Structural and Thermal Characterization of Milled Wood Lignin from Bamboo (Phyllostachys pubescens) Grown in Korea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Bamboo
2.2. Chemical and Structural Characterization of Bamboo MWL
2.2.1. Elemental Composition of MWL
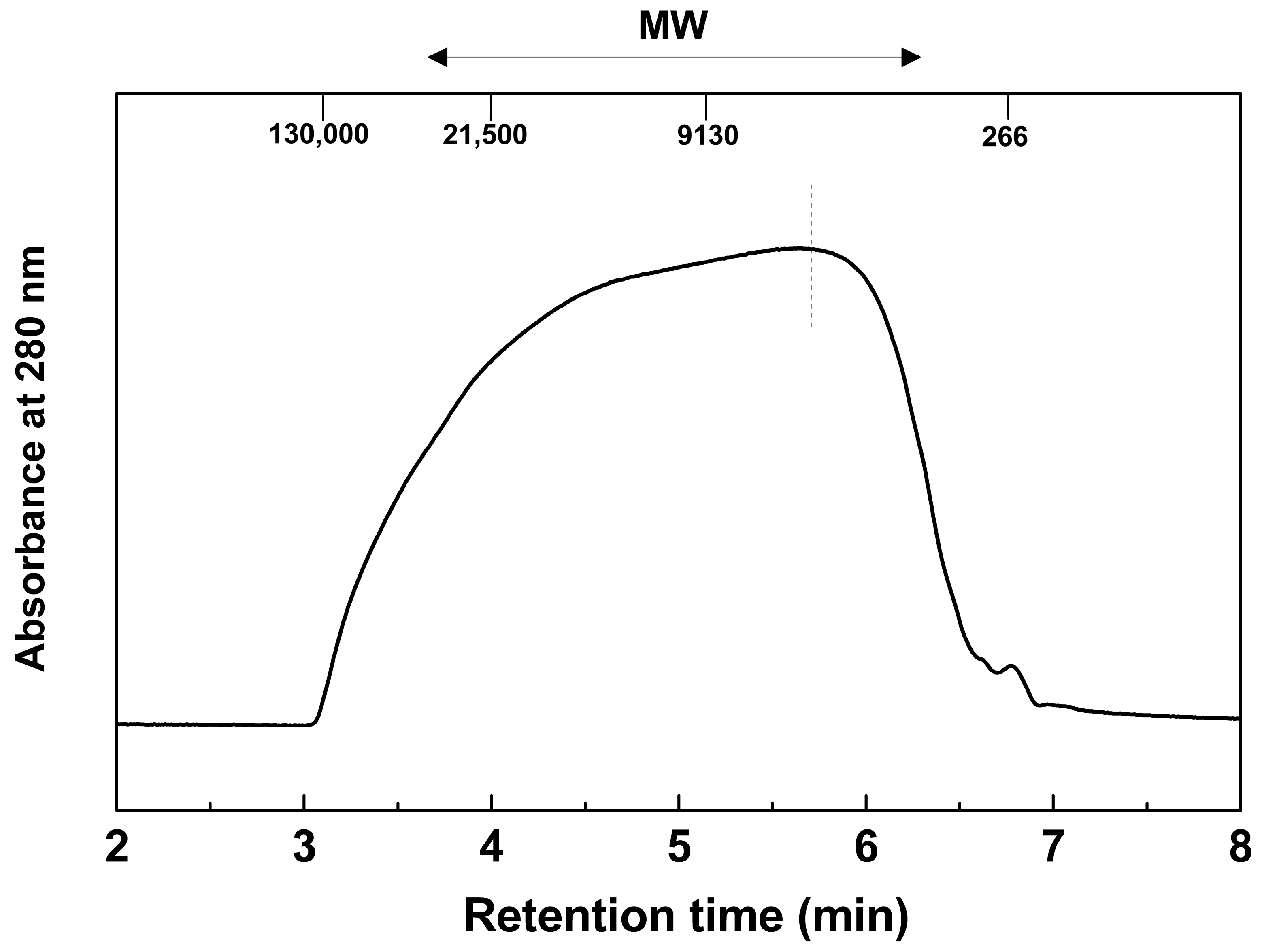
2.2.2. Molecular Weight (MW) Distribution, Average MW, and Polydispersity
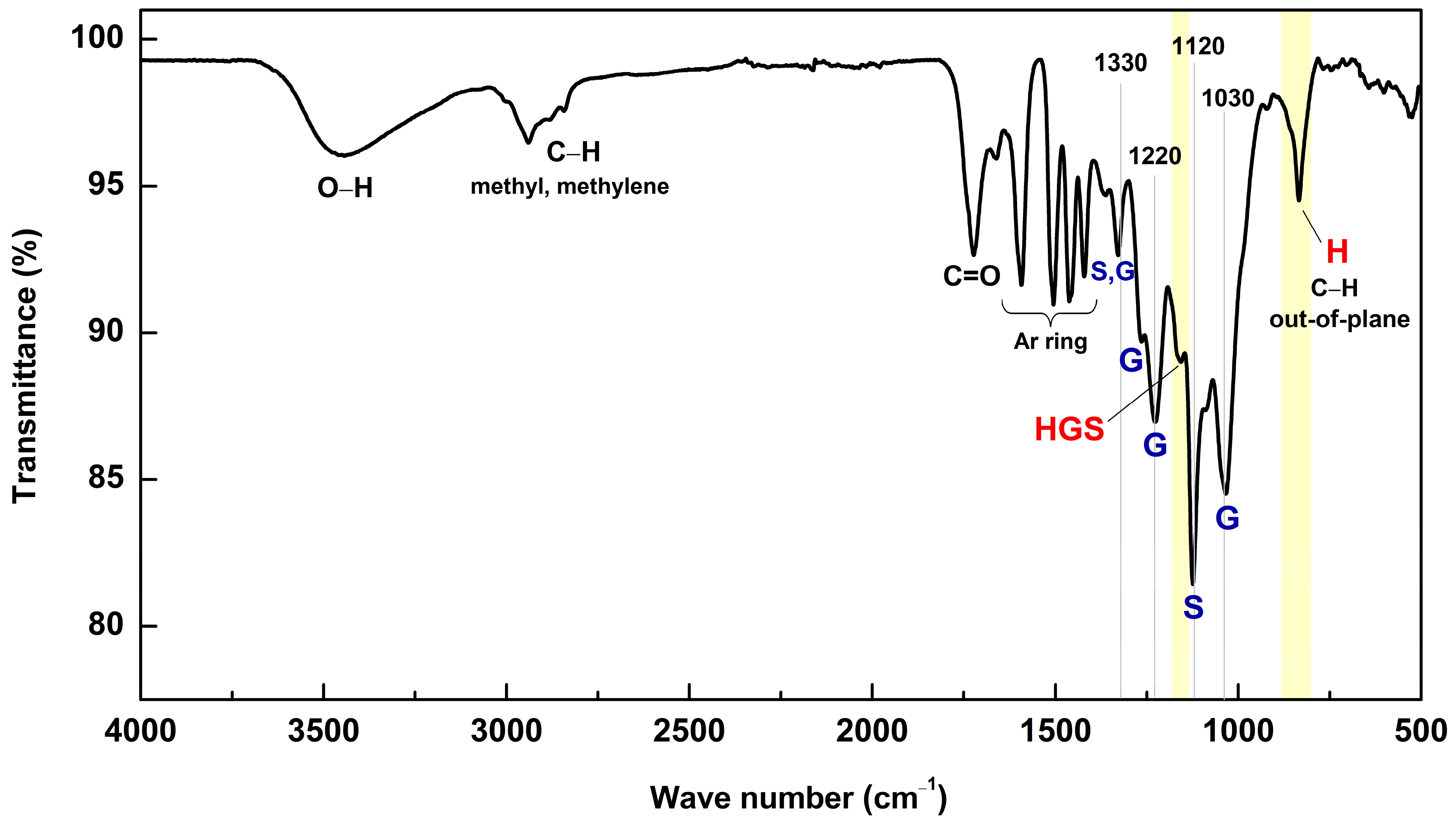
2.2.3. FT-IR Spectroscopy
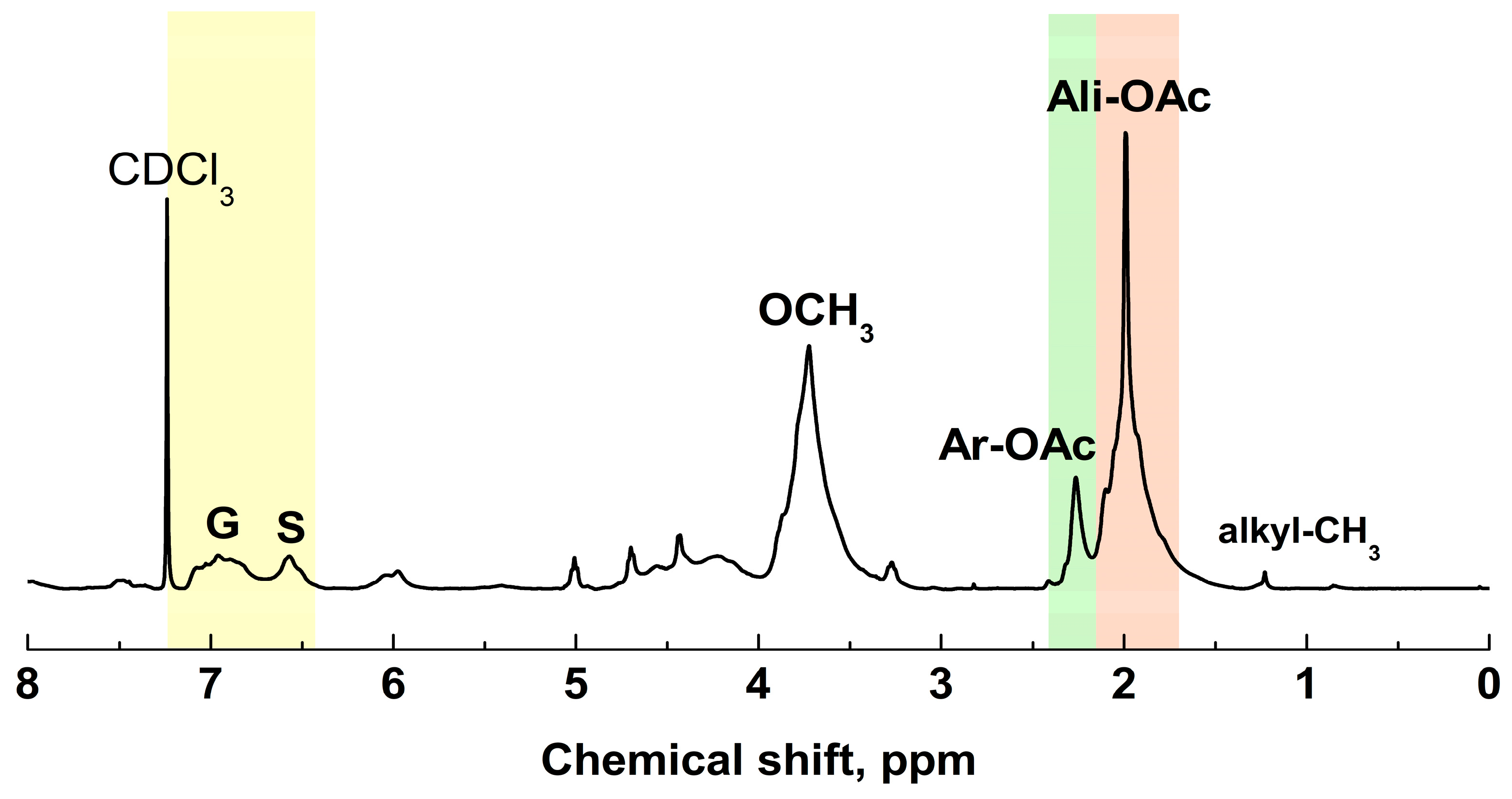
2.2.4. 1H NMR Analysis
2.2.5. 13C NMR Analysis
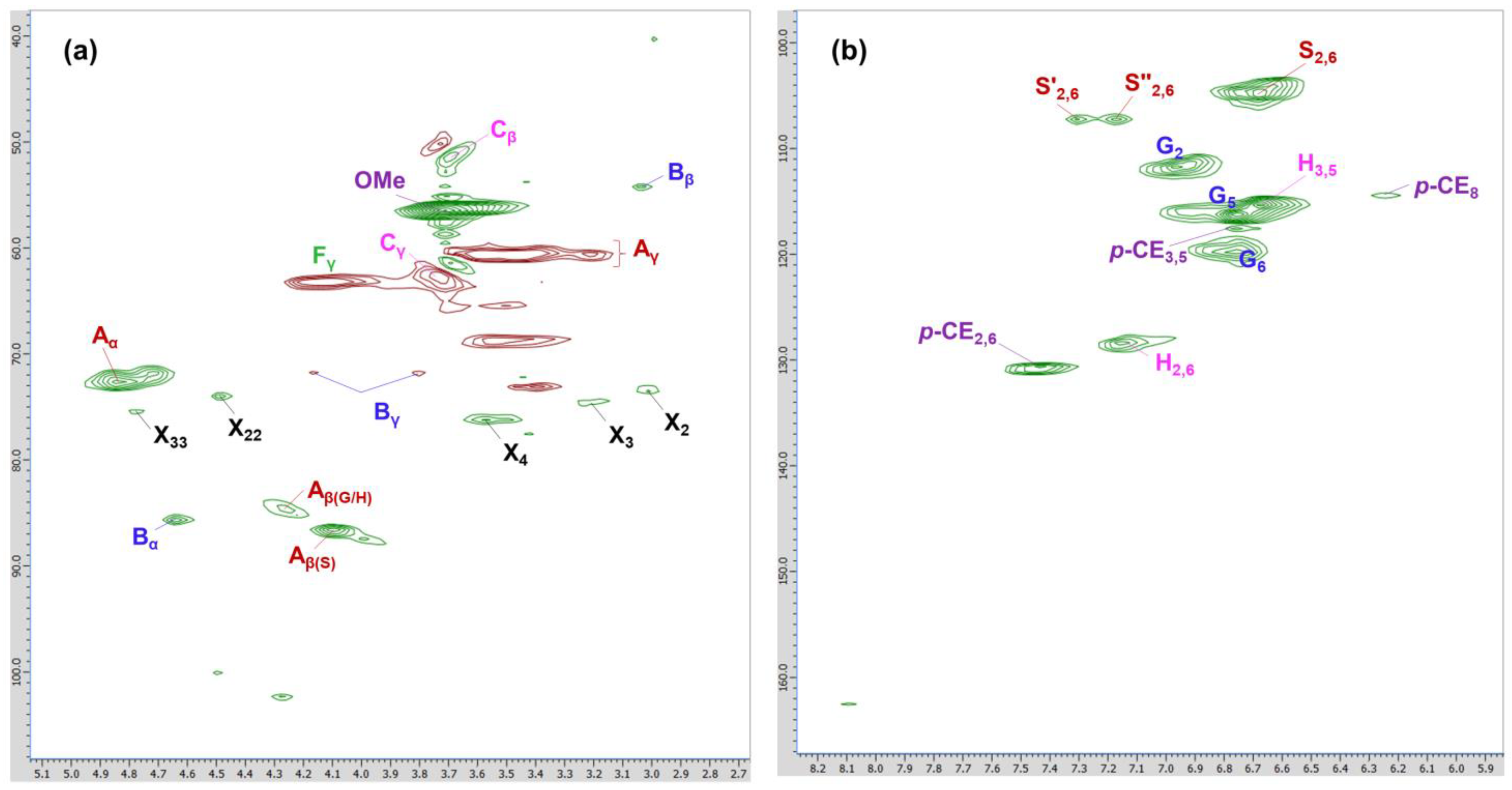
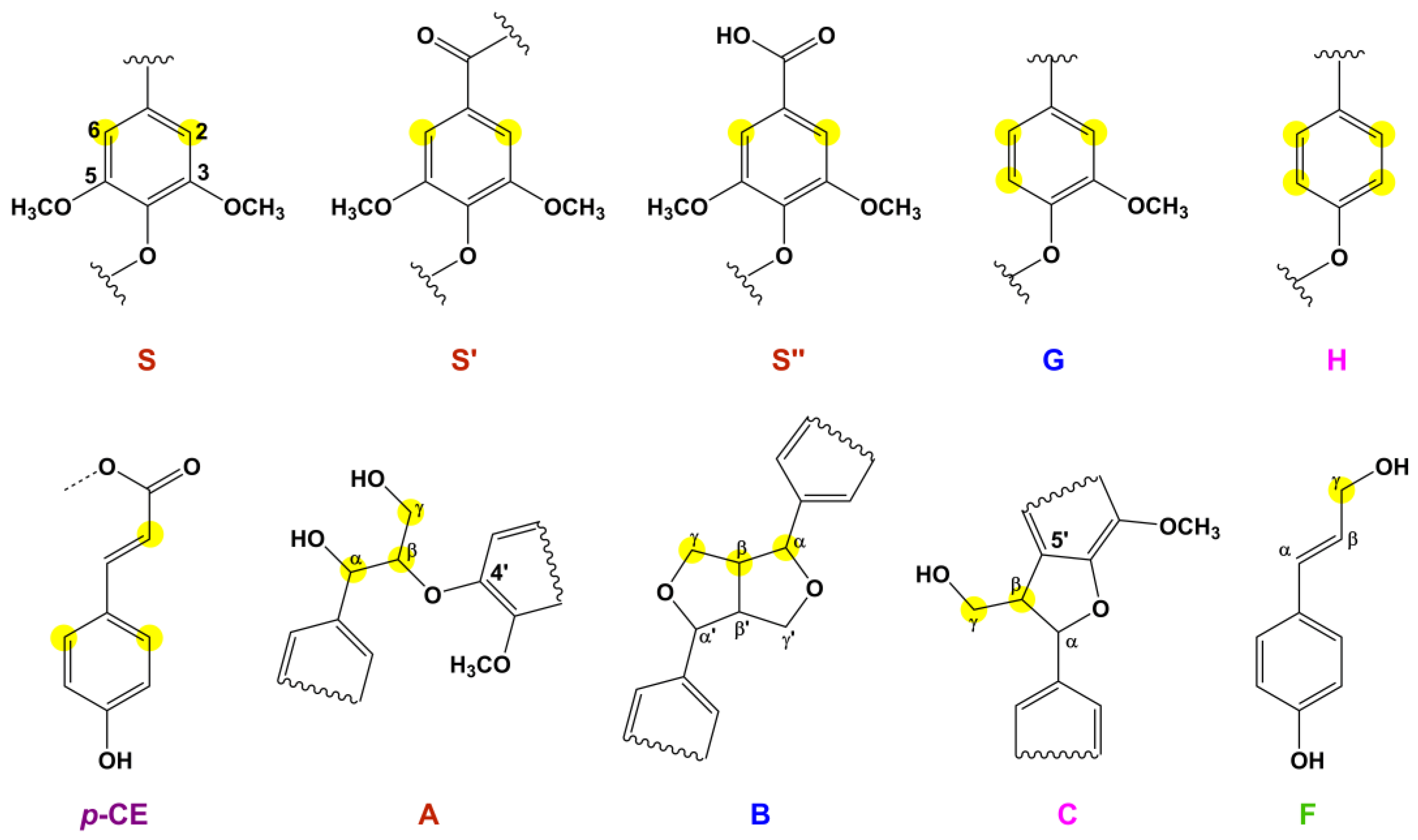
2.2.6. 2D HSQC NMR Analysis
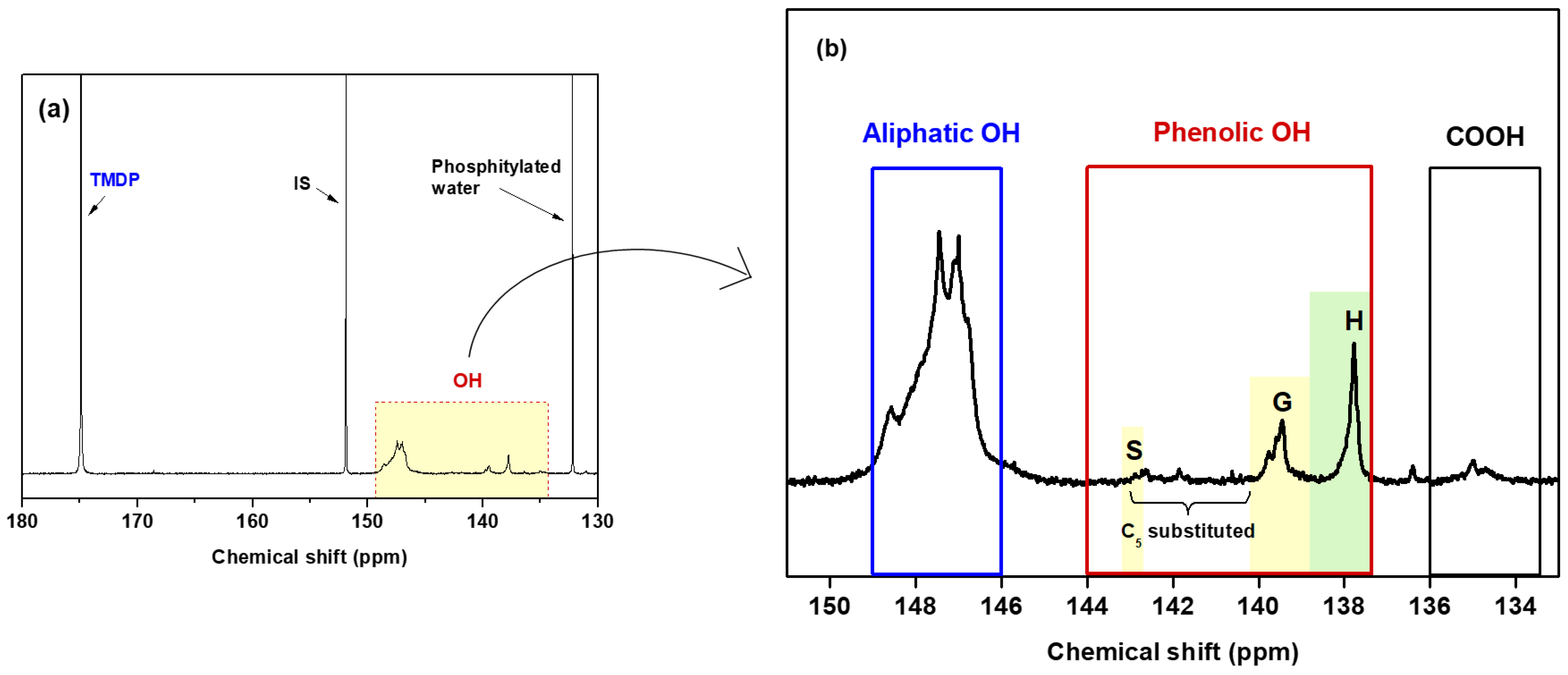
2.2.7. 31P NMR Analysis
2.3. Thermal Characterization of Bamboo MWL
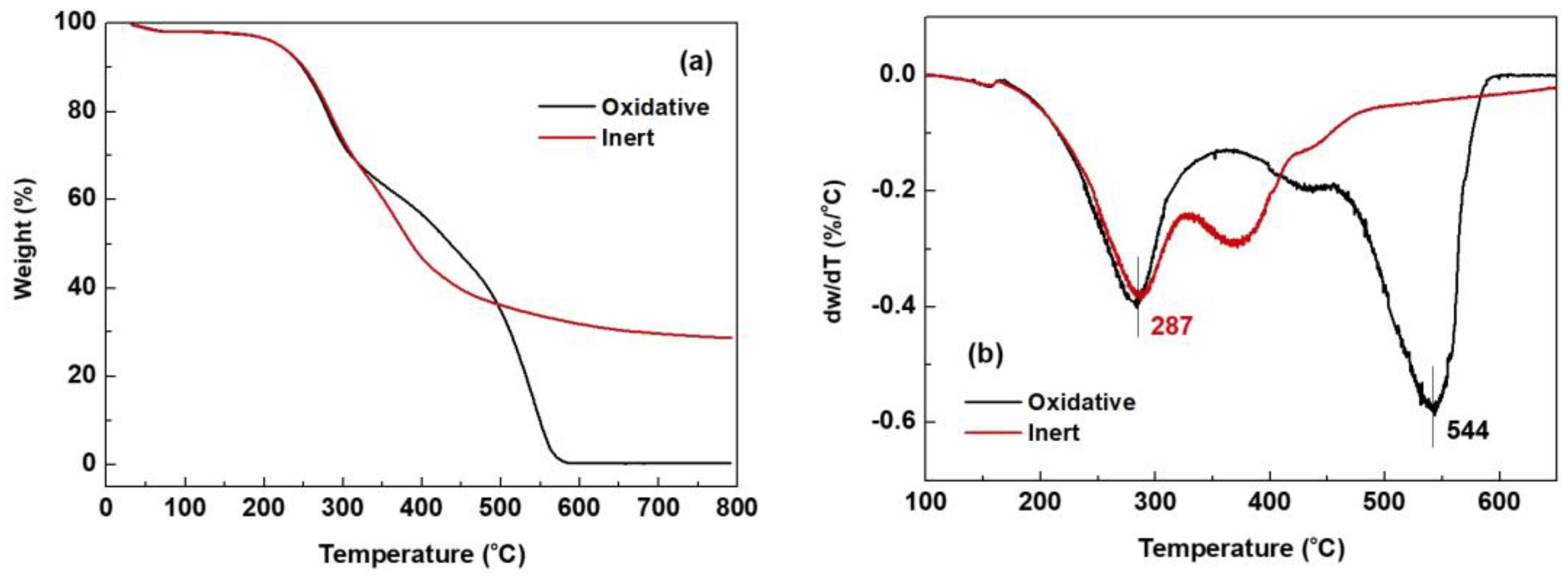
2.3.1. TGA
2.3.2. DSC
2.3.3. Pyrolysis GC/MS (Py-GC/MS)
3. Materials and Methods
3.1. Materials
3.2. Chemical Composition of Bamboo
3.3. Preparation of MWL
3.4. Elemental Analysis
3.5. Acetylation of MWL
3.6. Determination of MW
3.7. FT-IR Spectroscopy
3.8. 1H NMR Analysis
3.9. 13C and 2D HSQC NMR Analysis
3.10. 31P NMR Analysis
3.11. TGA
3.12. DSC
3.13. Pyrolysis GC/MS (Py-GC/MS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of the World’s Forests. Available online: https://www.Fao.org/3/ca8642en/online/ca8642en.html (accessed on 15 December 2023).
- Climate Change 2007: The Physical Science Basis; Contribution of Working Group I Contribution to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007.
- Dlamini, L.C.; Fakudze, S.; Makombe, G.G.; Muse, S.; Zhu, J. Bamboo as a valuable resource and its utilization in historical and modern-day China. BioResources 2022, 17, 1926–1938. [Google Scholar] [CrossRef]
- Chaowana, P. Bamboo: An alternative raw material for wood and wood-based composites. J. Mater. Sci. Res. 2013, 2, 90–102. [Google Scholar] [CrossRef]
- Li, W.; He, S. Research on the utilization and development of bamboo resources through problem analysis and assessment. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 052028. [Google Scholar] [CrossRef]
- Lobovikov, M.; Paudel, S.; Piazza, M.; Ren, H.; Wu, J. World Bamboo Resources: A Thematic Study Prepared in the Framework of the Global Forest Resources Assessment 2005; Food and Agriculture Organization (FAO): Rome, Italy, 2007. [Google Scholar]
- Yeromiyan, T. The Culture and History of Chinese Bamboo, The Chinese Language Institute. Available online: https://studycli.org/chinese-culture/chinese-bamboo (accessed on 17 August 2023).
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- National Institute of Forest Science (NIFoS). Distribution Status of Bamboo Forest Resources in Korea; National Institute of Forest Science: Seoul, Republic of Korea, 2016. [Google Scholar]
- Sharma, B.; Gatóo, A.; Bock, M.; Ramage, M. Engineered bamboo for structural applications. Constr. Build. Mater. 2015, 81, 66–73. [Google Scholar] [CrossRef]
- Liu, X.; Smith, G.D.; Jiang, Z.; Bock, M.C.D.; Boeck, F.; Frith, O.; Gatóo, A.; Liu, K.; Mulligan, H.; Semple, K.E.; et al. Nomenclature for engineered bamboo. BioResources 2016, 11, 1141–1161. [Google Scholar] [CrossRef]
- Nayak, L.; Mishra, S.P. Prospect of bamboo as a renewable textile fiber, historical overview, labeling, controversies and regulation. Fash. Text. 2016, 3, 2. [Google Scholar] [CrossRef]
- Okokpujie, I.P.; Akinlabi, E.T.; Fayomi, O.O. Assessing the policy issues relating to the use of bamboo in the construction industry in Nigeria. Heliyon 2020, 6, e04042. [Google Scholar] [CrossRef]
- Hidayati, S.; Suroso, E.; Satyajaya, W.; Iryani, D.A. Chemistry and structure characterization of bamboo pulp with formacell pulping. IOP Conf. Ser. Mater. Sci. Eng. 2019, 532, 012024. [Google Scholar] [CrossRef]
- Bonfatti Júnior, E.A.; Lengowski, E.C.; de Andrade, A.S.; Venson, I.; Klock, U.; da Silva Júnior, F.G.; Gonçalez, J.C.; de Muñiz, G.I.B. Bamboo kraft pulping. Adv. For. Sci. 2019, 6, 791–796. [Google Scholar] [CrossRef]
- Nimz, H.H.; Robert, D.; Faix, O.; Nemr, M. 13C NMR spectra of lignins, 8. Structural differences between lignins of hardwoods, softwoods, grasses and compression wood. Holzforschung 1981, 35, 16–26. [Google Scholar] [CrossRef]
- Li, X.B.; Shupe, T.F.; Peter, G.F.; Hse, C.Y.; Eberhardt, T.L. Chemical changes with maturation of the bamboo species Phyllostachys pubescens. J. Trop. For. Sci. 2007, 19, 6–12. [Google Scholar]
- Abreu, H.D.S.; Freire, M.D.F.I. Methoxyl content determination of lignins by 1H NMR. Ann. Acad. Bras. Ciênc. 1995, 67, 379–382. [Google Scholar]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Formic acid based organosolv pulping of bamboo (Phyllostachys acuta): Comparative characterization of the dissolved lignins with milled wood lignin. Chem. Eng. J. 2012, 179, 80–89. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.Z.; Li, M.F. Structural Characterization of bamboo lignin isolated with formic acid and alkaline peroxide by gel permeation chromatography and pyrolysis gas chromatography mass spectrometry. Ann. Chromatogr. Sep. Tech. 2015, 1, 1006. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Quantitative structural characterization of the lignins from the stem and pith of bamboo (Phyllostachys pubescens). Holzforschung 2013, 67, 613–627. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Xiao, L.P.; Shi, Z.J.; Sun, R.C. Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Qin, M.H.; Xu, W.Y.; Fu, Y.J.; Wang, Z.J.; Li, Z.Q.; Willför, S.; Xu, C.L.; Hou, Q.X. Structural changes of bamboo-derived lignin in an integrated process of autohydrolysis and formic acid inducing rapid delignification. Ind. Crops Prod. 2018, 115, 194–201. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Lundquist, K. NMR studies on lignin. 2. Interpretation of the 1H NMR spectrum of acetylated birch lignin. Acta Chem. Scand. B 1979, 33, 27–30. [Google Scholar] [CrossRef]
- Lundquist, K. NMR studies on lignin. 4. Investigation of spruce lignin by 1H NMR spectroscopy. Acta Chem. Scand. B 1980, 34, 21–26. [Google Scholar] [CrossRef]
- Jahan, M.S.; Mun, S.P. Characteristics of dioxane lignins isolated at different ages of nalita wood (Trema orientalis). J. Wood Chem. Technol. 2007, 27, 83–98. [Google Scholar] [CrossRef]
- Sun, R.C.; Xiao, B.; Lawther, J.M. Fractional and structural characterization of ball-milled and enzyme lignins from wheat straw. J. Appl. Polym. Sci. 1998, 68, 1633–1641. [Google Scholar] [CrossRef]
- Shi, Z.J.; Xu, G.F.; Deng, J.; Dong, M.Y.; Murugadoss, V.; Liu, C.T.; Shao, Q.; Wu, S.D.; Guo, Z.H. Structural characterization of lignin from D. sinicus by FTIR and NMR techniques. Green Chem. Lett. Rev. 2019, 12, 235–243. [Google Scholar] [CrossRef]
- Nakatsubo, F.; Tanahashi, M.; Higuchi, T. Acidolysis of bamboo lignin II. Isolation and identification of acidolysis products. Wood Res. 1972, 53, 9–18. [Google Scholar]
- Higuchi, T.; Tanahashi, M.; Nakatsubo, F. Acidolysis of bamboo lignin III. Estimation of arylglycerol-β-aryl ether groups in lignins. Wood Res. 1972, 54, 9–18. [Google Scholar]
- Tanahashi, M.; Nakatsubo, F.; Higuchi, T. Structural elucidation of bamboo lignin by acidolysis and ozonolysis I. Wood Res. 1975, 58, 9–18. [Google Scholar]
- Nakamura, Y.; Higuchi, T. Ester linkage of p-coumaric acid in bamboo lignin. Holzforschung 1976, 30, 87–191. [Google Scholar] [CrossRef]
- Seca, A.M.; Cavaleiro, J.A.; Domingues, F.M.; Silvestre, A.J.; Evtuguin, D.; Neto, C.P. Structural characterization of the lignin from the nodes and internodes of Arundo donax reed. J. Agric Food Chem. 2000, 48, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C. Unveiling the atructural heterogeneity of bamboo lignin by in situ HSQC NMR technique. Bioenerg. Res. 2012, 5, 886–903. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org. Biomol. Chem. 2010, 8, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.S.; Pajer, N.; Crestini, C. Quantitative 31P NMR analysis of lignins and tannins. J. Vis. Exp. 2021, 174, e62696. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.L.; Chen, T.Y.; Qin, Z.Y.; Peng, W.X.; Wen, J.L. Understanding the mechanism of self-bonding of bamboo binderless boards: Investigating the structural changes of lignin macromolecule during the molding pressing process. BioResources 2017, 12, 514–532. [Google Scholar] [CrossRef]
- Amit, T.A.; Roy, R.; Raynie, D.E. Thermal and structural characterization of two commercially available technical lignins for potential depolymerization via hydrothermal liquefaction. Curr. Res. Green Sustain. Chem. 2021, 4, 100106. [Google Scholar] [CrossRef]
- Wörmeyer, K.; Ingram, T.; Saake, B.; Brunner, G.; Smirnova, I. Comparison of different pretreatment methods for lignocellulosic materials. Part II. Influence of pretreatment on the properties of rye straw lignin. Bioresour. Technol. 2011, 102, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Lu, Q.; Sun, X.F. Physico-chemical and thermal characterization of lignins from Caligonum monogoliacum and Tamarix spp. Polym. Degrad. Stab. 2001, 72, 229–238. [Google Scholar] [CrossRef]
- Pe, J.A.; Mun, J.S.; Mun, S.P. Thermal characterization of kraft lignin prepared from mixed hardwoods. BioResources 2023, 18, 926–936. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Yoshida, H.; Mörck, R.; Kringstad, K.P.; Hatakeyama, H. Fractionation of kraft lignin by successive extraction with organic solvents. II. Thermal properties of kraft lignin fractions. Holzforschung 1987, 41, 171–176. [Google Scholar] [CrossRef]
- Faix, O.; Meier, D.; Fortmann, I. Thermal degradation products of wood. A collection of electron-impact (EI) mass spectra of monomeric lignin derived products. Holz. Roh. Werkst. 1990, 48, 351–354. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D. Pyrolysis–GC–MS characterization of forage materials. J. Agric. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; De Leeuw, J.W. Lignin pyrolysis products: Their structures and their significance as biomarkers. Org. Geochem. 1986, 10, 869–876. [Google Scholar] [CrossRef]
- TAPPI T 211 om-02; Ash in Wood, Pulp, Paper, and Paperboard, TAPPI Test Methods. TAPPI Press: Atlanta, GA, USA, 2002. Available online: https://www.tappi.org/content/sarg/t211.pdf (accessed on 15 March 2021).
- TAPPI T 207 cm-08; Water Solubility of Wood and Pulp. TAPPI Press: Atlanta, GA, USA, 2008.
- TAPPI T 204 os-76; Alcohol-Benzene and Dichloromethane Solubles in Wood and Pulp. TAPPI Press: Atlanta, GA, USA, 1988.
- TAPPI T 212 om-02; One Percent Sodium Hydroxide Solubility of Wood and Pulp, TAPPI Test Methods. TAPPI Press: Atlanta, GA, USA, 2002. Available online: https://tappi.micronexx.com/CD/TESTMETHODS/T212.pdf (accessed on 15 March 2021).
- TAPPI T 222 om-02; Acid-Insoluble Lignin in Wood and Pulp, TAPPI Test Methods. TAPPI Press: Atlanta, GA, USA, 2006. Available online: https://www.tappi.org/content/sarg/t222.pdf (accessed on 15 March 2021).
- TAPPI UM 250; Acid-Soluble Lignin in Wood and Pulp, TAPPI Useful Methods. TAPPI Press: Atlanta, GA, USA, 1991.
- Wise, L.E.; Murphy, M.; Daddieco, A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicellulose. Tech. Assoc. Pap. 1946, 29, 210–218. [Google Scholar]
- TAPPI T 203 om-83; Alpha-, Beta-, and Gamma-Cellulose in Pulp. TAPPI Press: Atlanta, GA, USA, 1988.
- Björkman, A. Isolation of lignin from finely divided wood with neutral solvents. Nature 1954, 174, 1057–1058. [Google Scholar] [CrossRef]
- Mun, J.S.; Pe, J.A.; Mun, S.P. Chemical characterization of kraft lignin prepared from mixed hardwoods. Molecules 2021, 26, 4861. [Google Scholar] [CrossRef]



| Ash (%) | 1.24 ± 0.01 |
| Extracts (%) | |
| Cold water | 5.77 ± 0.02 |
| Hot water | 9.85 ± 0.04 |
| 1% NaOH | 29.69 ± 0.10 |
| Alcohol-benzene | 4.77 ± 0.07 |
| Carbohydrate (%) | |
| Holocellulose | 69.25 ± 0.44 |
| α-Cellulose | 44.31 ± 0.16 |
| Hemicellulose * | 24.94 |
| Lignin (%) | |
| Klason | 27.20 ± 0.09 |
| Acid-soluble | 0.51 ± 0.01 |
| Total | 27.71 |
| Elemental Analysis (%) | Reference | ||||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | S | OCH3 | ||
| MWL | 58.39 | 5.66 | 34.66 | 0.12 | - | 20.47 | This study |
| MWL-A | 58.78 | 5.96 | 34.97 | 0.28 | - | 19.48 | [19] |
| MWL-X | 63.10 | 5.67 | 31.23 | - | - | 17.74 | [20] |
| C9 Formula | Formular Weight (Da) | Reference | |
|---|---|---|---|
| MWL | C9H7.76O3.23N0.02(OCH3)1.41 | 214.17 | This study |
| MWL-A | C9H7.67O2.72(OCH3)1.52 | 206.38 | [19] |
| MWL-X | C9H7.53O2.65(OCH3)1.10 | 191.59 | [20] |
| w (Da) | n (Da) | n) | References | |
|---|---|---|---|---|
| Ac-MWL | 13,279 | 4436 | 3.0 | This study |
| Ac-MWL-A | 12,090 | 5410 | 2.2 | [19] |
| Ac-MWL-S | 6080 | 3230 | 1.9 | [21] |
| Ac-MWL-P | 6050 | 3400 | 1.8 | [21] |
| Ac-MWL-B | 7692 | 4406 | 1.8 | [22] |
| Ac-MWL-N | 9420 | 7458 | 1.3 | [23] |
| Band (cm−1) | Assignments |
|---|---|
| 3441 | O–H stretching |
| 2843–2937 | C–H stretching in methyl, methylene groups |
| 1718 | C=O stretching in unconjugated ketone, carbonyl, and ester groups |
| 1664 | C=O stretching in conjugated p-substituted aryl ketone |
| 1594 | Aromatic skeleton vibration plus C=O stretching; S > G: Gcondensed > Getherified |
| 1503 | Aromatic skeleton vibration (G > S) |
| 1462 | C–H deformations (asymm in –CH3 and –CH2–) |
| 1419 | Aromatic skeleton vibration combined with C–H in plane deformations |
| 1365 | Aliphatic C–H stretching in CH3 and phenolic OH |
| 1330 | Condensed S and G ring (G ring bound via position 5) |
| 1266 | G ring plus C=O stretching (G-methoxyl C–O) |
| 1222 | C–O + C–O + C=O stretching (Gcondensed > Getherified) |
| 1160 | Typical for HGS lignins; C=O in ester groups (conj.) |
| 1123 | Aromatic C–H in-plane deformation (S) |
| 1089 | C–O deformation in sec-alcohols and aliphatic ethers |
| 1033 | Aromatic C–H in-plane deformation (G > S) + C–O deformation in primary alcohols + C–H stretching (unconjugated) |
| 921 | C–H out of plane (aromatic ring) |
| 834 | C–H out of plane in positions (2 and 6 of S + in all positions of H units) |
| Ppm | Main Assignments | Ac-MWL |
|---|---|---|
| 7.20–6.80 * | Aromatic proton in G units | 1.05 |
| 6.80–6.25 | Aromatic proton in S units | 0.93 |
| 6.25–5.75 | Hα of β-O-4 and β-1 structures | 0.47 |
| 5.75–5.24 | Hα of β-5 structures | 0.24 |
| 5.20–4.90 | H of xylan residues | 0.29 |
| 4.90–4.30 | Hα and Hβ of β-O-4 structures | 1.47 |
| 4.30–4.00 | Hα of β-β structures, H of xylan residues | 0.88 |
| 4.00–3.48 | H of methoxyl groups | 4.23 |
| 2.50–2.22 | H of aromatic acetates | 0.76 |
| 2.22–1.60 | H of aliphatic acetates | 4.38 |
| Signal No. | ppm | Assignments |
|---|---|---|
| 1 | 170.1 | Acetyl C=O in alcohols/phenols |
| 2 | 169.5 | |
| 3 | 166.3 | C-9 in p-CE |
| 4 | 162.0 | C-4 in H |
| 5 | 159.9 | C-4 in p-CE |
| 6 | 152.2 | C-3/C-5 in etherified S |
| 7 | 149.2 | C-4 in etherified G, C-3 in etherified G with α-CO |
| 8 | 147.1 | C-3 in G, C-3/C-5 in nonetherified S, C-3 in 5-5 biphenyl |
| 9 | 145.4 | C-4 in nonetherified G, Cα in p-CE |
| 10 | 138.0 | C-4 in etherified S |
| 11 | 134.9 | C-1 in etherified S, C-4 in nonetherified S |
| 12 | 134.4 | C-1 in etherified G |
| 13 | 133.3 | C-1 in nonetherified G |
| 14 | 132.3 | C-1 in nonetherified S |
| 15 | 130.2 | C-2/C-6 in p-CE |
| 16 | 127.9 | C-2/C-6 in H |
| 17 | 125.0 | C-1 in p-CE |
| 18 | 119.1 | C-6 in G |
| 19 | 115.8 | C-3/C-5 in p-CE |
| 20 | 115.2 | C-5 in G, C-3/C-5 in H, C-8 in p-CE |
| 21 | 111.2 | C-2 in G |
| 22 | 106.5 | C-2/C-6 in S with α-CO |
| 23 | 104.2 | C-2/C-6 in S, C-4 in β-β resinol |
| 24 | 103.4 | C-2/C-6 in S |
| 25, 26 | 101.8, 99.5 | Residual carbohydrates |
| 27 | 86.9 | C-α in β-5 phenylcoumaran |
| 28 | 86.1 | C-β in β-O-4 |
| 29 | 85.0 | C-α in β-β resinol |
| 30 | 84.5–81.3 | C-β in β-O-4 |
| 31 | 75.6 | Residual carbohydrates |
| 32 | 75.3 | C-α in β-1, residual carbohydrates |
| 33 | 73.4 | C-α in β-O-4, residual carbohydrates |
| 34 | 72.2 | C-α in β-O-4 |
| 35 | 71.7 | C-γ in β-β resinol |
| 36 | 68.2 | NA * |
| 37 | 64.9 | C-γ in β-5 phenylcoumaran |
| 38 | 62.7 | C-γ in β-5 phenylcoumaran, β-O-4 with α-CO |
| 39 | 60.1 | C-γ in β-O-4 |
| 40 | 55.8 | OCH3 in S and G |
| 41 | 29.0 | CH2 in aliphatic side chain |
| 42 | 20.9 | CH3 in acetyl |
| Notation | δC/δH | Main Assignments |
|---|---|---|
| Cβ | 51.5/3.70 | Cβ–Hβ in β-5 phenylcoumaran (E) |
| Bβ | 54.1/3.04 | Cβ–Hβ in β-β resinol (B) |
| Ome | 56.6/3.71 | C–H in methoxyls |
| Aγ | 60.2/3.38–3.89 | Cγ–Hγ in β-O-4 (A) |
| Cγ | 62.8/3.73 | Cγ–Hγ in β-5 phenylcoumaran (E) |
| Fγ | 63.3/4.11 | Cγ–Hγ in p-hydroxycinnamyl alcohol end-group (F) |
| Bγ | 71.8/4.16, 72.1/3.80 | Cγ–Hγ in β-β resinol (B) |
| Aα | 72.8/4.85 | Cα–Hα in β-O-4 (A) |
| Aβ(G/H) | 82.4/4.34 | Cβ–Hβ in β-O-4 (A) linked to a G/H units |
| Bα | 85.8/4.64 | Cα–Hα in β-β resinol (B) |
| Aβ(S) | 86.6/4.10 | Cβ–Hβ in β-O-4 (A) linked to a S units |
| S2,6 | 104.8/6.69 | C2,6–H2,6 in syringyl units (S) |
| S″2,6 | 107.0/7.30 | C2,6–H2,6 in oxidized (CαOOH) syringyl units (S″) |
| S′2,6 | 107.2/7.19 | C2,6–H2,6 in oxidized (Cα=O) syringyl units (S) |
| G2 | 111.9/6.97 | C2–H2 in guaiacyl units (G) |
| H3,5 | 113.8/6.67 | C3,5–H3,5 in H units (H) |
| G5 | 115.4/6.68 | C5–H5 in guaiacyl units (G) |
| p-CE3,5 | 116.1/6.78 | C3,5–H3,5 in p-coumarate (PCE) |
| p-CE8 | 116.2/6.26 | C8–H8 in p-coumarate (PCE) |
| G6 | 119.8/6.80 | C6–H6 in guaiacyl units (G) |
| H2,6 | 128.3/7.17 | C2,6–H2,6 in H units (H) |
| p-CE2,6 | 130.6/7.48 | C2,6–H2,6 in p-coumarate (PCE) |
| X2 | 73.1/3.05 | C2–H2 in β-D-xylopyranoside |
| X3 | 74.6/3.26 | C3–H3 in β-D-xylopyranoside |
| X4 | 76.0/3.50 | C4–H4 in β-D-xylopyranoside |
| X22 | 74.0/4.49 | C2–H2 in 2-O-acetyl-β-D-xylopyranoside |
| X33 | 75.4/4.78 | C3–H3 in 3-O-acetyl-β-D-xylopyranoside |
| Amount (mmol/g MWL) | References | |||||||
|---|---|---|---|---|---|---|---|---|
| Ali OH | Ph OH | C5-sub OH + S OH | G OH | H OH | Total OH * | COOH | ||
| MWL | 6.74 | 1.45 | 0.18 | 0.51 | 0.76 | 8.19 | 0.23 | This study |
| MWL-N | 4.52 | 1.50 | 0.28 | 0.48 | 0.74 | 6.02 | 0.24 | [23] |
| MWL-Y | 3.71 | 1.93 | 0.59 | 0.58 | 0.76 | 5.64 | 0.30 | [39] |
| Condition | Composition (%) | Temperature (°C) | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| 120 °C (Volatiles) | 400 °C | 500 °C | 800 °C (Ash/Residue) | Onset | DTGmax | |||
| MWL | Oxidative | 1.9 | 56.7 | 34.9 | 0.3 | 234 | 544 | This study |
| MWL | Inert | 1.8 | 89.4 | 63.9 | 28.7 | 235 | 287 | |
| MWL-N | - | - | - | 32.9 | - | 367 | [23] | |
| MWL-Y | - | - | - | 21.0 | - | 360 | [39] | |
| No. | Compound | Type | Formula | RRT a | MW | m/z b | Relative Composition (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2-Methylphenol | H | C7H8O | 0.91 | 108 | 108, 77 | 3.5 |
| 2 | 4-Methylphenol | H | C7H8O | 0.96 | 107 | 107, 77 | 7.0 |
| 3 | Guaiacol (G) | G | C7H8O2 | 1.00 | 124 | 124, 109, 81 | 10.3 |
| 4 | 4-Ethylphenol | H | C8H10O | 1.17 | 122 | 122, 107 | 4.5 |
| 5 | 4-Methylguaiacol | G | C8H10O2 | 1.24 | 138 | 138, 123, 95 | 3.4 |
| 6 | 4-Vinylphenol | H/PCA | C8H8O | 1.29 | 120 | 120, 91 | 31.7 |
| 7 | 3-Methoxycatechol | S | C7H8O3 | 1.39 | 140 | 140, 125, 97 | 1.4 |
| 8 | 4-Vinylguaiacol | G/FA | C9H10O2 | 1.50 | 150 | 150, 135 | 11.3 |
| 9 | Syringol (S) | S | C8H10O3 | 1.57 | 154 | 154, 139, 93 | 7.3 |
| 10 | Vanillin | G | C8H8O3 | 1.66 | 152 | 152, 151 | 5.2 |
| 11 | (E)-Isoeugenol | G | C10H12O2 | 1.76 | 164 | 164, 149 | 2.9 |
| 12 | 4-Propylguaiacol | G | C10H14O2 | 1.77 | 166 | 166, 137 | 1.5 |
| 13 | Acetylguaiacol | G | C9H12O2 | 1.82 | 166 | 166, 151 | 2.6 |
| 14 | 4-Allylsyringol | S | C11H14O3 | 2.02 | 194 | 194, 91 | 1.2 |
| 15 | Syringaldehyde | S | C9H10O4 | 2.11 | 182 | 182, 181 | 4.1 |
| 16 | Acetosyringone | S | C10H12O4 | 2.23 | 196 | 196, 181 | 2.0 |
| GPC Configuration | Waters (Acquity APC) System, USA |
|---|---|
| Columns | Acquity APC 2.5 µm XT 125, Acquity APC 1.7 µm XT 200 (4.6 × 150 mm, Waters, Wexford, Ireland) |
| Flow rate | 0.6 mL/min |
| Sample injection volume | 10 μL |
| Eluent | THF |
| Column oven temperature | 30 °C |
| Detector | UV (254 nm: polystyrene standards; 280 nm: sample) |
| Analysis time | 10 min |
| MW polystyrene standards | Red: 130,000-21,500-6540-1250 Da White: 35,500-9130-2280-266 Da |
| Stage | Equipment/Condition | |
|---|---|---|
| Pyrolysis | Equipment | Curie-point pyrolyzer (JCI-21, Japan Analytical Industry), pyrofoil (F670, JAI) |
| GC/MS | GCMS-QP2010 Ultra, Shimadzu | |
| GC | Pyrolysis temp. | 670 °C for 5 s |
| Interface temp. | 300 °C | |
| GC | Column | J & W DB-5MS (30 m × 0.25 mm ID × 0.25 μm, Agilent Techn.) |
| Carrier gas | He, 1 mL/min | |
| Injector/Detector temp. | 250 °C | |
| Split ratio | 1:30 | |
| Oven temp. | 50 °C (1 min) → ramping (5 °C/min) → 320 °C (5 min) | |
| MS | Ionization | Electron impact method, 70 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, J.-S.; Mun, S.-P. Structural and Thermal Characterization of Milled Wood Lignin from Bamboo (Phyllostachys pubescens) Grown in Korea. Molecules 2024, 29, 183. https://doi.org/10.3390/molecules29010183
Mun J-S, Mun S-P. Structural and Thermal Characterization of Milled Wood Lignin from Bamboo (Phyllostachys pubescens) Grown in Korea. Molecules. 2024; 29(1):183. https://doi.org/10.3390/molecules29010183
Chicago/Turabian StyleMun, Ji-Sun, and Sung-Phil Mun. 2024. "Structural and Thermal Characterization of Milled Wood Lignin from Bamboo (Phyllostachys pubescens) Grown in Korea" Molecules 29, no. 1: 183. https://doi.org/10.3390/molecules29010183






