Elucidating Flavonoid and Antioxidant Activity in Edible and Medicinal Herbs Woodwardia japonica (L.f.) Sm. Based on HPLC-ESI-TOF-MS and Artificial Neural Network Model: Response to Climatic Factors
Abstract
:1. Introduction
2. Results and Discussion
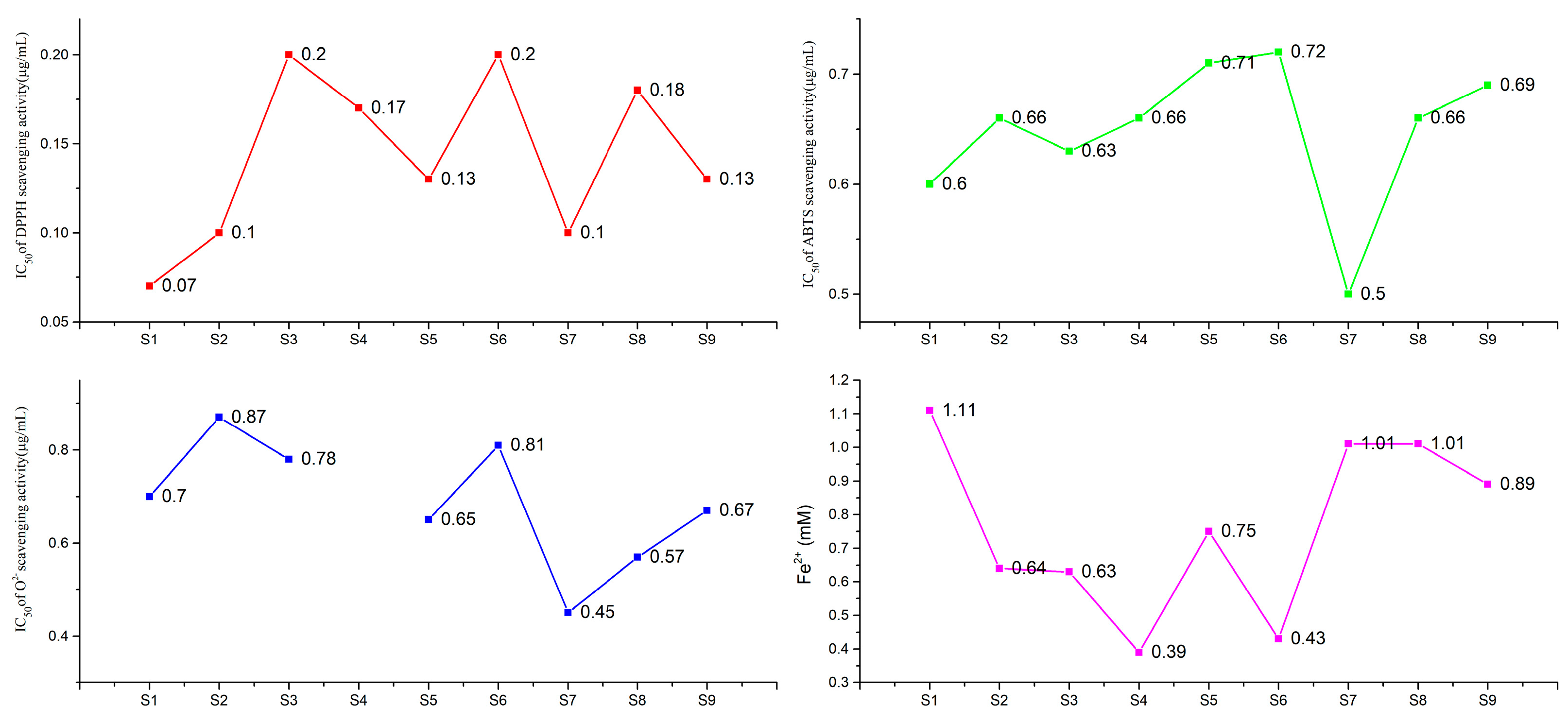
2.1. TFC and Antioxidant Activities of W. japonica from Nine Main Production Areas
2.2. Flavonoids of W. japonica from Different Districts
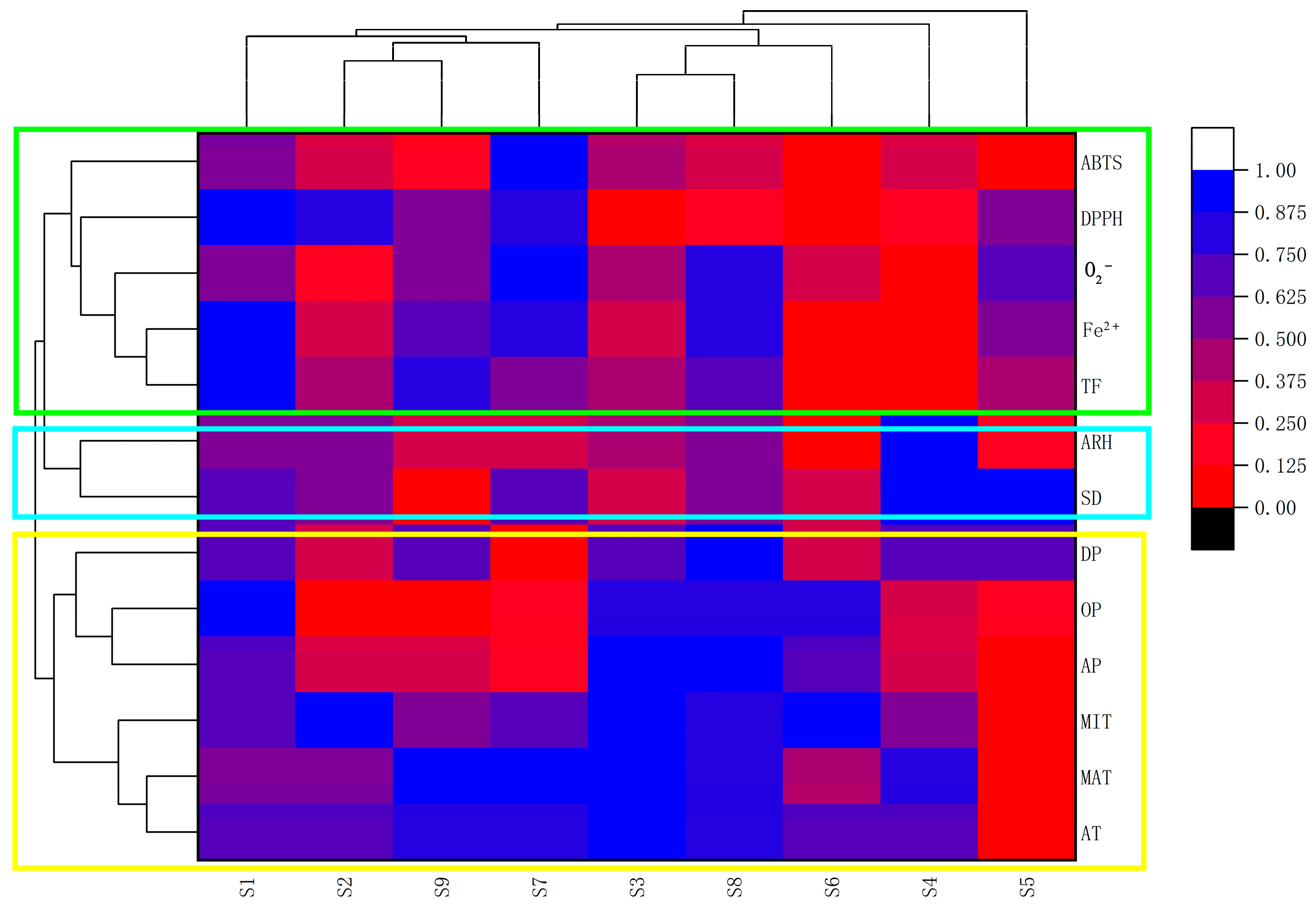
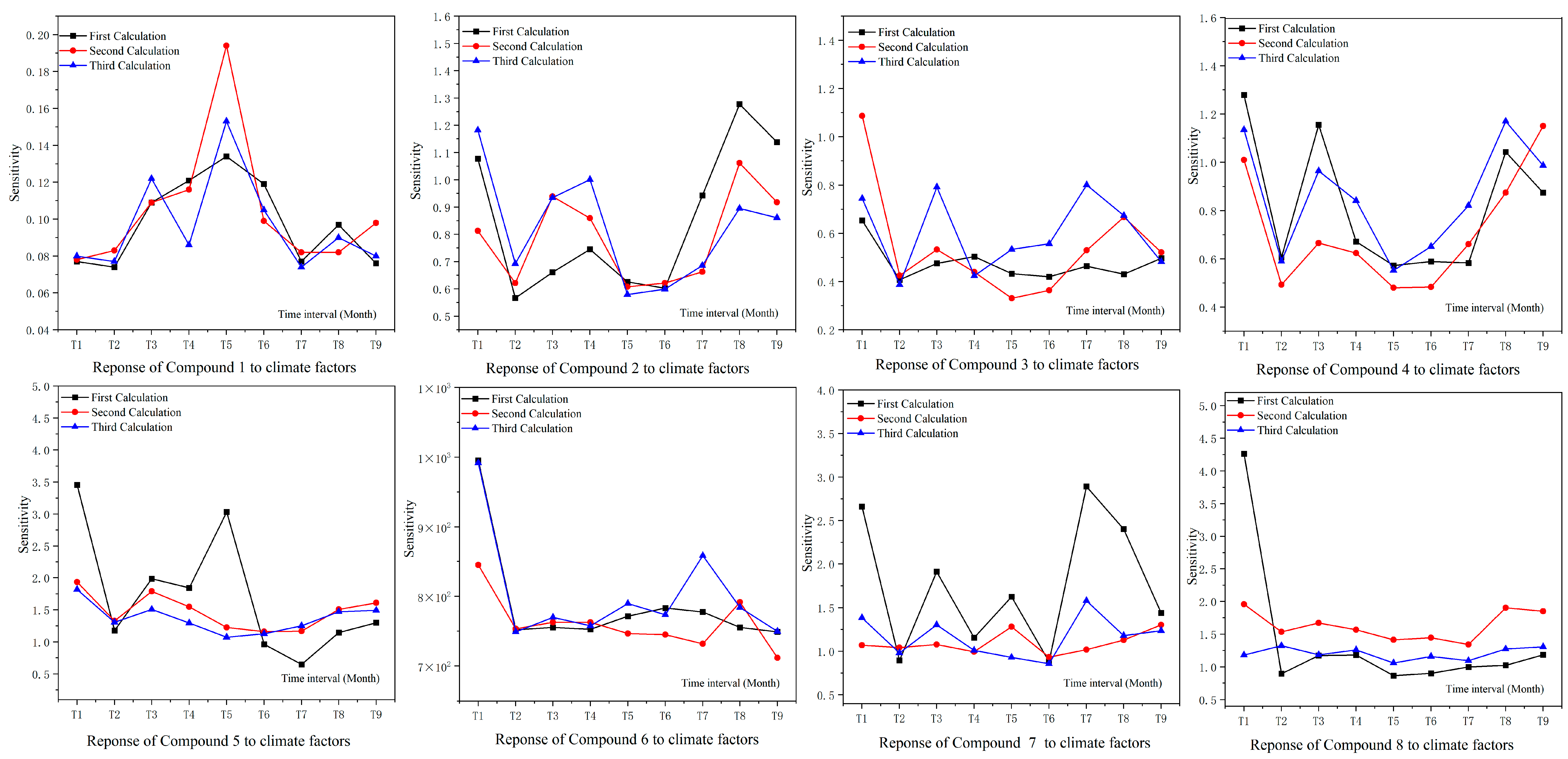
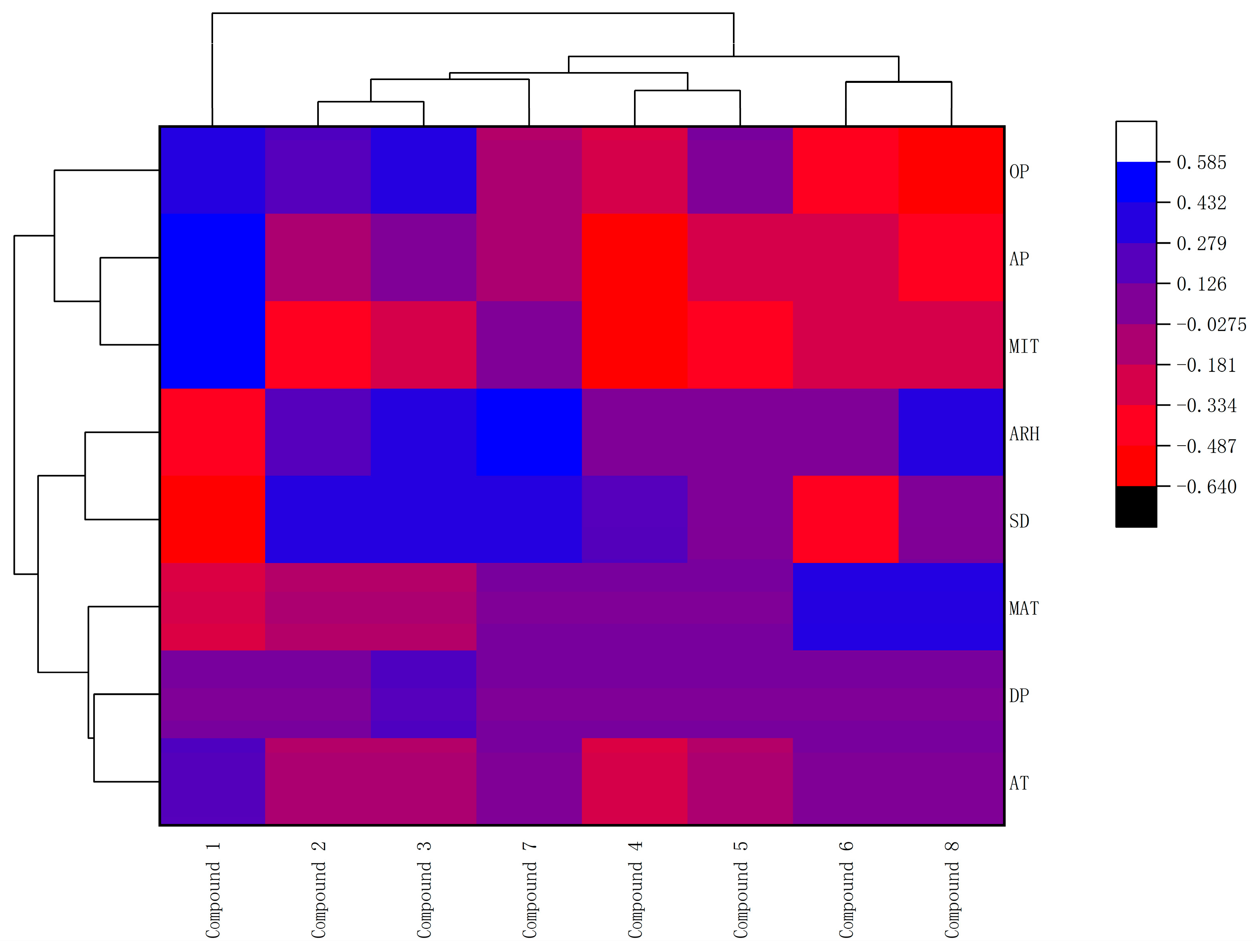
2.3. Evaluating for the Effects of Climate Factors on Flavonoids and Antioxidant Activities by the Method of ANN, the Correlation Coefficient Matrix and Hierarchical Cluster Analysis (HCA)
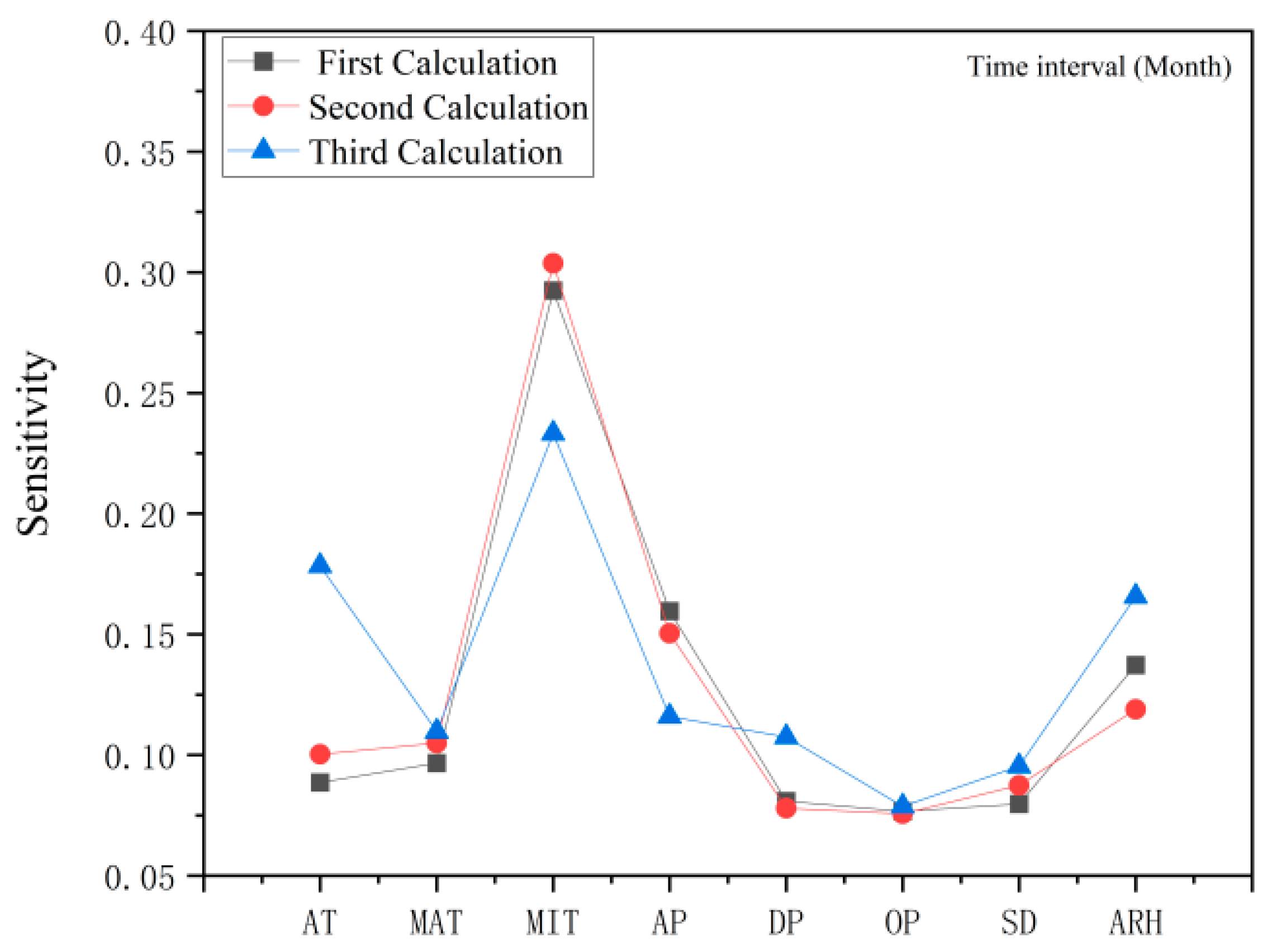
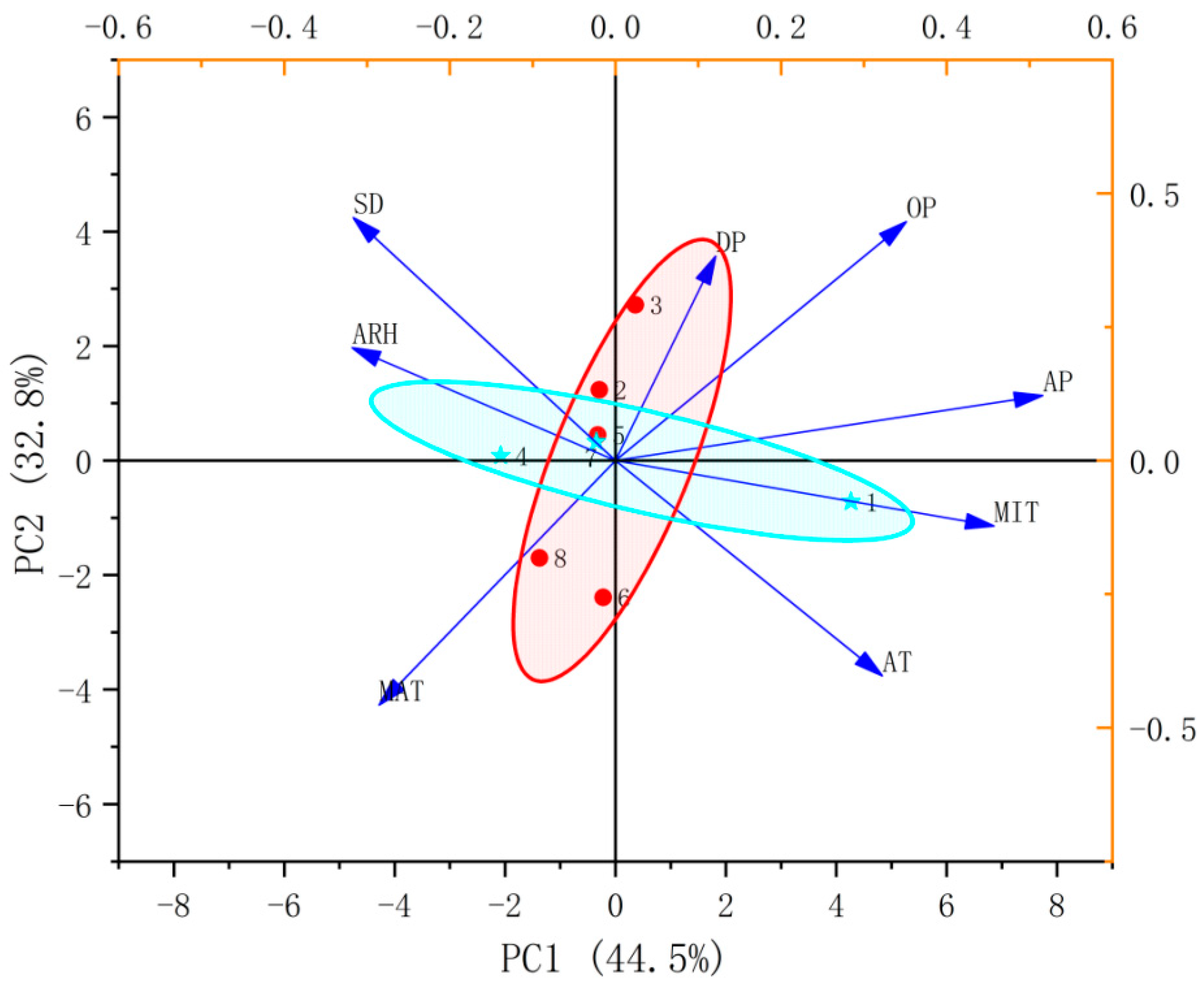
2.4. Response of Flavonoid Type of W. japonica to Climatic Factors with ANN, Pearson Correlation Coefficient, PCA and HCA
3. Materials and Methods
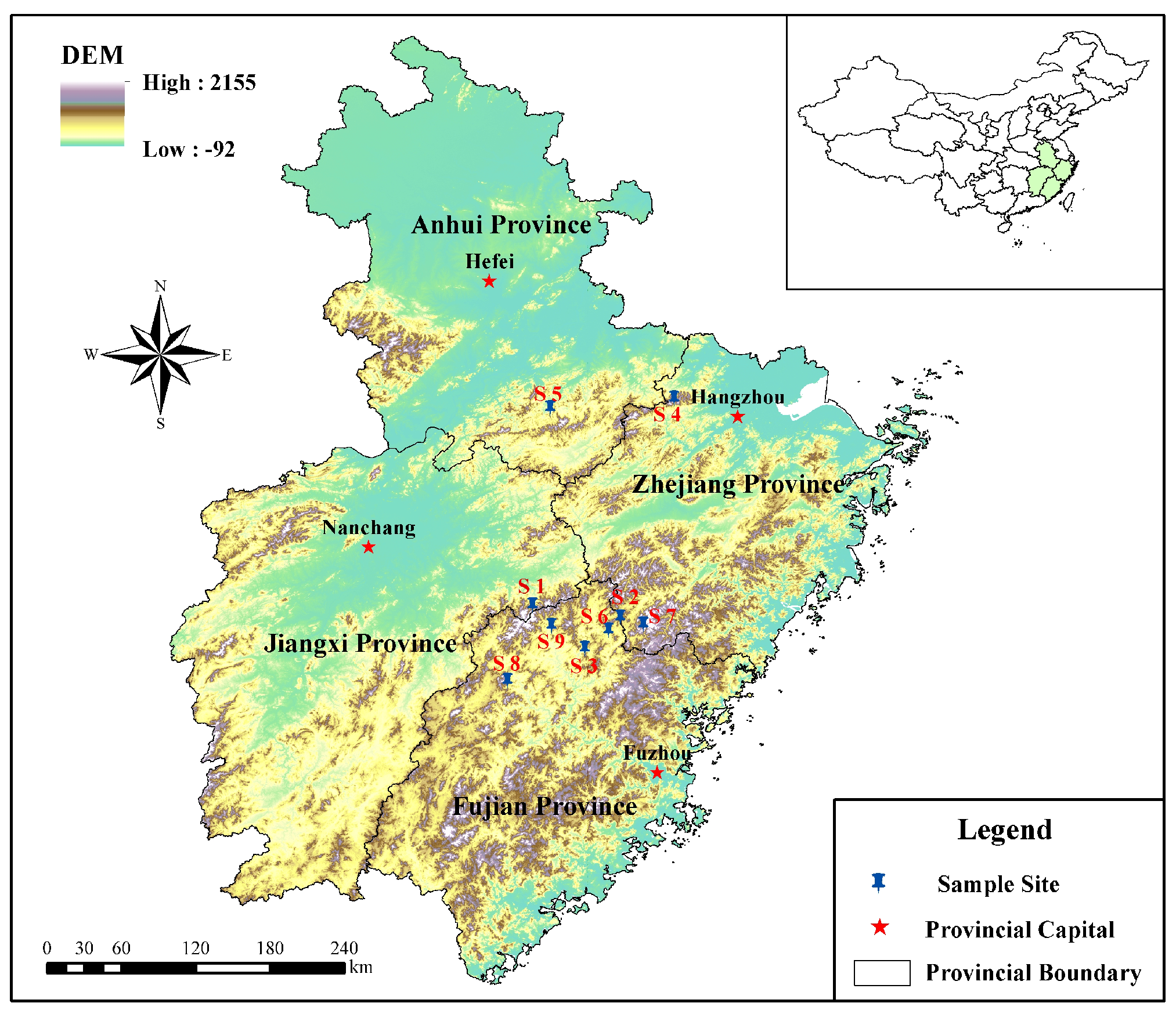
3.1. Plant Materials
3.2. Chemicals
3.3. Preparation of Plant Extracts
3.4. Determination of TFCs
3.5. Antioxidant Activity
3.5.1. DPPH· Scavenging Assay
3.5.2. ABTS· Scavenging Assay
3.5.3. Superoxide Anion (O2−) Scavenging Assay
3.5.4. Reducing Force on Fe3+ Assay
3.6. HPLC-ESI-TOF-MS Analysis
3.7. Model Construction
3.7.1. Sample Organization
3.7.2. The Selection of Parameter
3.7.3. Sensitivity Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chettri, S. Nutrient and Elemental Composition of Wild Edible Ferns of the Himalaya. Am. Fern. J. 2018, 108, 95–106. [Google Scholar] [CrossRef]
- Nekrasov, E.V.; Svetashev, V.I. Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids. Foods 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B. Dietary flavonoid aglycones and their glycosides: What show better biological benefits? Crit. Rev. Food Sci. 2017, 57, 1874–1905. [Google Scholar]
- Liu, Y.; Wujisguleng, W.; Long, C. Food uses of ferns in China: A review. Acta Soc. Bot. Pol. 2012, 81, 263–270. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.L.; Cao, J.G.; Wu, Y.H.; Xiao, J.B.; Wang, Q.X. Analysis of flavonoids and antioxidants in extracts of ferns from Tianmu Mountain in Zhejiang Province (China). Ind. Crop. Prod. 2017, 97, 137–145. [Google Scholar] [CrossRef]
- Cao, H.; Chai, T.T.; Wang, X.; Morais-Braga, M.F.B.; Yang, J.H.; Wong, F.C.; Wang, R.B.; Yao, H.K.; Cao, J.G.; Cornara, L.; et al. Phytochemicals from fern species: Potential for medicine applications. Phytochem. Rev. 2017, 16, 379–440. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.G.; Xia, X.; Chen, X.F.; Xiao, J.B.; Wang, Q.X. Characterization of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food Chem. Toxicol. 2013, 51, 242–250. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.Y.; Chen, L.; Zhang, Y.B.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J.B. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Andrae-Marobela, K.; Ghislain, F.W.; Okatch, H.; Majinda, R.R. Polyphenols: A diverse class of multi-target anti-HIV-1 agents. Curr. Drug Metab. 2013, 14, 392–413. [Google Scholar] [CrossRef]
- Deng, B.; Cao, Y.N.; Fang, S.Z.; Shang, X.L.; Yang, W.X.; Qian, C.Y. Variation and stability of growth and leaf favonoid content in Cyclocarya paliurus across environments. Ind. Crop. Prod. 2015, 76, 386–393. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.B.; Cao, F.L.; Zhu, C.C.; Wang, G.Y.; EI-Kassaby, Y.A. Light intensity affects the growth and flavonol biosynthesis of Ginkgo (Ginkgo biloba L.). New Forest 2014, 45, 765–776. [Google Scholar] [CrossRef]
- Herrera, J.C.; Hochberg, U.; Degu, A.; Sabbatini, P.; Lazarovitch, N.; Castellarin, S.D.; Fait, A.; Alberti, G.; Peterlunger, E. Grape metabolic response to postveraison water deficit is affected by interseason weather variability. J. Agric. Food Chem. 2017, 65, 5868–5878. [Google Scholar] [CrossRef]
- Zheng, T.; Sun, J.Q.; Shi, X.J.; Liu, D.L.; Sun, B.Y.; Deng, Y.J.; Zhang, D.L.; Liu, S.M. Evaluation of climate factors affecting the quality of red huajiao (Zanthoxylum bungeanum maxim.) based on UPLC-MS/MS and MaxEnt model. Food Chem. X 2022, 16, 100522. [Google Scholar] [CrossRef]
- Zhou, C.C.; Yin, G.F.; Hu, X.B. Multi-objective optimization of material selection for sustainable products: Artificial neural networks and genetic algorithm approach. Mater. Des. 2009, 30, 1209–1215. [Google Scholar] [CrossRef]
- Samaraweera, M.A.; Hall, L.M.; Hill, D.W.; Grant, D.F. Evaluation of an artificial neural network retention index model for chemical structure identification in nontargeted metabolomics. Anal. Chem. 2018, 90, 12752–12760. [Google Scholar] [CrossRef] [PubMed]
- Jahirul, M.I.; Rasul, M.G.; Brown, R.J. Investigation of correlation between chemical composition and properties of biodiesel using principal component analysis (PCA) and artificial neural network (ANN). Renew. Energ. 2021, 168, 632–646. [Google Scholar] [CrossRef]
- Xia, X.; Cao, J.G.; Zheng, Y.X.; Wang, Q.X.; Xiao, J.B. Flavonoid concentrations and bioactivity of flavonoid extracts from 19 species of ferns from China. Ind. Crop. Prod. 2014, 58, 91–98. [Google Scholar] [CrossRef]
- Takuli, P.; Khulbe, K.; Kumar, P.; Parki, A.; Syed, A.; Elgorban, A.M. Phytochemical profiling, antioxidant and antibacterial efficacy of a native Himalayan Fern: Woodwardia unigemmata (Makino) Nakai. Saudi J. Biol. Sci. 2020, 27, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.T.; Fang, Y.S.; Tai, Z.G.; Yang, M.H.; Xu, Y.Q.; Li, F.; Cao, Q.E. Phenolic content and radical scavenging capacity of 31 species of ferns. Fitoterapia 2008, 79, 581–583. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–4. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Bafor, E.E.; Eze, C.; Omoruyi, O.; Elvis-Offiah, B.U.; Viegelmann, C.; Edrada-Ebel, R.A. Green tea inhibits uterine contractility in ex vivo (non-pregnant) mice models. Trop. J. Nat. Prod. Res. 2018, 2, 254–261. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Parat, M.O.; Hodson, M.P.; Pan, J.; Shaw, P.N.; Hewavitharana, A.K. Chemical characterization and in vitro cytotoxicity on squamous cell carcinoma cells of Carica papaya leaf extracts. Toxins 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Sarais, G.; Lai, C.; Pizza, C.; Montoro, P. LC-MS based metabolomics study of different parts of myrtle berry from Sardinia (Italy). J. Berry Res. 2017, 7, 217–229. [Google Scholar] [CrossRef]
- Console, L.; Giangregorio, N.; Cellamare, S.; Bolognino, I.; Palasciano, M.; Indiveri, C.; Incampo, G.; Campana, S.; Tonazzi, A. Human mitochondrial carnitine acylcarnitine carrier: Molecular target of dietary bioactive polyphenols from sweet cherry (Prunus avium L.). Chem. Biol. Interact. 2019, 307, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman; Rasheed, H.M.; Bashir, K.; Gurgul, A.; Wahid, F.; Che, C.T.; Shahzadi, I.; Khan, T.; Rasheed, H. UHPLC-MS/MS-GNPS based phytochemical investigation of Dryopteris ramosa (Hope) C. Chr. and evaluation of cytotoxicity against liver and prostate cancer cell lines. Heliyon 2022, 8, e11286. [Google Scholar] [CrossRef] [PubMed]
- Maia, I.R.D.O.; Trevisan, M.T.S.; Silva, M.G.D.V.; Klika, K.D.; Brito, E.D.; Silva, L.M.A.E.; Pinto, F.D.C.L.; Breuer, A.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in Senna splendida from the northeast of Brazil. Nat. Prod. Commun. 2018, 13, 1934578X1801300614. [Google Scholar]
- Hu, J.F.; Wunderlich, D.; Sattler, I.; Thiericke, R.; Grabley, S.; Feng, X.Z. New 2-O-methylrhamno-isoflavones from Streptomyces sp. Nat. Prod. Res. 2003, 17, 451–458. [Google Scholar] [CrossRef]
- Ma, W.Y.; Ma, L.P.; Yi, B.; Zhang, M.; Feng, S.X.; Tian, L.P. Antidiabetic activity of Callicarpa nudiflora extract in type 2 diabetic rats via activation of the AMPK-ACC pathway. Asian Pac. J. Trop. Bio. 2019, 9, 456. [Google Scholar]
- Caldwell, C.R.; Britz, S.J.; Mirecki, R.M. Effect of temperature, elevated carbon dioxide, and drought during seed development on the isoflavone content of dwarf soybean [Glycine max (L.) Merrill] grown in controlled environments. J. Agric. Food Chem. 2005, 53, 1125–1129. [Google Scholar] [CrossRef]
- Neugebauer, A.; Schieberle, P.; Granvogl, M. Characterization of the key odorants causing the musty and fusty/muddy sediment off-flavors in olive oils. J. Agric. Food Chem. 2021, 69, 14878–14892. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Berger, J.M.; Itagaki, Y.; Nakanishi, K. The effect of ultraviolet-depleted light on the flavonol contents of the cactus species Opuntia wilcoxii and Opuntia violacea. Chem. Biodivers 2007, 4, 1525–1532. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Bhatia, C.; Pandey, A.; Gaddam, S.R.; Hoecker, U.; Trivedi, P.K. Low temperature- enhanced flavonol synthesis requires light-associated regulatory components in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 2099–2112. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.F.; Song, C.Z.; Falginella, L.G.; Castellarin, S.D. Day temperature has a stronger effect than night temperature on anthocyanin and flavonol accumulation in ‘Merlot’ (Vitis vinifera L.) grapes during ripening. Front. Plant Sci. 2020, 11, 1095. [Google Scholar] [CrossRef]
- Pastore, C.; Allegro, G.; Valentini, G.; Muzzi, E.; Filippetti, I. Anthocyanin and flavonol composition response to veraison leaf removal on Cabernet Sauvignon, Nero d’Avola, Raboso Piave and Sangiovese Vitis vinifera L. cultivars. Sci. Hortic-Amst. 2017, 218, 147–150. [Google Scholar] [CrossRef]
- Yang, B.; Zheng, J.; Laaksonen, O.; Tahvonen, R.; Kallio, H. Effects of latitude and weather conditions on phenolic compounds in currant (Ribes spp.) cultivars. J. Agric. Food Chem. 2013, 61, 3517–3532. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N. The influence of organic and conventional crop management, variety and year on the yield and flavonoid level in common buckwheat groats. Food Chem. 2011, 127, 602–608. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Camilo, C.J.; Nonato, C.F.A.; Rodrigues, F.F.G.; Menezes, I.R.A.; Ribeiro-Filho, J.; Xiao, J.B.; Souza, M.M.A. Influence of seasonal variation on phenolic content and in vitro antioxidant activity of Secondatia floribunda A. DC. (Apocynaceae). Food Chem. 2020, 315, 126277. [Google Scholar] [CrossRef]
- Dong, J.E.; Ma, X.H.; Wei, Q.; Peng, S.B.; Zhang, S.C. Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of Eucommia ulmoides. Ind. Crop. Prod. 2011, 34, 1607–1614. [Google Scholar] [CrossRef]
- Wang, X.; Cao, J.G.; Wu, Y.H.; Wang, Q.X.; Xiao, J.B. Flavonoids, antioxidant potential, and acetylcholinesterase inhibition activity of the extracts from the gametophyte and archegoniophore of Marchantia polymorpha L. Molecules 2016, 21, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ding, G.H.; Liu, B.D.; Wang, Q.X. Flavonoids and antioxidant activity of rare and endangered fern: Isoetes sinensis. PLoS ONE 2020, 15, e0232185. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, H.Y.; Li, F.P.; Li, X.B.; Du, X.Q.; Ye, X.Y. Identification of key influence factors and an empirical formula for spring snowmelt-runoff: A case study in mid-temperate zone of northeast China. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]

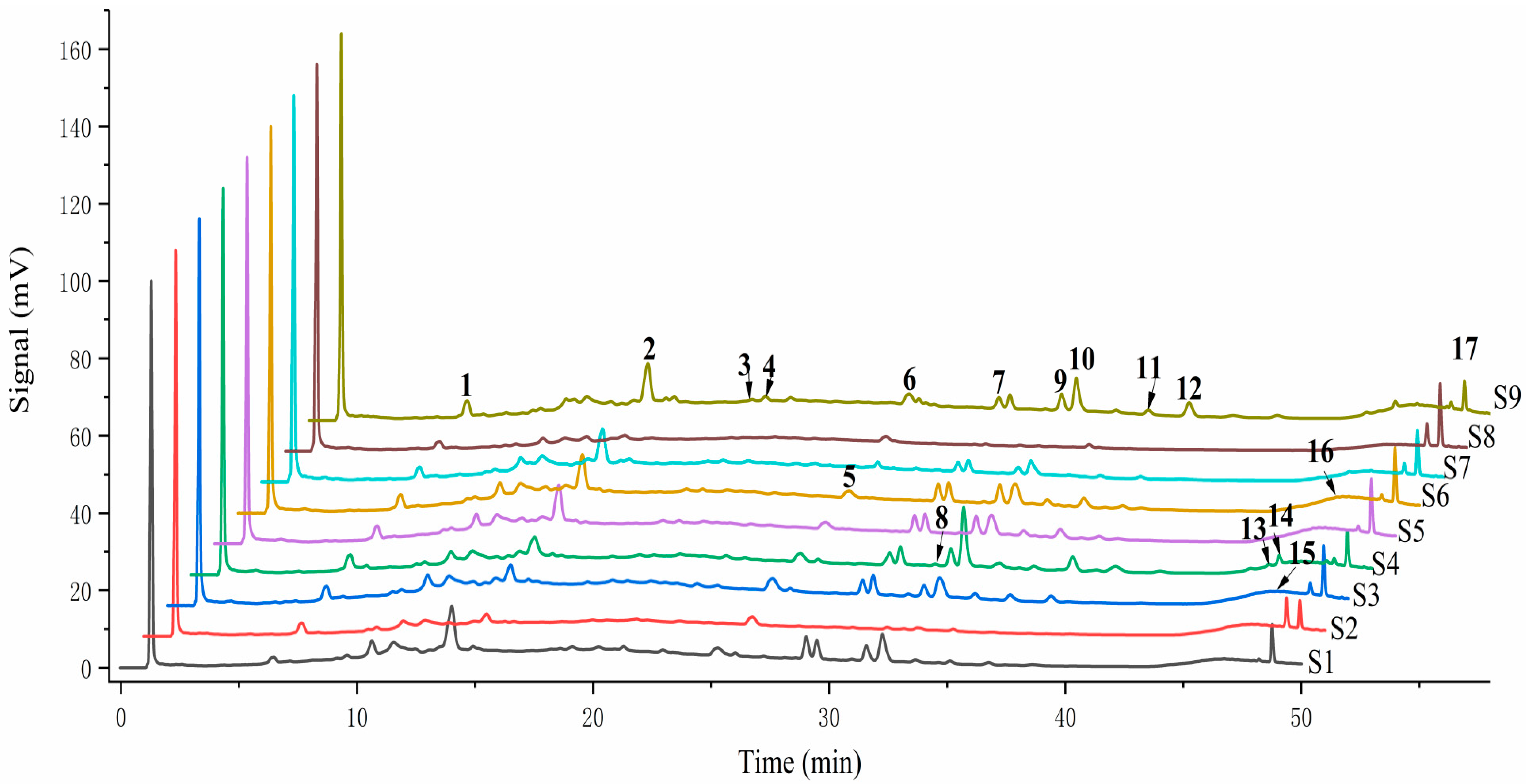



| No. | RT(Min) | Compound Formula | Molar Mass | Experimental m/z | Error | UV (nm) λmax | Identification | |
|---|---|---|---|---|---|---|---|---|
| (ppm) | (mDa) | |||||||
| 1 | 6.74 | C7H6O4 | 154.0269 | [M-H]−153.0193, [M+H]+155.0394 | −1.59 | −0.24 | 280 | Protocatechuate |
| 2 | 14.76 | C16H18O9 | 354.0954 | [M-H]−353.0877, [2M-H]−707.1832 [M+H]+355.1027 | −0.79 | −0.28 | 285,325 | Chlorogenic acid |
| 3 | 19.45 | C32H36O16 | 676.1999 | [M-H]−675.1931, [M-C7H12O2]−547.1466 [M-C7H12O2-C10H10O5]−338.0996 | 0.69 | 0.47 | 283,313 | Unknown |
| 4 | 21.50 | C16H16O8 | 336.0845 | [M-H]−335.0772 | 0.17 | 0.06 | 230,280 | Unknown |
| 5 | 25.04 | C18H26O8 | 370.1626 | [M-H]−369.1555 | 0.5 | 0.19 | 230,285 | Unknown |
| 6 | 25.87 | C21H20O12 | 464.0954 | [M-H]−463.0882, [M+H]+465.1026 [2M-H]−927.1837 | −0.22 | −0.1 | 283 | Isotrifolin (flavonol) |
| 7 | 29.58 | C27H30O16 | 610.1527 | [M-H]−609.1461, [M+H]+611.1621 | 1.16 | 0.71 | 265,285,350 | Rutin (flavonol) |
| 8 | 31.17 | C33H48O16 | 700.2945 | [M-H]−699.287 | −0.42 | −0.29 | 230,280 | Unknown |
| 9 | 31.7 | C21H20O12 | 464.0954 | [M-H]−463.0882, [2M-H]−827.1829 [M+H]+465.1041 | 0.08 | 0.04 | 270,280,355 | Myricetin deoxyhexoside (flavonol) |
| 10 | 32.35 | C27H30O16 | 610.1533 | [M-H]−609.1461, [M+H]+611.1613 | 0.15 | 0.09 | 270,285,335 | Quercetin-3-rutinoside (flavonol) |
| 11 | 35.28 | C21H20O11 | 448.1011 | [M-H]−447.0933, [M+H]+449.1095 [2M-H]−895.1918 | −1.26 | −0.57 | 270,280,340 | Luteolin 6-C-glucoside (flavone) |
| 12 | 37.44 | C21H20O11 | 448.101 | [M-H]−447.0933, [2M-H]−895.1927 [M+H]+449.1094 | −1.02 | −0.46 | 240,265,350 | Quercitrin (flavonol) |
| 13 | 45.69 | C28H32O13 | 576.1841 | [M-H]−575.177, [M+H]+577.1912 | 0.38 | 0.22 | 235,285 | Genestein G 2 (isoflavone) |
| 14 | 46.16 | C30H26O14 | 610.1326 | [M-H]−609.125, [M+H]+611.1388 | −0.57 | −0.35 | 235,280,335 | Luteolin-4′-O-(6″-trans-caffeoyl)-β-d-glucopyranoside (flavone) |
| 15 | 46.59 | C20H36O12 | 468.2209 | [M-H]−467.2134 | −0.58 | −0.27 | 235,280 | Unknown |
| 16 | 47.84 | C22H38O12 | 494.2372 | [M-H]−493.2291 | −1.8 | −0.89 | 238,280 | Rhodioloside B |
| 17 | 49.04 | C28H46O8 | 510.3189 | [M-H]−509.312 | 0.71 | 0.36 | 245,280 | Pladienolides F/Pladienolides G |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Cao, J.; Tian, L.; Liu, B.; Fan, Y.; Wang, Q. Elucidating Flavonoid and Antioxidant Activity in Edible and Medicinal Herbs Woodwardia japonica (L.f.) Sm. Based on HPLC-ESI-TOF-MS and Artificial Neural Network Model: Response to Climatic Factors. Molecules 2023, 28, 1985. https://doi.org/10.3390/molecules28041985
Wang X, Cao J, Tian L, Liu B, Fan Y, Wang Q. Elucidating Flavonoid and Antioxidant Activity in Edible and Medicinal Herbs Woodwardia japonica (L.f.) Sm. Based on HPLC-ESI-TOF-MS and Artificial Neural Network Model: Response to Climatic Factors. Molecules. 2023; 28(4):1985. https://doi.org/10.3390/molecules28041985
Chicago/Turabian StyleWang, Xin, Jianguo Cao, Lin Tian, Baodong Liu, Yawen Fan, and Quanxi Wang. 2023. "Elucidating Flavonoid and Antioxidant Activity in Edible and Medicinal Herbs Woodwardia japonica (L.f.) Sm. Based on HPLC-ESI-TOF-MS and Artificial Neural Network Model: Response to Climatic Factors" Molecules 28, no. 4: 1985. https://doi.org/10.3390/molecules28041985





