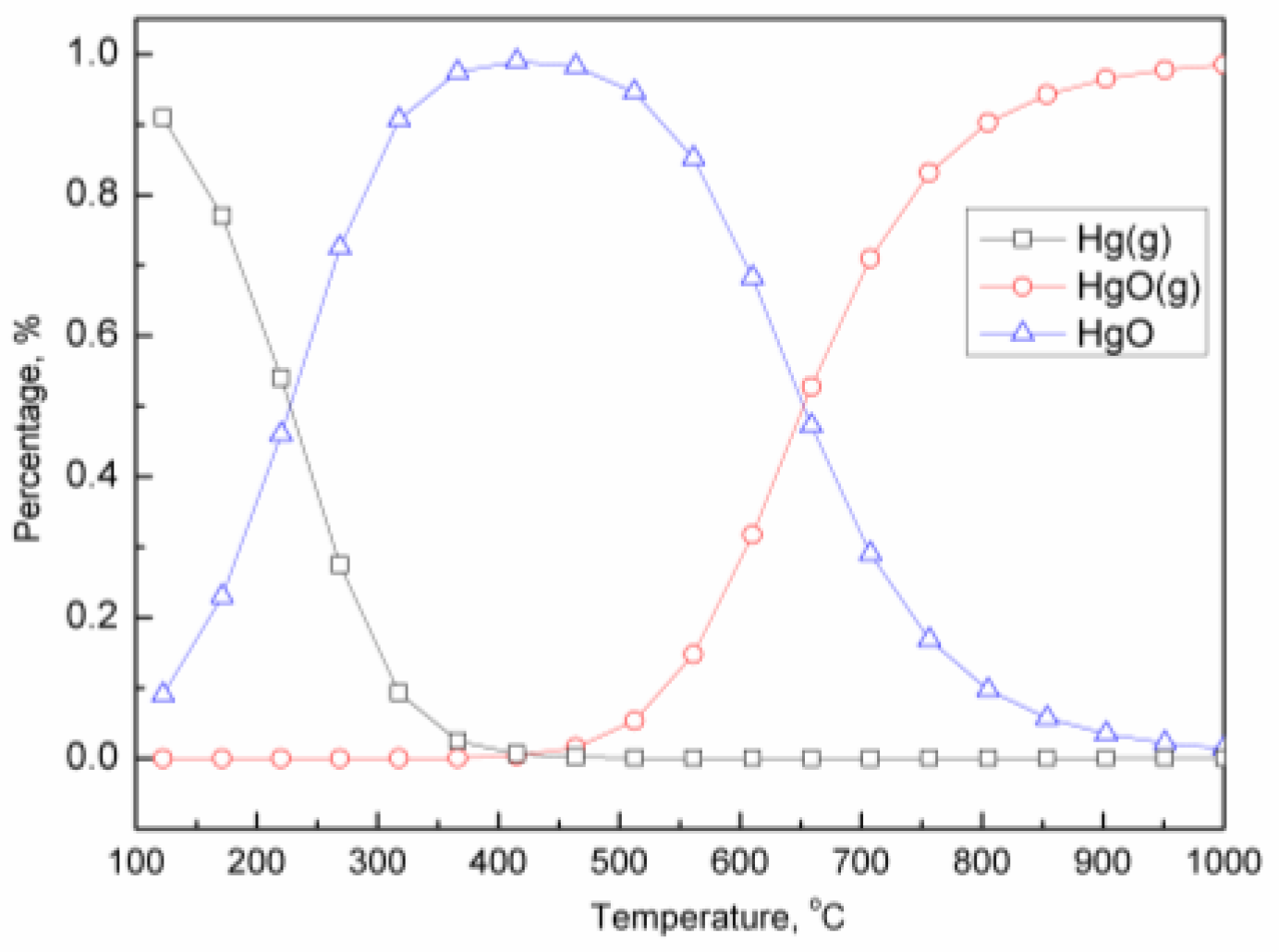
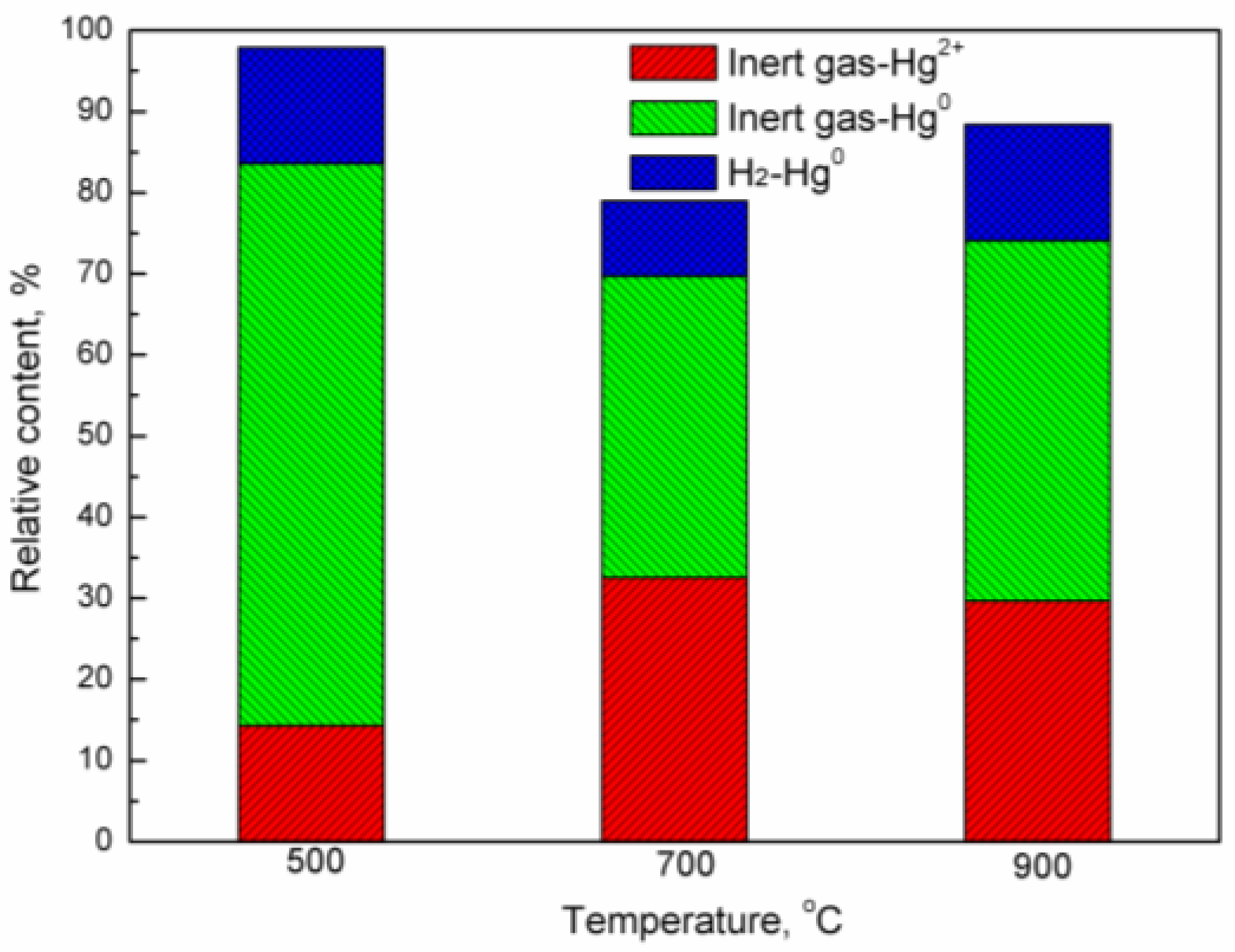
2.3. Oxidation of Mercury Vapor by Iron-Based Oxygen Carrier
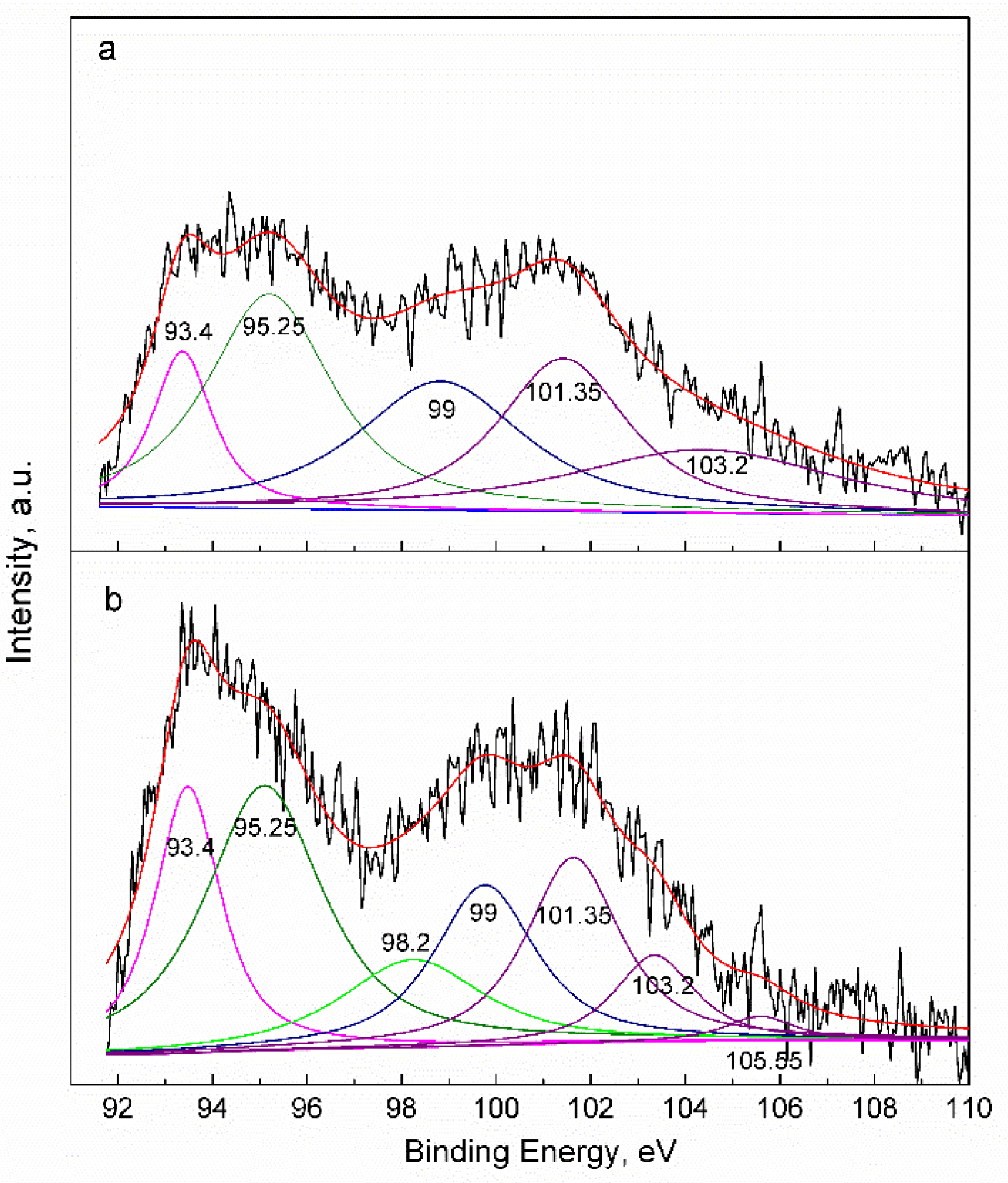
2.4. Effect Mechanism of Iron-Based Oxygen Carrier on Mercury
3. Experimental and Methods
3.1. Preparation and Characterization of Materials
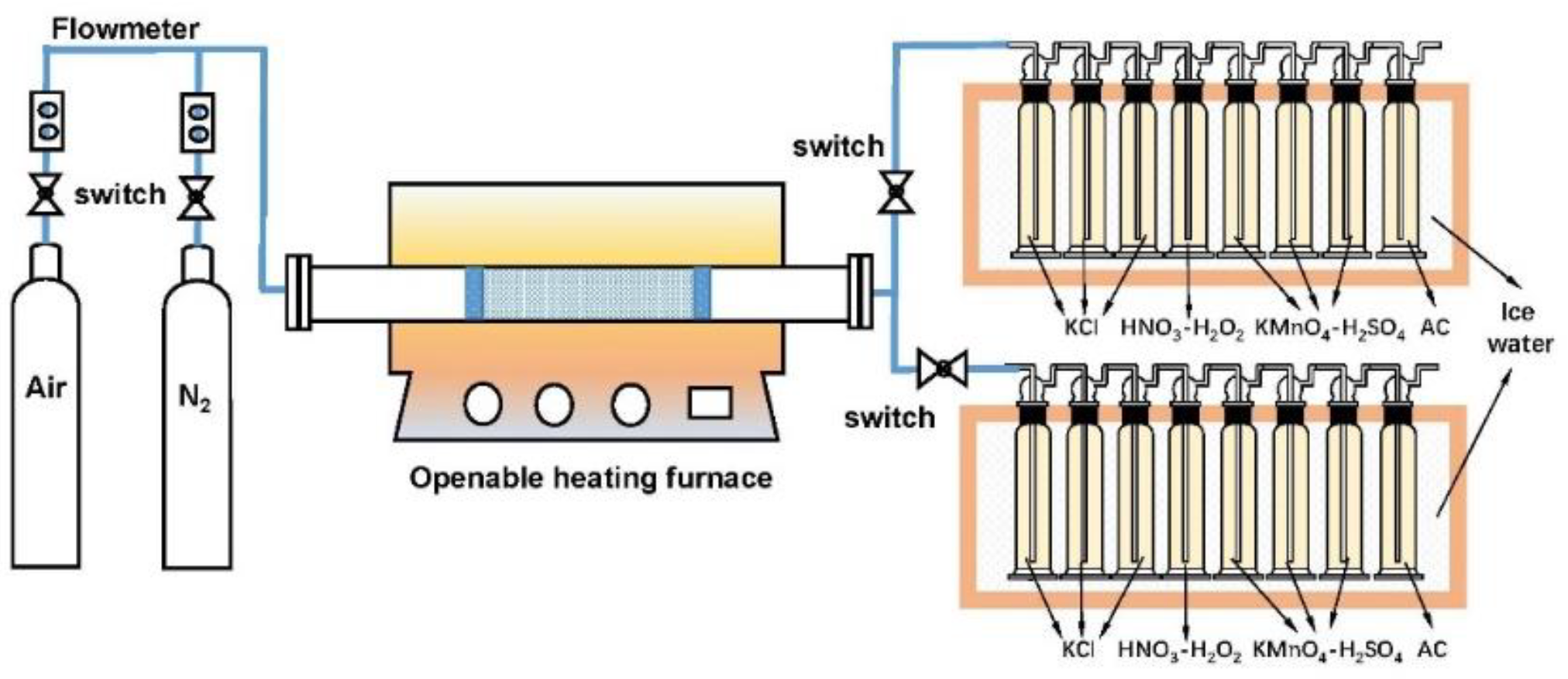
3.2. Experimental Setup and Procedure
3.3. Data Evaluation
4. Conclusions
- (1)
- The effect of an iron-based oxygen carrier on mercury release in chemical looping conversion is attributed to three aspects: the enhanced release rate of mercury from coal, the adsorption of mercury on the surface of the oxygen carrier, and the oxidation of gaseous mercury from Hg0 to Hg2+.
- (2)
- With the increasing temperature, the adsorbance of mercury by the iron-based oxygen carrier decreases, while its oxidation of mercury enhances. Even at 900 °C, the adsorbance of mercury by the oxygen carrier remained at 0.1687 g/g, with a relative content of Hg2+ at 22.55%.
- (3)
- The mercury adsorption on the surface of the iron-based oxygen carrier involves both chemisorption and physical absorption. Physical adsorption includes both Hg0 and Hg2+, while chemisorption refers to complex-compound formation between mercury and the iron-based oxygen carrier.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evan, J.G.; Henry, W.P. Constance Senior. Mercury Control for Coal-Derived Gas Streams; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015; pp. 51–66. [Google Scholar]
- Wu, C.L.; Cao, Y.; Dong, Z.B.; Cheng, C.M.; Li, H.X.; Pan, W.P. Evaluation of mercury speciation and removal through air pollution control devices of a 190 MW boiler. J. Environ. Sci. 2010, 22, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.-H.; Negreira, A.S.; Kirchofer, A.; Feng, F.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90–91, 4–20. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.Y.; Zhao, Y.C.; Zheng, C.G. Volatility and speciation of mercury during pyrolysis and gasification of five Chinese coals. Energy Fuel 2011, 25, 3988–3996. [Google Scholar] [CrossRef]
- Zhu, X.; Donat QI, F.; Müller, C.R.; Li, F.X. Chemical looping beyond combustion–a perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energ. Combust. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.H.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Guo, Q.J. Investigation into syngas generation from solid fuel using CaSO4-based chemical looping gasification process. Chin. J. Chem. Eng. 2013, 21, 127–134. [Google Scholar]
- Liu, Y.Z.; Jia, W.H.; Guo, Q.J.; Ryu, H.J. Effect of the gasifying medium on the coal chemical looping gasification with CaSO4 as oxygen carrier. Chin. J. Chem. Eng. 2014, 22, 1208–1214. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Zhou, L.; Li, B.; Wang, T.; Liu, H. A review on mercury removal in chemical looping combustion of coal. Sep. Purif. Technol. 2024, 337, 126352. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Fan, Y.; Liu, Q.; Wang, X.; Xu, K.; Jin, J.; Ma, J.; Ma, J. Mercury transformation and removal in chemical looping combustion of coal: A review. Fuel 2023, 347, 128440. [Google Scholar] [CrossRef]
- Mendiara, T.; Izquierdo, M.T.; Abad, A.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Adánez, J. Mercury release and speciation in chemical looping combustion of coal. Energy Fuels 2014, 28, 2786–2794. [Google Scholar] [CrossRef]
- Mendiara, T.; Izquierdo, M.T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Release of pollutant components in CLC of lignite. Int. J. Greenh. Gas Control 2014, 22, 15–24. [Google Scholar] [CrossRef]
- Pérez, V.R.; Adánez, R.I.; Gayán, P.; Izquierdo, M.T.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Adánez, J. Sulphur, nitrogen and mercury emissions from coal combustion with CO2 capture in chemical looping with oxygen uncoupling (CLOU). Int. J. Greenh. Gas Control 2016, 46, 28–38. [Google Scholar] [CrossRef]
- Ma, J.C.; Mei, D.F.; Tian, X.; Zhang, S.B.; Yang, J.P.; Wang, C.Q.; Chen, G.P.; Zhao, Y.C.; Zheng, C.G.; Zhao, H.B. Fate of mercury in volatiles and char during in situ gasification chemical-looping combustion of coal. Environ. Sci. Technol. 2019, 53, 7887–7892. [Google Scholar] [CrossRef]
- An, M.; Ma, J.J.; Guo, Q.J. Transformation and migration of mercury during chemical-looping gasification of coal. Ind. Eng. Chem. Res. 2019, 58, 20481–20490. [Google Scholar] [CrossRef]
- An, M.; Guo, Q.J.; Wei, X.Y. Reaction mechanism of H2S with Hg0 on CuFe2O4 oxygen carrier with oxygen vacancy structure during coal chemical looping gasification. Fuel 2023, 333, 126477. [Google Scholar] [CrossRef]
- Ghorishi, S.B.; Lee, C.W.; Jozewicz, W.S.; Kilgroe, J.D. Effects of fly ash transition metal content and flue gas HCl/SO2 ratio on mercury speciation in waste combustion. Environ. Eng. Sci. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Galbreath, K.C.; Zygrliche, C.J.; Tibbetts, J.E.; Schulz, R.L.; Dunham, G.E. Effects of NOx, α-Fe2O3, γ-Fe2O3, and HCl on mercury transformations in a 7-kW coal combustion system. Fuel Process. Technol. 2005, 86, 429–448. [Google Scholar] [CrossRef]
- Ni, M.G.; Liu, D.Y.; Jin, J.; Feng, L.; Liu, Z. Ranking Oxygen Carriers for Elemental Mercury Oxidation in Coal-Fired Chemical-Looping Combustion: A Thermodynamic Approach. Energy Fuels 2020, 34, 2355–2365. [Google Scholar] [CrossRef]
- Li, J.H.; Lin, C.F.; Qin, W.; Xiao, X.B.; Li, W. Synergetic effect of mercury adsorption on the catalytic decomposition of CO over perfect and reduced Fe2O3[001] Surface. Acta Phys.-Chim. Sin. 2016, 32, 2717–2723. [Google Scholar] [CrossRef]
- Zhang, J.J.; Qin, W.; Dong, C.Q.; Yang, Y.P. Density functional theory study of elemental mercury adsorption on Fe2O3[104] and it’s effect on carbon deposit during chemical looping combustion. Energy Fuels 2016, 30, 3413–3418. [Google Scholar] [CrossRef]
- Guo, P.; Guo, X.; Zheng, C.G. Roles of γ-Fe2O3 in fly ash for mercury removal: Results of density functional theory study. Appl. Surf. Sci. 2010, 256, 6991–6996. [Google Scholar] [CrossRef]
- Jung, J.E.; Geatches, D.; Lee, K.; Aboud, S.; Brown, G.E., Jr.; Wilcox, J. First-Principles investigation of mercury adsorption on the α-Fe2O3(11̅02) surface. J. Phys. Chem. C 2015, 119, 26512–26518. [Google Scholar] [CrossRef]
- Yin, L.B.; Zhuo, Y.Q.; Xu, Q.S. Mercury emission from coal-fired power plants in China. Proc. CSEE 2013, 33, 1–9. [Google Scholar]
- Guo, Q.J.; Cheng, Y.; Liu, Y.Z.; Jia, W.H.; Ryu, H.-J. Coal chemical looping gasification for syngas generation using an iron-based oxygen carrier. Ind. Eng. Chem. Res. 2014, 53, 78–86. [Google Scholar] [CrossRef]
- Zhang, J.S.; Guo, Q.J.; Liu, Y.Z.; Cheng, Y. Preparation and characterization of Fe2O3/Al2O3 using the solution combustion approach for chemical looping combustion. Ind. Eng. Chem. Res. 2012, 51, 12773–12781. [Google Scholar] [CrossRef]
- ASTMD678416; Standard Test Method for Mercury from Coal-Fired Stationary Sources (Ontario Hydro Method). EPA: Washington, DC, USA, 1999.
- Liu, Y.Z.; Guo, Q.J.; Cheng, Y.; Ryu, H.-J. Reaction mechanism of coal chemical looping process for syngas production with CaSO4 oxygen carrier in CO2 atmosphere. Ind. Eng. Chem. Res. 2012, 51, 10364–10373. [Google Scholar] [CrossRef]



| Proximate Analysis, Wad/% | Ultimate Analysis, Wad/% | Hg, μg·g−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | O | N | S | ||
| JYC | 1.88 | 8.36 | 34.08 | 55.68 | 74.74 | 4.58 | 8.66 | 1.28 | 0.50 | 0.229 |
| ZTC | 22.38 | 23.5 | 30.98 | 23.14 | 35.6 | 2.21 | 14.49 | 0.93 | 0.89 | 0.252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Zhao, S.; Gao, M.; Liu, Y. Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process. Molecules 2024, 29, 2195. https://doi.org/10.3390/molecules29102195
Hu G, Zhao S, Gao M, Liu Y. Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process. Molecules. 2024; 29(10):2195. https://doi.org/10.3390/molecules29102195
Chicago/Turabian StyleHu, Guochao, Shuju Zhao, Minggang Gao, and Yongzhuo Liu. 2024. "Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process" Molecules 29, no. 10: 2195. https://doi.org/10.3390/molecules29102195





