Oscillatoria limnetica Mediated Green Synthesis of Iron Oxide (Fe2O3) Nanoparticles and Their Diverse In Vitro Bioactivities
Abstract
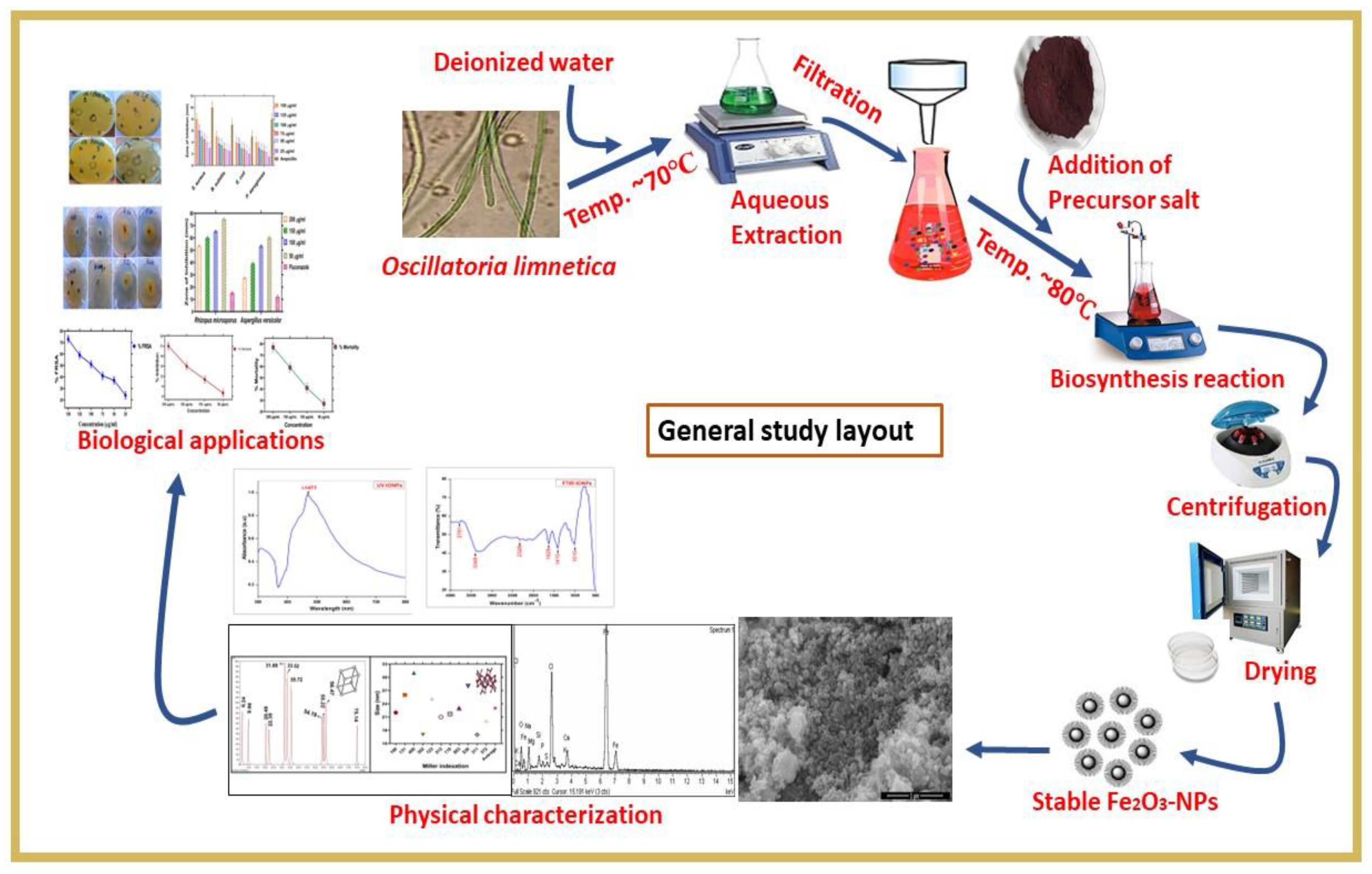
:1. Introduction
2. Results and Discussion
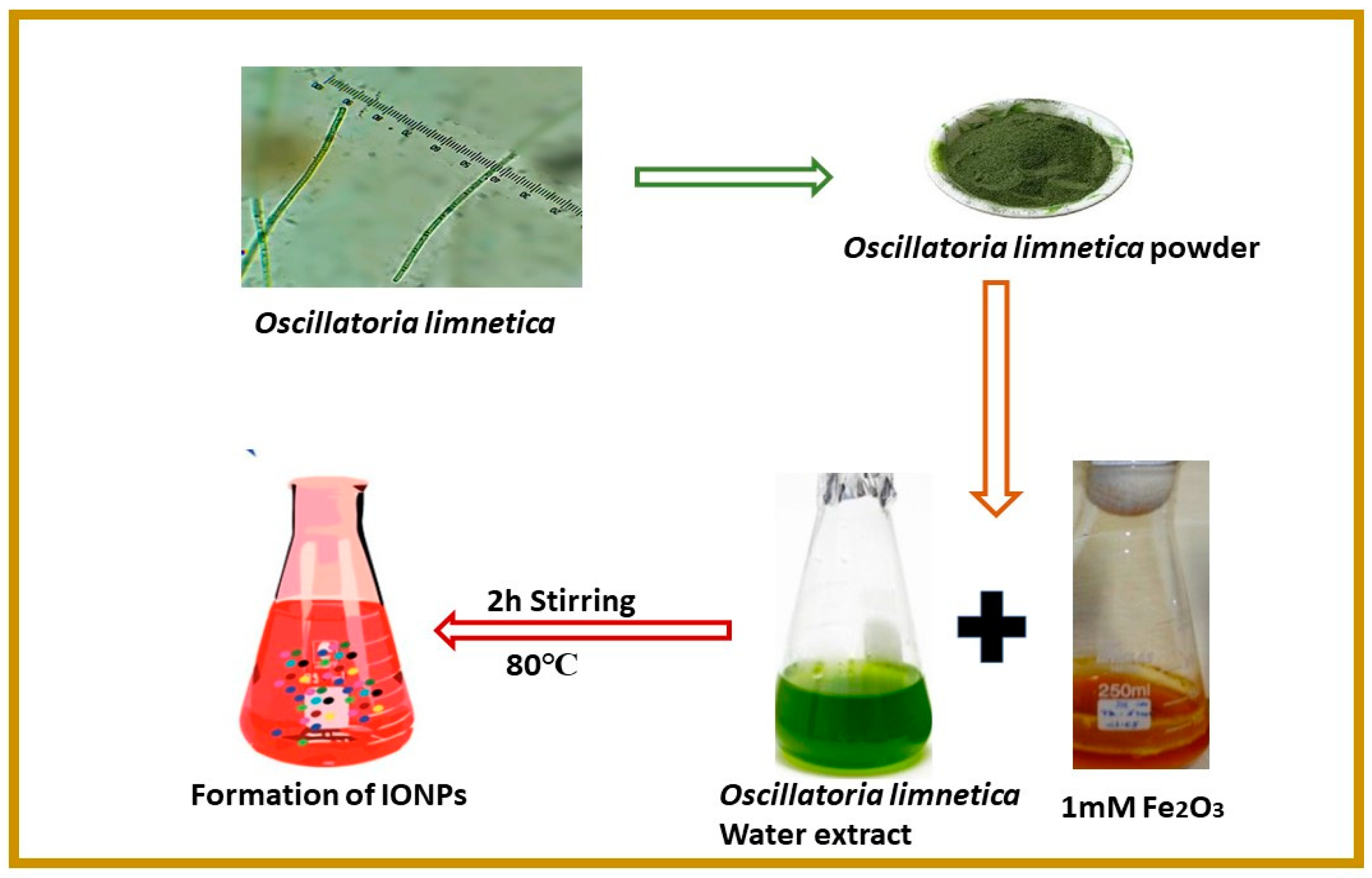
2.1. Biosynthesis and Characterization of IONPs
2.2. UV-Visible Spectroscopy
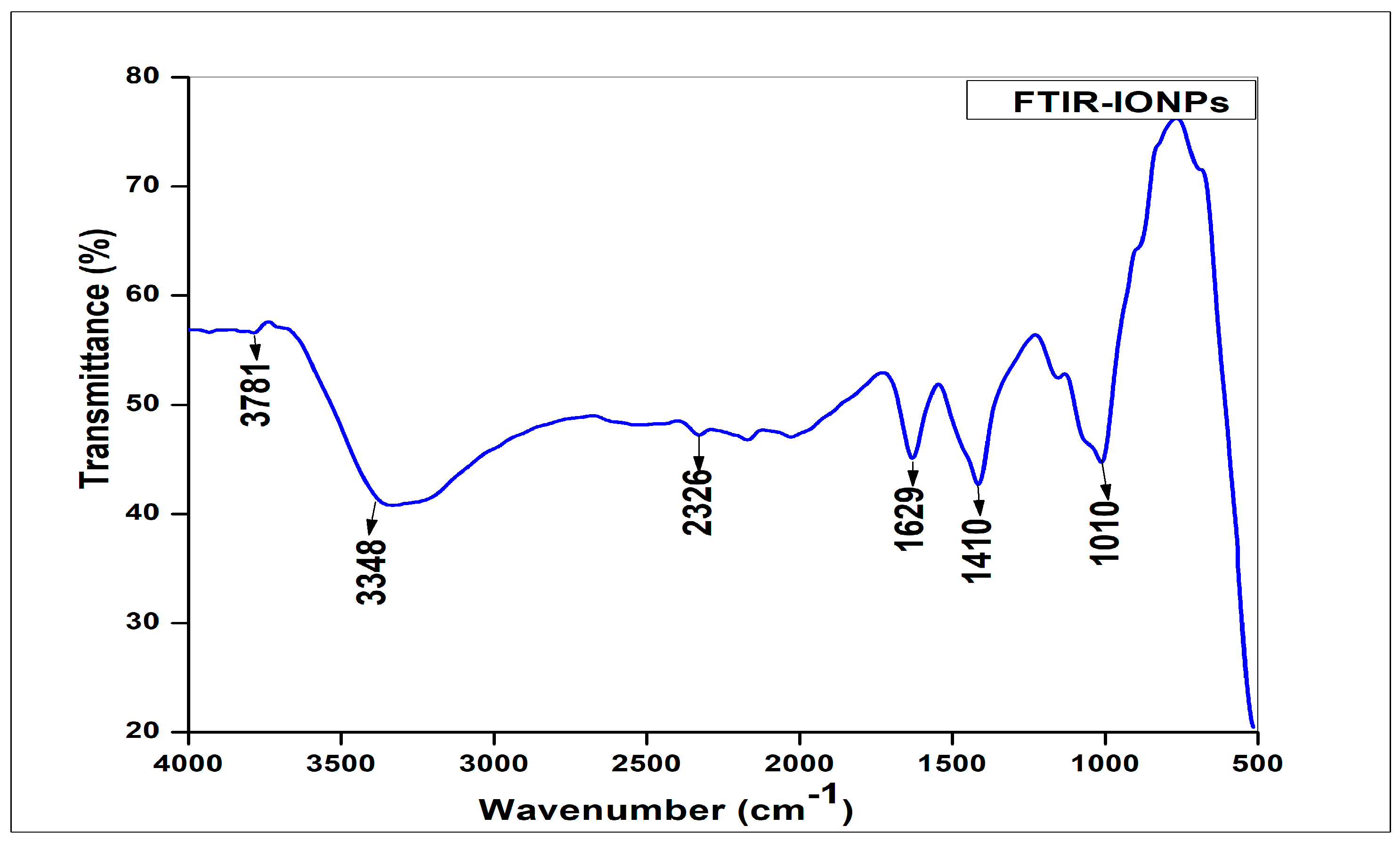
2.3. Fourier Transform Infrared Spectroscopy
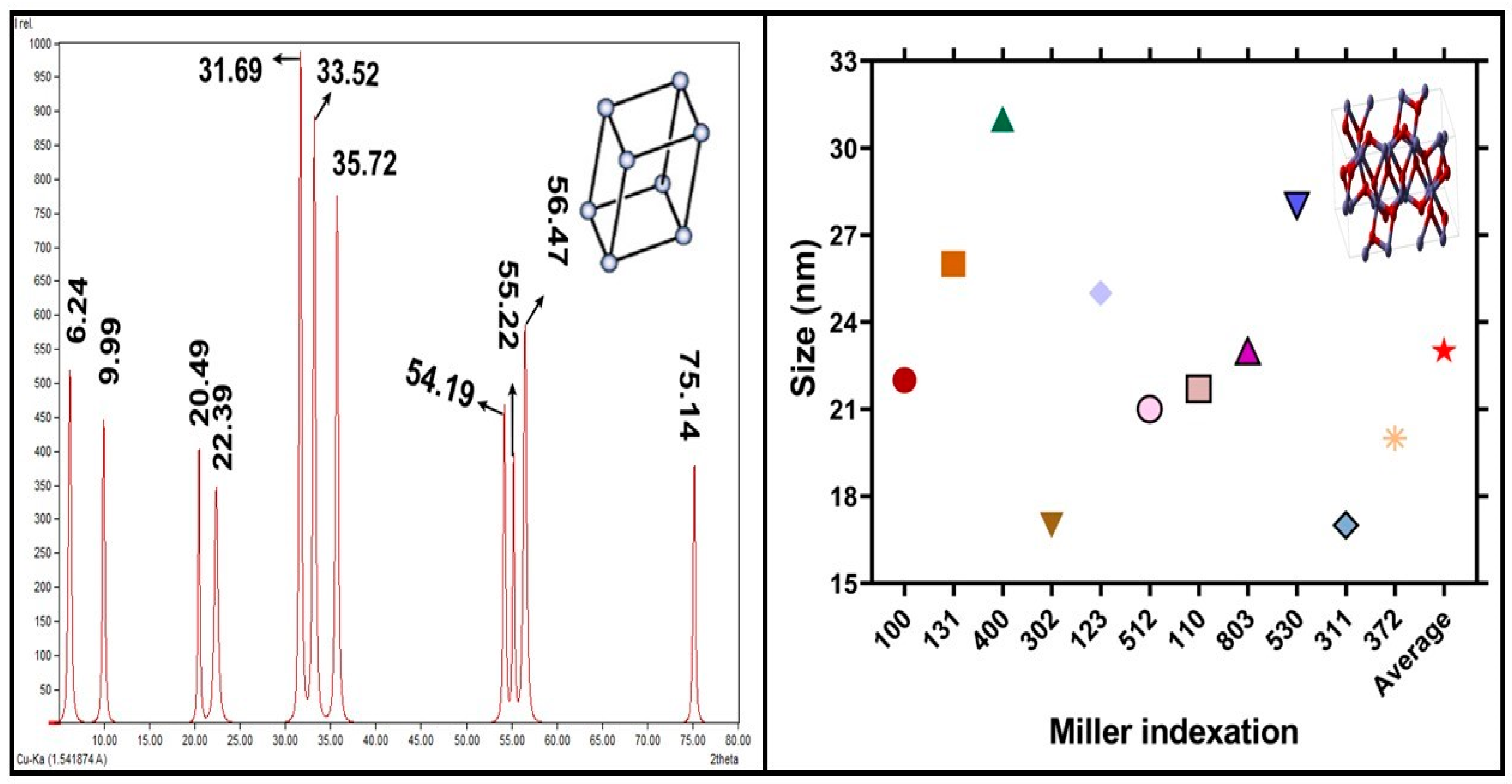
2.4. X-ray Diffractive Analysis
2.5. Scanning Electron Microscopy
2.6. Energy Dispersive X-ray Analysis (EDX) Analysis
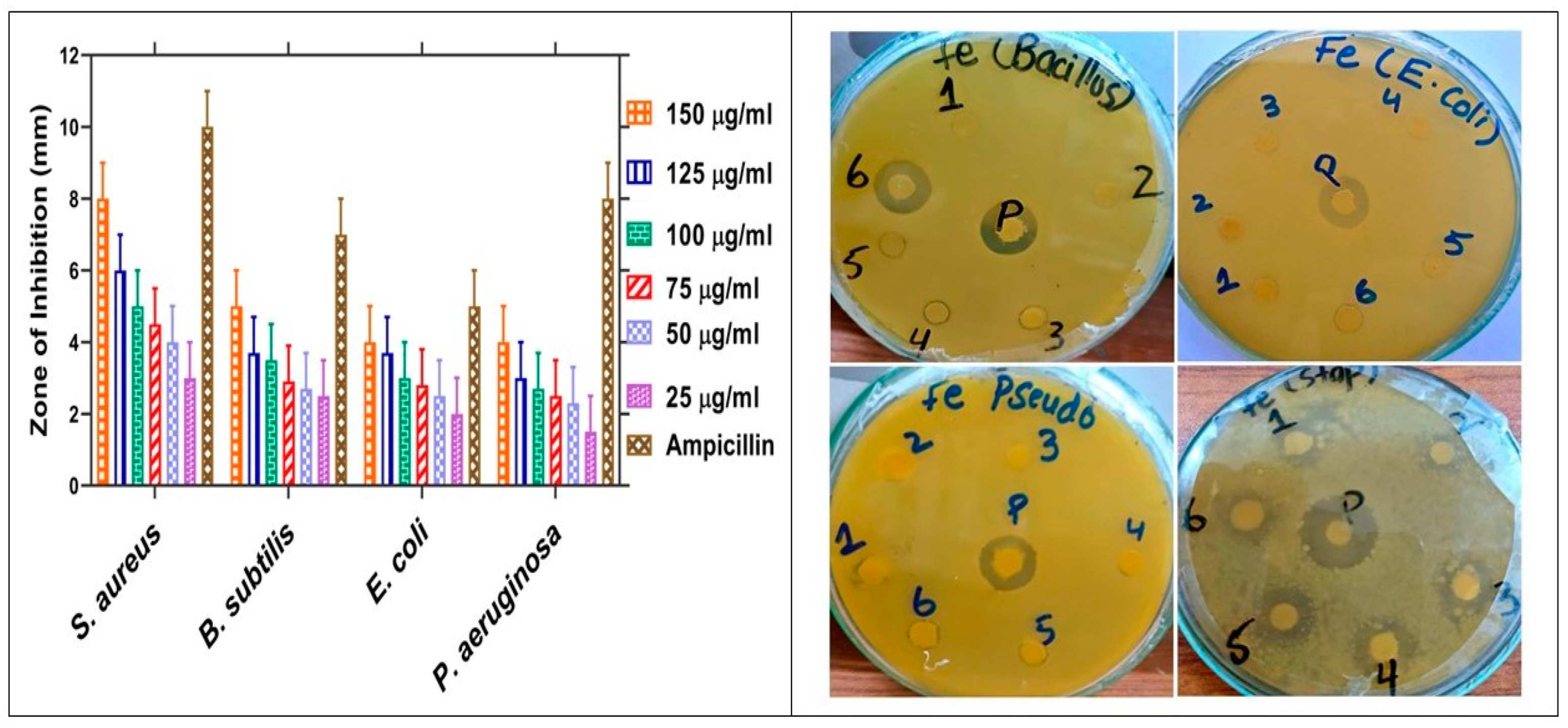
2.7. Antibacterial Assay
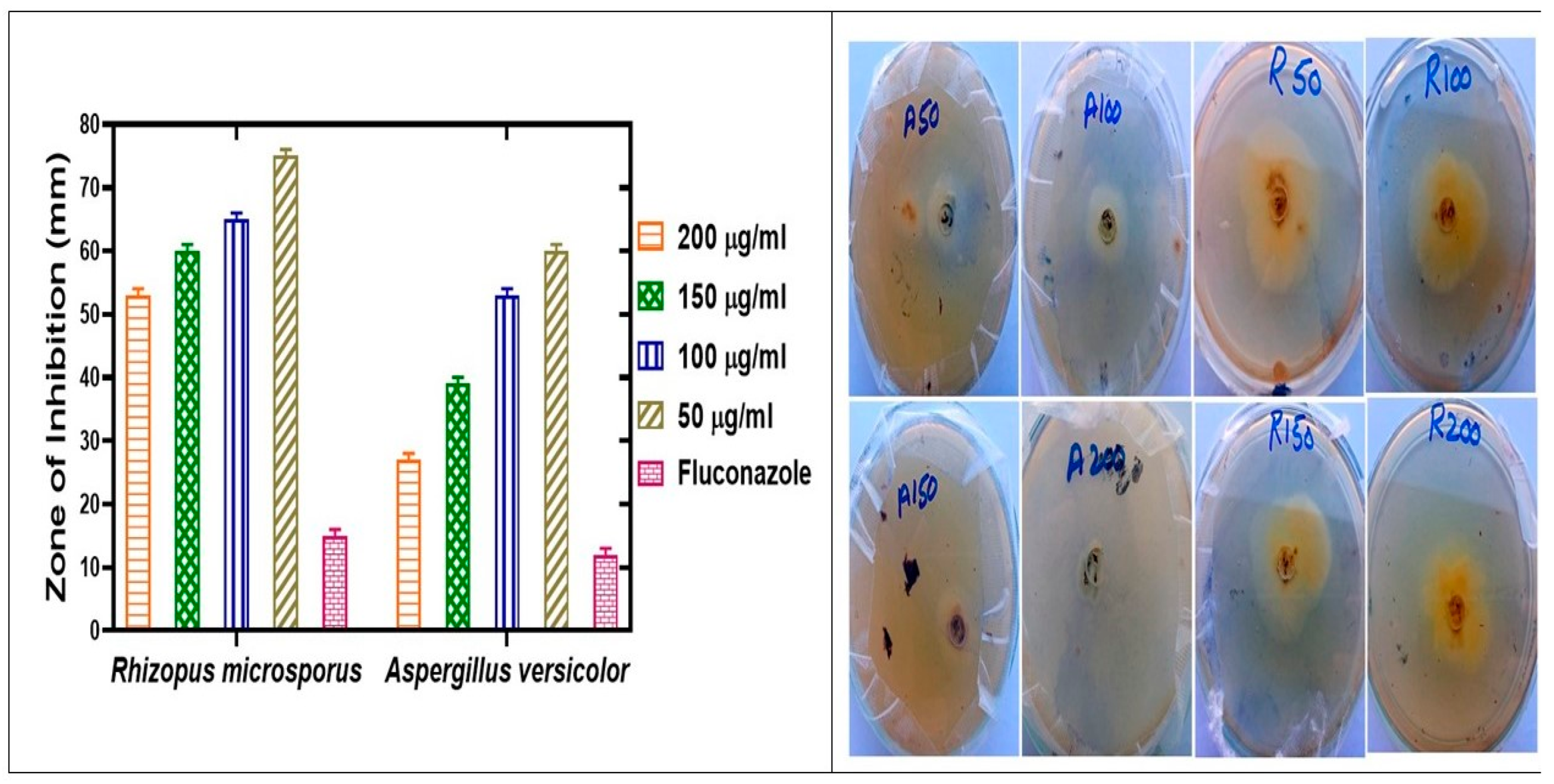
2.8. Antifungal Assay
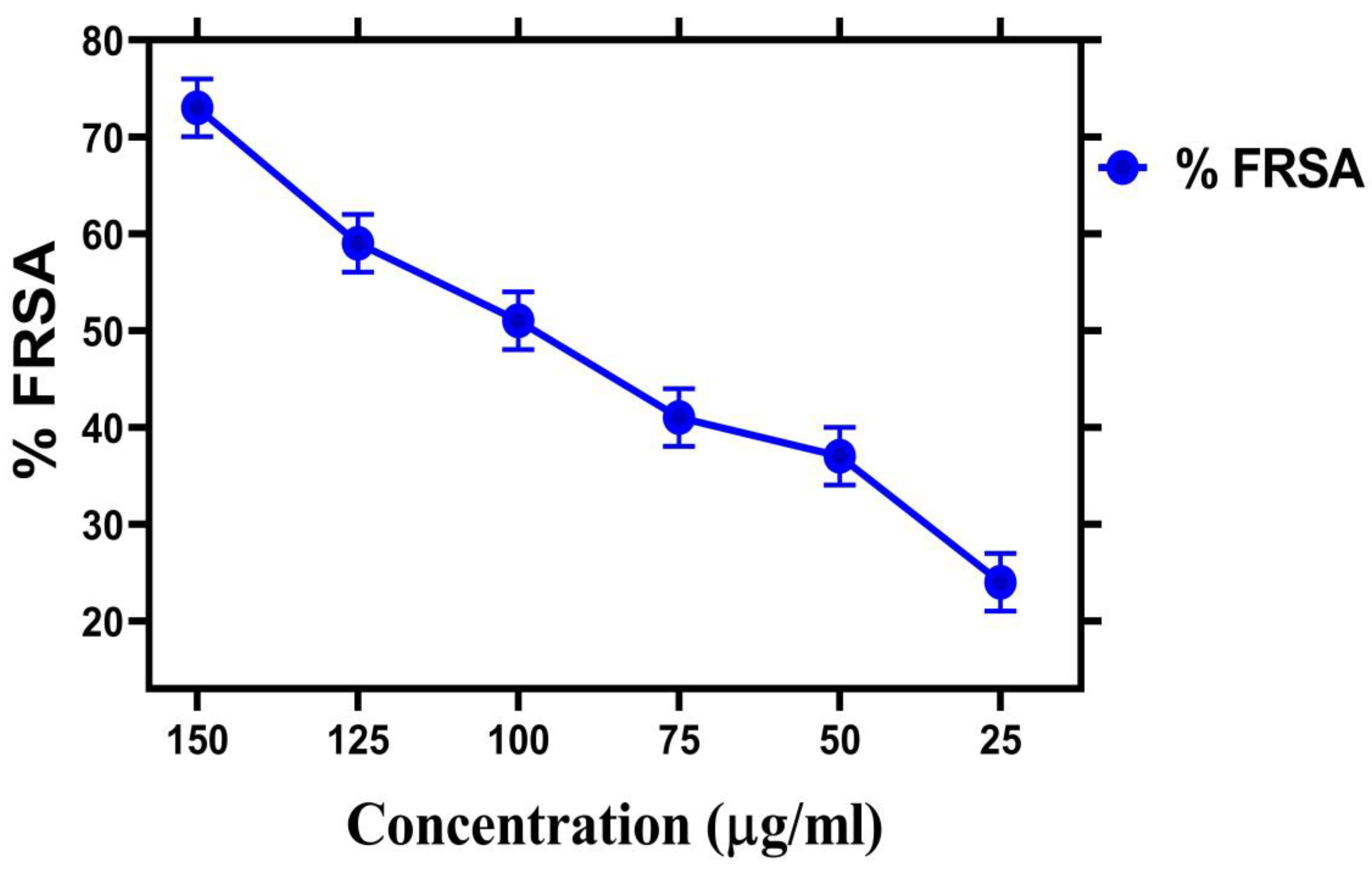
2.9. Antioxidant Assay
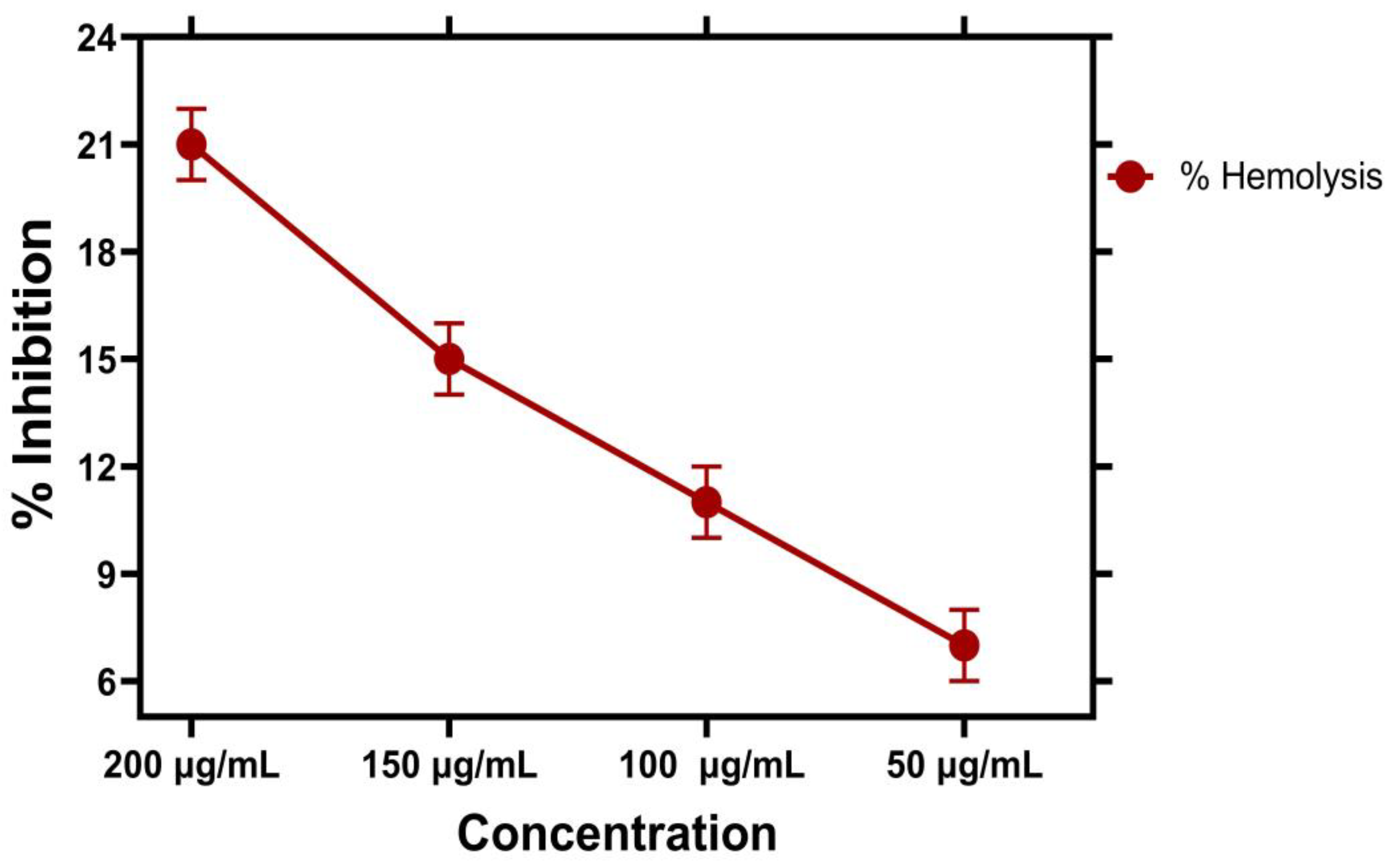
2.10. Hemolytic Assay
Biocompatibility against Human RBCs
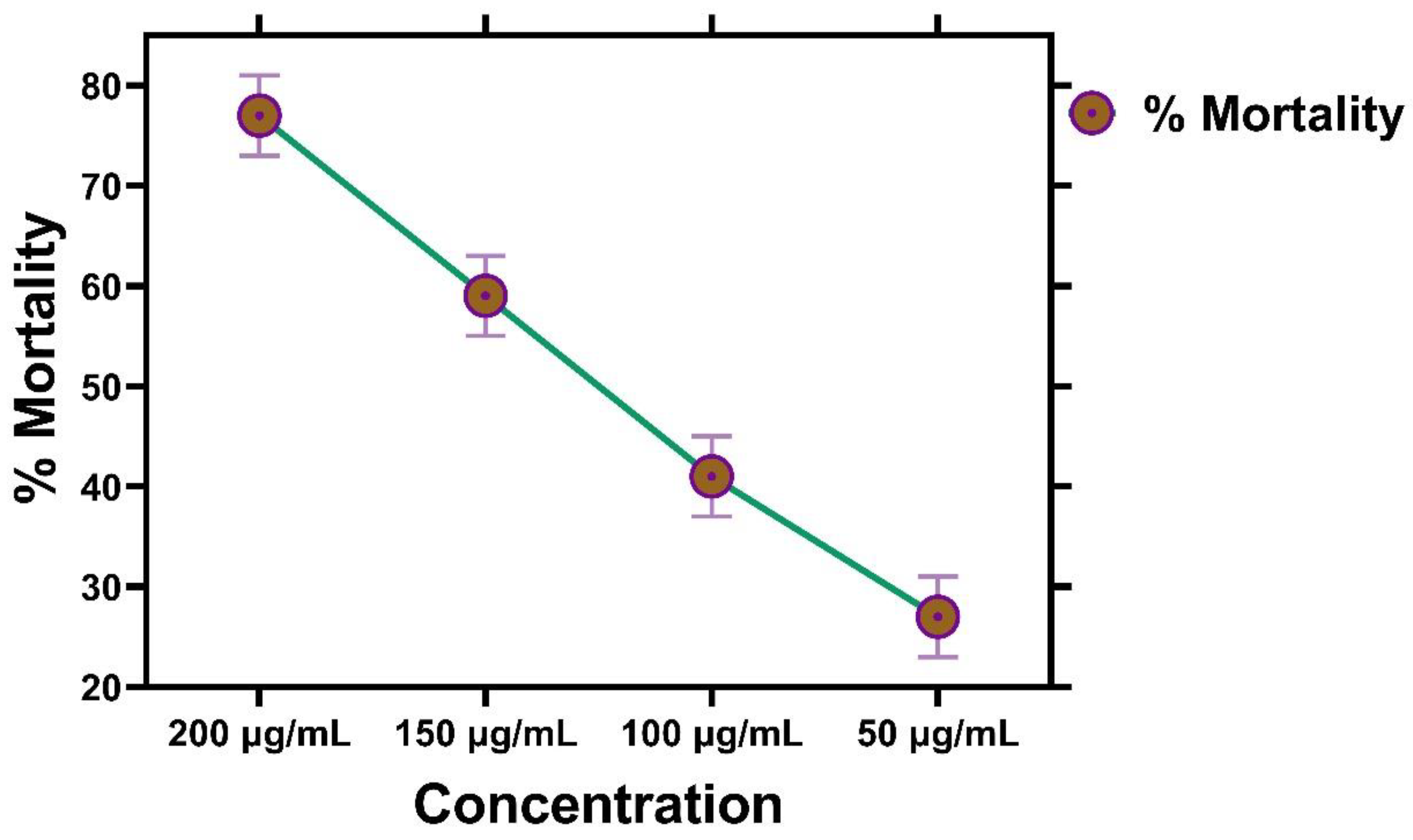
2.11. Brine Shrimp Cytotoxicity Assay
3. Materials and Methods
3.1. Chemicals
3.2. Collection and Preparation of Algal Extract
3.3. Synthesis of IONPs
3.4. Characterization of (IONPs)
3.4.1. UV-Visible Spectroscopy
3.4.2. Fourier Transform Infrared Spectroscopy
3.4.3. X-ray Diffraction Analysis
3.4.4. Scanning Electron Microscopy
3.4.5. Energy Dispersive X-ray Analysis
3.5. Antibacterial Assay
3.6. Antifungal Assay
3.7. Antioxidant Assay
3.8. Hemolytic Assay
Biocompatibility against Human (RBCs)
3.9. Cytotoxicity Assay
Brine Shrimp Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abbasi, B.A.; Iqbal, J.; Yaseen, T.; Zahra, S.A.; Ali, S.; Uddin, S.; Mahmood, T.; Kanwal, S.; El-Serehy, H.A.; Chalgham, W. Exploring Physical Characterization and Different Bio-Applications of Elaeagnus angustifolia Orchestrated Nickel Oxide Nanoparticles. Molecules 2023, 28, 654. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.B.; Saggu, J.I.; Gul, A.; Abbasi, B.A.; Iqbal, J.; Waris, S.; Jardan, Y.A.B.; Chalgham, W. Synthesis and characterization of silver and graphene nanocomposites and their antimicrobial and photocatalytic potentials. Molecules 2022, 27, 5184. [Google Scholar] [CrossRef] [PubMed]
- Shousha, W.G.; Aboulthana, W.M.; Salama, A.H.; Saleh, M.H.; Essawy, E.A. Evaluation of the biological activity of Moringa oleifera leaves extract after incorporating silver nanoparticles, in vitro study. Bull. Natl. Res. Cent. 2019, 43, 212. [Google Scholar] [CrossRef] [Green Version]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, T.; Liu, S.; Du, L.; Chen, Q.; Li, X.; Dong, J.; Zhang, Z.; Lu, S.; Gong, Y. Insights into the Synthesis, types and application of iron Nanoparticles: The overlooked significance of environmental effects. Environ. Int. 2022, 158, 106980. [Google Scholar] [CrossRef]
- Uddin, S.; Iqbal, J.; Safdar, L.B.; Ahmad, S.; Abbasi, B.A.; Capasso, R.; Kazi, M.; Quraihi, U.M. Green synthesis of BPL-NiONPs using leaf extract of Berberis pachyacantha: Characterization and multiple in vitro biological applications. Molecules 2022, 27, 2064. [Google Scholar] [CrossRef]
- Moraru, C.I.; Panchapakesan, C.P.; Huang, Q.; Takhistov, P.; Liu, S.; Kokini, J.L. Nanotechnology: A new frontier in food science understanding the special properties of materials of nanometer size will allow food scientists to design new, healthier, tastier, and safer foods. Food Technol. 2003, 57, 24–29. [Google Scholar]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxidative Med. Cell. Longev. 2020, 2020, 1215395. [Google Scholar] [CrossRef]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef]
- Raghib, F.; Naikoo, M.I.; Khan, F.A.; Alyemeni, M.N.; Ahmad, P. Interaction of ZnO nanoparticle and AM fungi mitigates Pb toxicity in wheat by upregulating antioxidants and restricted uptake of Pb. J. Biotechnol. 2020, 323, 254–263. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.A.; Iqbal, J.; Israr, M.; Yaseen, T.; Zahra, S.A.; Shahbaz, A.; Rahdar, A.; Raouf, B.; Khan, S.U.; Kanwal, S.; et al. Rhamnella gilgitica functionalized green synthesis of ZnONPs and their multiple therapeutic properties. Microsc. Res. Tech. 2022, 85, 2338–2350. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.H.; Ashraf, M.; Sabir, I.A.; Manzoor, M.A.; Malik, M.S.; Gulzar, S.; Ashraf, F.; Iqbal, J.; Niu, Q.; Zhang, Y. Green synthesis and Characterization of Copper oxide nanoparticles using Calotropis procera leaf extract and their different biological potentials. J. Mol. Struct. 2022, 1259, 132696. [Google Scholar] [CrossRef]
- Safat, S.; Buazar, F.; Albukhaty, S.; Matroodi, S. Enhanced sunlight photocatalytic activity and biosafety of marine-driven synthesized cerium oxide nanoparticles. Sci. Rep. 2021, 11, 14734. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Khalil, A.T.; Ali, M.; Iqbal, J.; Rahman, L.; Numan, M.; Khamlich, S.; Maaza, M.; Ullah, I.; Abbasi, B.A.; et al. Precursor effects on the physical, biological, and catalytic properties of Fagonia indica Burm. f. mediated zinc oxide nanoparticles. Microsc. Res. Tech. 2021, 84, 3087–3103. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Yaseen, T.; Zahra, S.A.; Shahbaz, A.; Shah, S.A.; Uddin, S.; Ma, X.; Raouf, B.; Kanwal, S. Green synthesis of zinc oxide nanoparticles using Elaeagnus angustifolia L. leaf extracts and their multiple in vitro biological applications. Sci. Rep. 2021, 11, 20988. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Jeffryes, C.; Mechouet, M.; Agathos, S.N. Biosynthesis of inorganic nanoparticles: A fresh look at the control of shape, size and composition. Bioengineering 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.T.; Khan, M.D.; Razzaque, S.; Afridi, S.; Ullah, I.; Iqbal, J.; Tasneem, S.; Shah, A.; Shinwari, Z.K.; Revaprasadu, N. Single precursor-based synthesis of transition metal sulfide nanoparticles and evaluation of their antimicrobial, antioxidant and cytotoxic potentials. Appl. Nanosci. 2021, 11, 2489–2502. [Google Scholar] [CrossRef]
- Uddin, S.; Safdar, L.B.; Iqbal, J.; Yaseen, T.; Laila, S.; Anwar, S.; Abbasi, B.A.; Saif, M.S.; Quraishi, U.M. Green synthesis of nickel oxide nanoparticles using leaf extract of Berberis balochistanica: Characterization, and diverse biological applications. Microsc. Res. Tech. 2021, 84, 2004–2016. [Google Scholar] [CrossRef]
- Chouhan, C.; Rajput, R.P.S.; Sahu, R.; Verma, P.; Sahu, S. An updated review on nanoparticle based approach for nanogel drug delivery system. J. Drug Deliv. Ther. 2020, 10, 254–266. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Uddin, S.; Safdar, L.B.; Anwar, S.; Iqbal, J.; Laila, S.; Abbasi, B.A.; Saif, M.S.; Ali, M.; Rehman, A.; Basit, A. Green synthesis of nickel oxide nanoparticles from Berberis balochistanica stem for investigating bioactivities. Molecules 2021, 26, 1548. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Folliero, V.; Chianese, A.; Zannella, C.; De Filippis, A.; Rosati, L.; Prisco, M.; Falanga, A.; Mali, A.; Galdiero, M. Synthesis of chitosan-coated silver nanoparticle bioconjugates and their antimicrobial activity against multidrug-resistant bacteria. Appl. Sci. 2021, 11, 9340. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Alsahli, A.A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Chang, S.-J.; Hsueh, T.-J.; Chen, I.-C.; Huang, B.-R. Highly sensitive ZnO nanowire CO sensors with the adsorption of Au nanoparticles. Nanotechnology 2008, 19, 175502. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ali, A.; Ullah, Z.; Sher, H.; Dai, D.-Q.; Ali, M.; Iqbal, J.; Zahoor, M.; Ali, I. Biosynthesized silver nanoparticles using Polygonatum geminiflorum efficiently control fusarium wilt disease of tomato. Front. Bioeng. Biotechnol. 2022, 10, 1679. [Google Scholar] [CrossRef]
- Lv, B.; Xu, Y.; Wu, D.; Sun, Y.J. Preparation of α-Fe2O3 nanodisks by blocking the growth of (001) plane. J. Mater. Sci. Technol. 2009, 25, 155. [Google Scholar]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Ni, Y.; Ge, X.; Zhang, Z.; Ye, Q. Fabrication and characterization of the plate-shaped γ-Fe2O3 nanocrystals. Chem. Mater. 2002, 14, 1048–1052. [Google Scholar] [CrossRef]
- Pereira, A.L.; Vasconcelos, V. Classification and phylogeny of the cyanobiont Anabaena azollae Strasburger: An answered question? Int. J. Syst. Evol. Microbiol. 2014, 64, 1830–1840. [Google Scholar] [CrossRef] [Green Version]
- Rico, M.; González, A.G.; Santana-Casiano, M.; González-Dávila, M.; Pérez-Almeida, N.; de Tangil, M.S. Production of primary and secondary metabolites using algae. In Prospects and Challenges in Algal Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 311–326. [Google Scholar]
- Ibrahim, H.; Reda, M.M.; Klingner, A. Preparation and characterization of green carboxymethylchitosan (CMCS)–Polyvinyl alcohol (PVA) electrospun nanofibers containing gold nanoparticles (AuNPs) and its potential use as biomaterials. Int. J. Biol. Macromol. 2020, 151, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Shahbaz, A.; Zahra, S.A.; Kanwal, S.; Munir, A.; Rabbani, A.; Mahmood, T. Biogenic synthesis of green and cost effective iron nanoparticles and evaluation of their potential biomedical properties. J. Mol. Struct. 2020, 1199, 126979. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M.; Alharbi, R.M.; Alkhulaifi, M.M. Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J. Biol. Sci. 2019, 26, 1207–1215. [Google Scholar] [CrossRef]
- Thema, F.; Manikandan, E.; Dhlamini, M.; Maaza, M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Hameed, S.; Munir, A.; Kanwal, S. Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl. Organomet. Chem. 2019, 33, e4950. [Google Scholar] [CrossRef]
- Salem, D.M.; Ismail, M.M.; Tadros, H.R. Evaluation of the antibiofilm activity of three seaweed species and their biosynthesized iron oxide nanoparticles (Fe3O4-NPs). Egypt. J. Aquat. Res. 2020, 46, 333–339. [Google Scholar] [CrossRef]
- Sarkar, R.; Kumbhakar, P.; Mitra, A. Green synthesis of silver nanoparticles and its optical properties. Dig. J. Nanomater. Biostructures 2010, 5, 491–496. [Google Scholar]
- Al-Radadi, N.S.; Hussain, T.; Faisal, S.; Shah, S.A.R. Novel biosynthesis, characterization and bio-catalytic potential of green algae (Spirogyra hyalina) mediated silver nanomaterials. Saudi J. Biol. Sci. 2022, 29, 411–419. [Google Scholar]
- Mahanty, S.; Bakshi, M.; Ghosh, S.; Chatterjee, S.; Bhattacharyya, S.; Das, P.; Das, S.; Chaudhuri, P. Green synthesis of iron oxide nanoparticles mediated by filamentous fungi isolated from Sundarban mangrove ecosystem, India. Bionanoscience 2019, 9, 637–651. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.; Ahmed, M. Silver and copper oxide nanoparticles-decorated graphene oxide via pulsed laser ablation technique: Preparation, characterization, and photoactivated antibacterial activity. Nano-Struct. Nano-Objects 2020, 22, 100464. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y. Reduced neutralization of SARS-CoV-2 B. 1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.e4213. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wu, Y.; Lu, B.; Zhu, G.; Gong, T.; Wang, R.; Peng, Q.; Li, Y. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) biofilm by cationic poly (D, L-lactide-co-glycolide) nanoparticles. J. Bioadhesion Biofilm Res. 2020, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Ali, M.; Zohra, T.; Salman, M.; Ikram, A.; Shinwari, Z.K.; Maaza, M. Bio-redox potential of Hyphaene thebaica in bio-fabrication of ultrafine maghemite phase iron oxide nanoparticles (Fe2O3 NPs) for therapeutic applications. Mater. Sci. Eng. C 2020, 112, 110890. [Google Scholar] [CrossRef]
- Antony, V.S.; Silky, V.; Raji, P.; SaiPriya, C.; Selvarani, J.A. Bioactivity Studies of Datura metel, Aegle marmelos, Annona reficulata and Saraca indica and their Green Synthesized Silver Nanoparticle. J. Pure Appl. Microbiol. 2019, 13, 329–338. [Google Scholar]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Maaza, M. Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 2017, 10, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raguvaran, R.; Manuja, A.; Manuja, B.K. Zinc oxide nanoparticles: Opportunities and challenges in veterinary sciences. Immunome Res. 2015, 11, 1. [Google Scholar]
- Sankarganesh, P.; Ganesh Kumar, A.; Parthasarathy, V.; Joseph, B.; Priyadharsini, G.; Anbarasan, R.J. Synthesis of Murraya koenigii Mediated Silver Nanoparticles and Their In Vitro and In Vivo Biological Potential. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2971–2979. [Google Scholar] [CrossRef]
- Ullah, M.O.; Haque, M.; Urmi, K.F.; Zulfiker, A.H.M.; Anita, E.S.; Begum, M.; Hamid, K. Anti–bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac. J. Trop. Biomed. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Selvi, S.S.; Praba, A.; Selvaraj, M.; Rani, L.M.; Suganthi, P.; Devi, B.S.; Govindaraju, M. Antibacterial activity and in-vitro cytotoxicity assay against brine shrimp using silver nanoparticles synthesized from Sargassum ilicifolium. Dig. J. Nanomater. Biostructures 2012, 7, 1447–1455. [Google Scholar]
- Cohen, Y.; Padan, E.; Shilo, M.J. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. J. Bacteriol. 1975, 123, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Hassan, D.; Khalil, A.T.; Saleem, J.; Diallo, A.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): Their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 693–707. [Google Scholar] [CrossRef] [Green Version]
- Kiani, B.H.; Haq, I.-U.; Alhodaib, A.; Basheer, S.; Fatima, H.; Naz, I.; Ur-Rehman, T. Comparative Evaluation of Biomedical Applications of Zinc Nanoparticles Synthesized by Using Withania somnifera Plant Extracts. Plants 2022, 11, 1525. [Google Scholar] [CrossRef]
- Ali, M.; Haroon, U.; Khizar, M.; Chaudhary, H.J.; Munis, M.F.H. Scanning electron microscopy of bio-fabricated Fe2O3 nanoparticles and their application to control brown rot of citrus. Microsc. Res. Tech. 2021, 84, 101–110. [Google Scholar] [CrossRef]
- Ali, M.; Haroon, U.; Khizar, M.; Chaudhary, H.J.; Munis, M.F.H. Facile single step preparations of phyto-nanoparticles of iron in Calotropis procera leaf extract to evaluate their antifungal potential against Alternaria alternata. Curr. Plant Biol. 2020, 23, 100157. [Google Scholar] [CrossRef]
- Hassan, D.; Khalil, A.T.; Solangi, A.R.; El-Mallul, A.; Shinwari, Z.K.; Maaza, M. Physiochemical properties and novel biological applications of Callistemon viminalis-mediated α-Cr2O3 nanoparticles. Appl. Organometal. Chem. 2019, 33, e5041. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Compound | Concentrations | Zone of Inhibition (ZOI) and (% Inhibition) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | ||||||||
| S. aureus | B. subtilis | E. coli | P. aeruginosa | ||||||
| ZOI (mm) | (% Inhibition) | ZOI (mm) | (% Inhibition) | ZOI (mm) | (% Inhibition) | ZOI (mm) | (% Inhibition) | ||
| IONPs | 25 µg/mL | 3 ± 0.03 | 20 ± 0.03 | 2.5 ± 0.03 | 14.4 ± 0.03 | 2 ± 0.03 | 35 ± 0.03 | 1.5 ± 0.03 | 10.7 ± 0.03 |
| 50 µg/mL | 4 ± 0.03 | 30 ± 0.03 | 2.7 ± 0.03 | 31.5 ± 0.03 | 2.5 ± 0.03 | 45 ± 0.03 | 2.3 ± 0.03 | 20.7 ± 0.03 | |
| 75 µg/mL | 4.5 ± 0.03 | 35 ± 0.03 | 2.9 ± 0.03 | 34.5 ± 0.03 | 2.8 ± 0.03 | 51 ± 0.03 | 2.5 ± 0.03 | 23.2 ± 0.03 | |
| 100 µg/mL | 5 ± 0.03 | 40 ± 0.03 | 3.5 ± 0.03 | 43 ± 0.03 | 3 ± 0.03 | 55 ± 0.03 | 2.7 ± 0.03 | 25.7 ± 0.03 | |
| 125 µg/mL | 6 ± 0.03 | 50 ± 0.03 | 3.7 ± 0.03 | 45.8 ± 0.03 | 3.7 ± 0.03 | 69 ± 0.03 | 3 ± 0.03 | 29.5 ± 0.03 | |
| 150 µg/mL | 8 ± 0.03 | 70 ± 0.03 | 5 ± 0.03 | 64.4 ± 0.03 | 4 ± 0.03 | 75 ± 0.03 | 4 ± 0.03 | 42 ± 0.03 | |
| Ampicillin | 5 mg/mL | 10 ± 0.05 | 100 ± 0.05 | 7 ± 0.05 | 100 ± 0.05 | 5 ± 0.05 | 100 ± 0.05 | 8 ± 0.05 | 100 ± 0.05 |
| Minimal inhibitory concentrations (MIC) | |||||||||
| MIC (µg/mL) | MIC (µg/mL) | MIC (µg/mL) | MIC (µg/mL) | ||||||
| IONPs | 20 ± 0.03 | 14.4 ± 0.03 | 35 ± 0.03 | 10.7 ± 0.03 | |||||
| Compound | Concentrations | Zone of Inhibition (ZOI) and (% Inhibition) | |||
|---|---|---|---|---|---|
| Fungal Strains | |||||
| Rhizopus microsporus | Aspergillus versicolor | ||||
| ZOI (mm) and (% Inhibition) | ZOI (mm) and (% Inhibition) | ||||
| IONPs | 50 µg/mL | 75 ± 0.03 | 25 ± 0.03 | 60 ± 0.03 | 40 ± 0.03 |
| 100 µg/mL | 65 ± 0.03 | 35 ± 0.03 | 53 ± 0.03 | 47 ± 0.03 | |
| 150 µg/mL | 60 ± 0.03 | 40 ± 0.03 | 39 ± 0.03 | 61 ± 0.03 | |
| 200 µg/mL | 53 ± 0.03 | 47 ± 0.03 | 27 ± 0.03 | 73 ± 0.03 | |
| (Fluconazole) | 5 mg/mL | 15 ± 0.05 | 100 ± 0 | 12 ± 0.05 | 100 ± 0 |
| Minimal inhibitory concentrations (MIC) | |||||
| MIC (µg/mL) | MIC (µg/mL) | ||||
| IONPs | 53 ± 0.03 | 27 ± 0.03 | |||
| Concentration of NPs | % FRSA | IC50 |
|---|---|---|
| 25 µg/mL | 24 ± 0.003 | 1.32 |
| 50 µg/mL | 37 ± 0.002 | 3.9 |
| 75 µg/mL | 41.8 ± 0.03 | 6.4 |
| 100 µg/mL | 51 ± 0.03 | 9.02 |
| 125 µg/mL | 59 ± 0 | 11.6 |
| 150 µg/mL | 73 ± 0.03 | 14.2 |
| Concentrations of NPs | % Hemolysis | Triton X-100 | IC50 |
|---|---|---|---|
| 50 µg/mL | 7.1 ± 0.003 | 0.200 ± 0.40 | 49.5 |
| 100 µg/mL | 11.3 ± 0.005 | 0.62 ± 0.90 | 99.5 |
| 150 µg/mL | 14.8 ± 0.006 | 0.79 ± 0.01 | 149.5 |
| 200 µg/mL | 21.5 ± 0.003 | 0.180 ± 0.20 | 199.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haris, M.; Fatima, N.; Iqbal, J.; Chalgham, W.; Mumtaz, A.S.; El-Sheikh, M.A.; Tavafoghi, M. Oscillatoria limnetica Mediated Green Synthesis of Iron Oxide (Fe2O3) Nanoparticles and Their Diverse In Vitro Bioactivities. Molecules 2023, 28, 2091. https://doi.org/10.3390/molecules28052091
Haris M, Fatima N, Iqbal J, Chalgham W, Mumtaz AS, El-Sheikh MA, Tavafoghi M. Oscillatoria limnetica Mediated Green Synthesis of Iron Oxide (Fe2O3) Nanoparticles and Their Diverse In Vitro Bioactivities. Molecules. 2023; 28(5):2091. https://doi.org/10.3390/molecules28052091
Chicago/Turabian StyleHaris, Muhammad, Namra Fatima, Javed Iqbal, Wadie Chalgham, Abdul Samad Mumtaz, Mohamed A. El-Sheikh, and Maryam Tavafoghi. 2023. "Oscillatoria limnetica Mediated Green Synthesis of Iron Oxide (Fe2O3) Nanoparticles and Their Diverse In Vitro Bioactivities" Molecules 28, no. 5: 2091. https://doi.org/10.3390/molecules28052091





