Biosynthesis of Silver Nanoparticles Utilizing Leaf Extract of Trigonella foenum-graecum L. for Catalytic Dyes Degradation and Colorimetric Sensing of Fe3+/Hg2+
Abstract
:1. Introduction
2. Results and Discussions
2.1. Synthesis of AgNPs
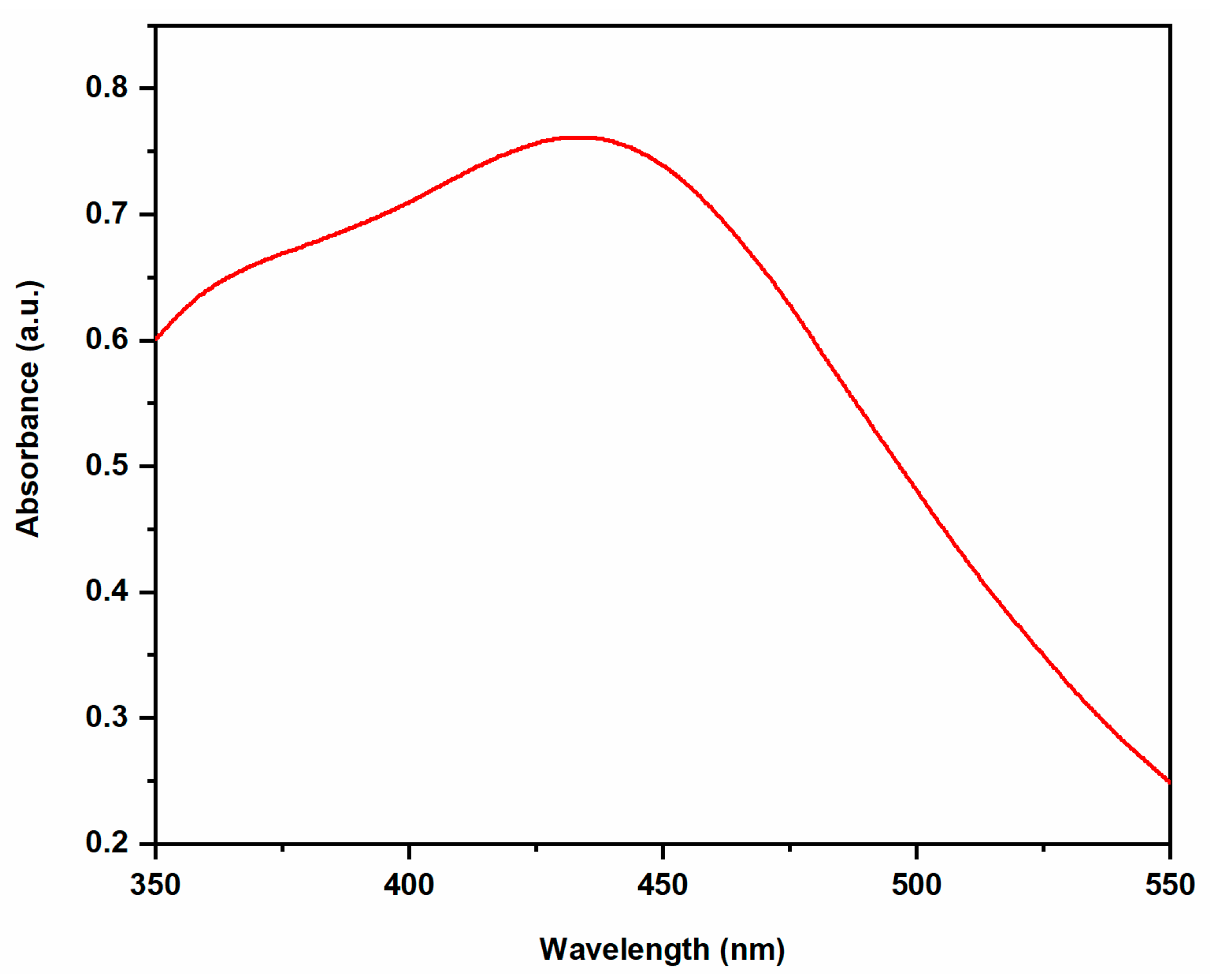
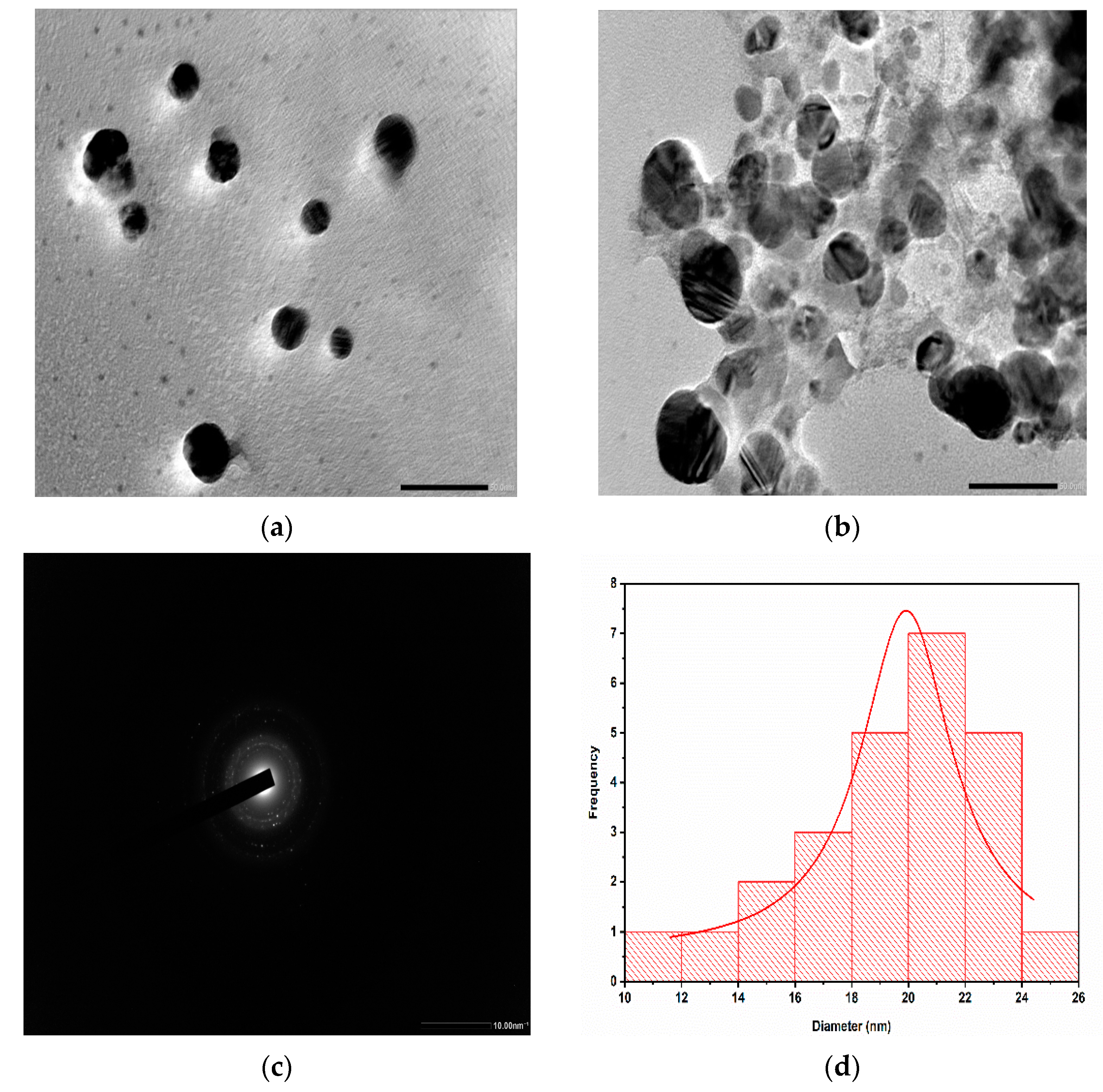
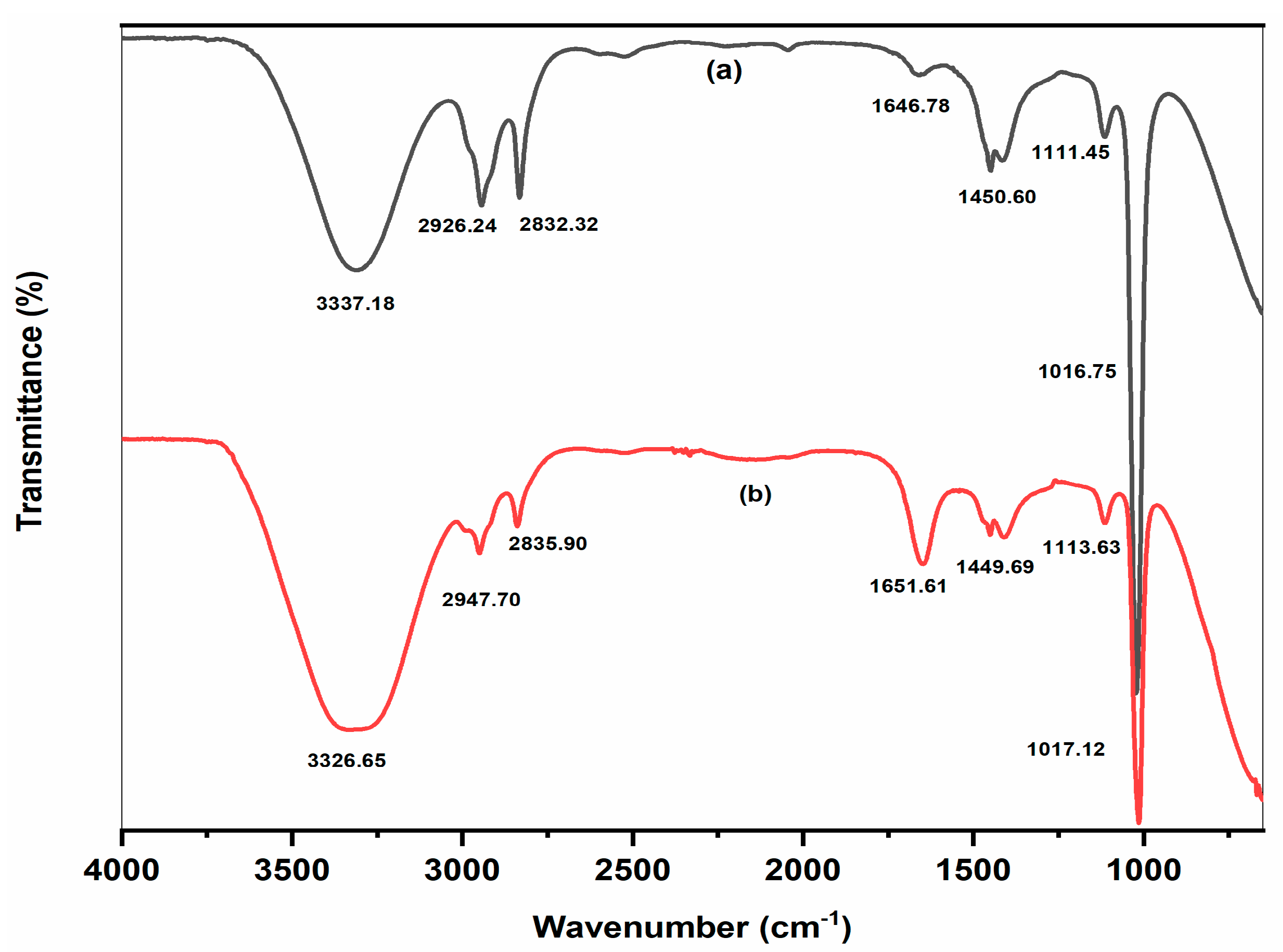
2.2. Characterization of AgNPs
2.3. Catalytic Degradation of Dyes
2.3.1. Degradation of Methylene Blue
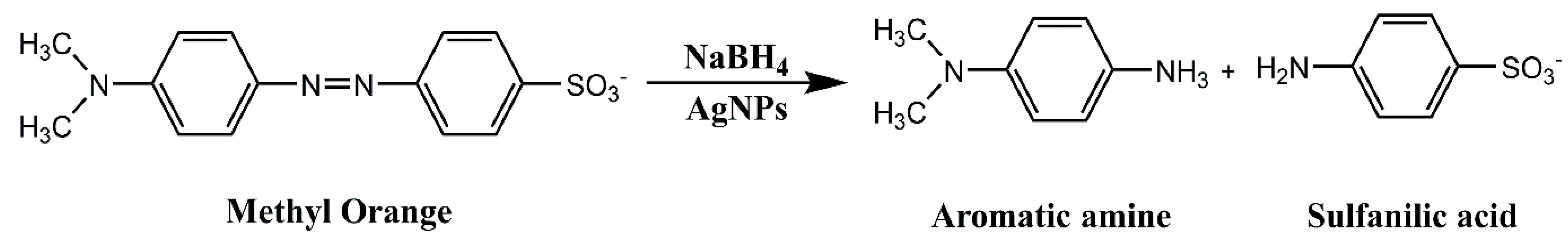
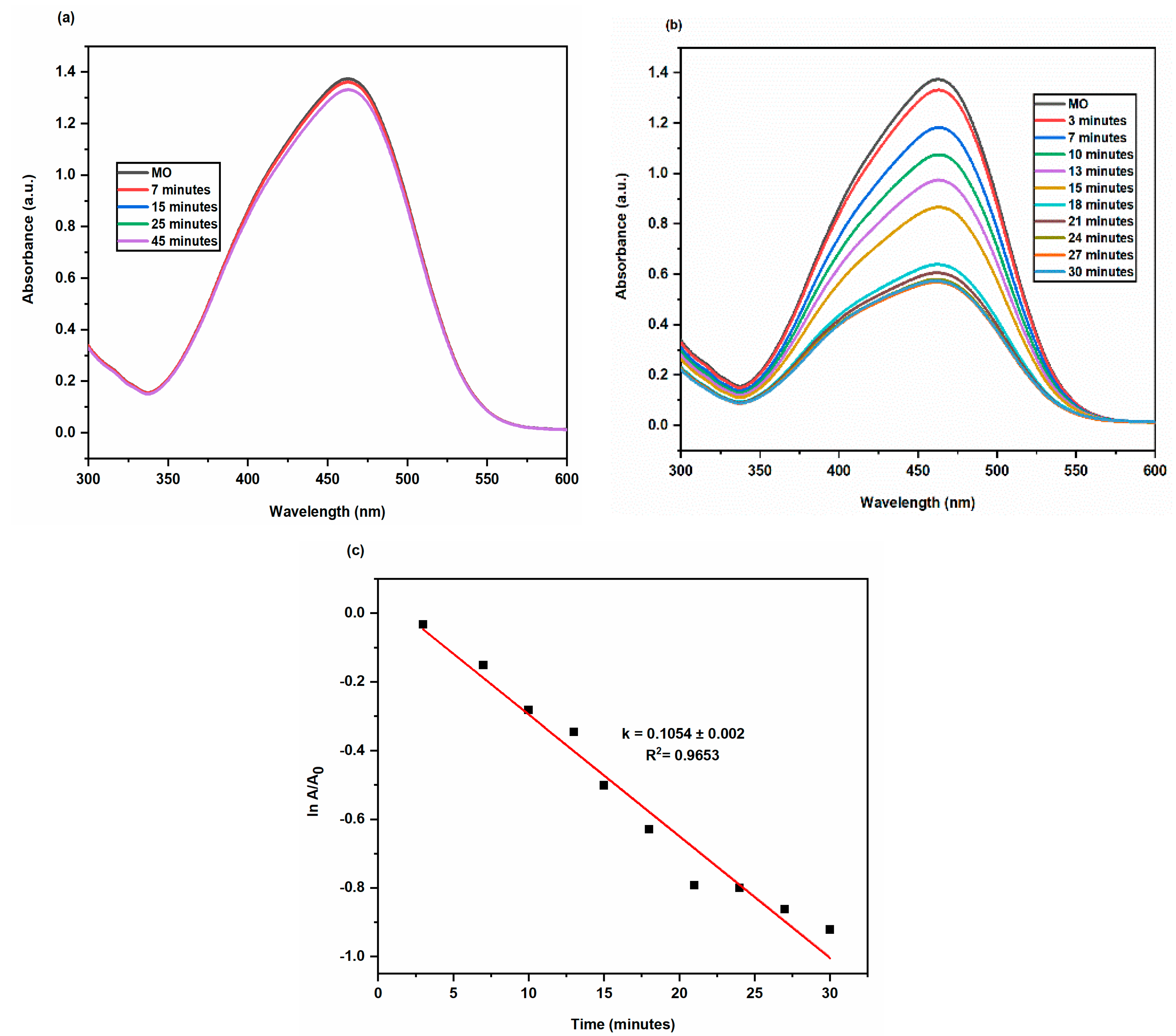
2.3.2. Degradation of Methyl Orange
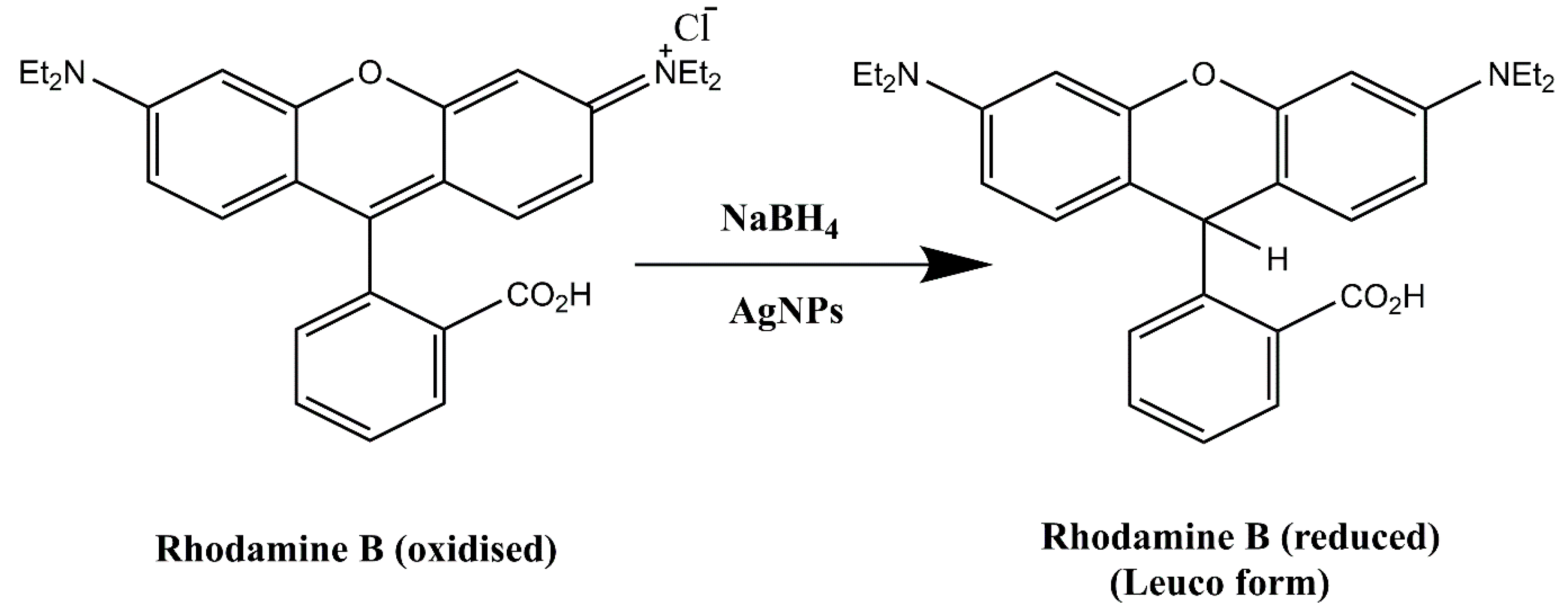
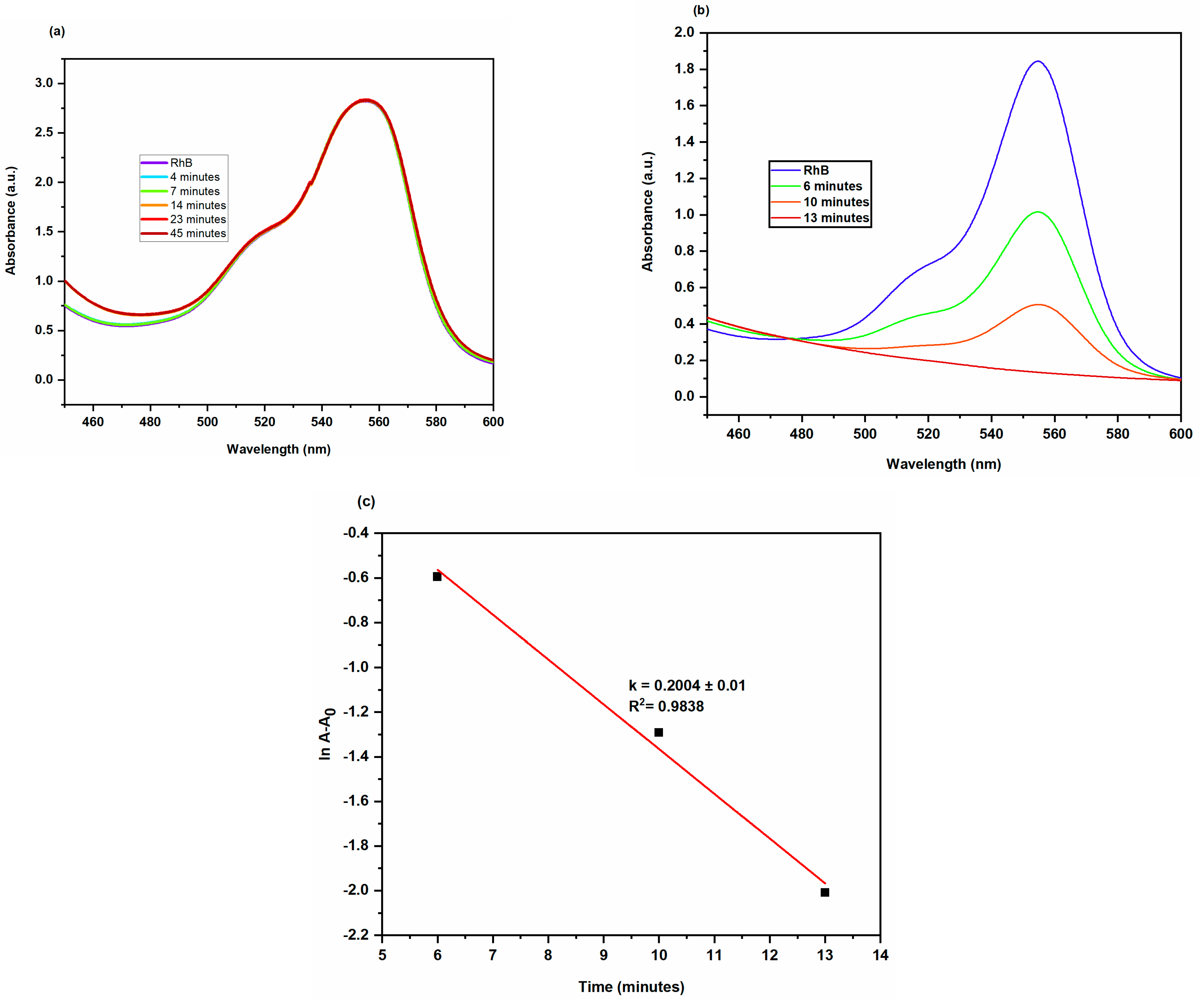
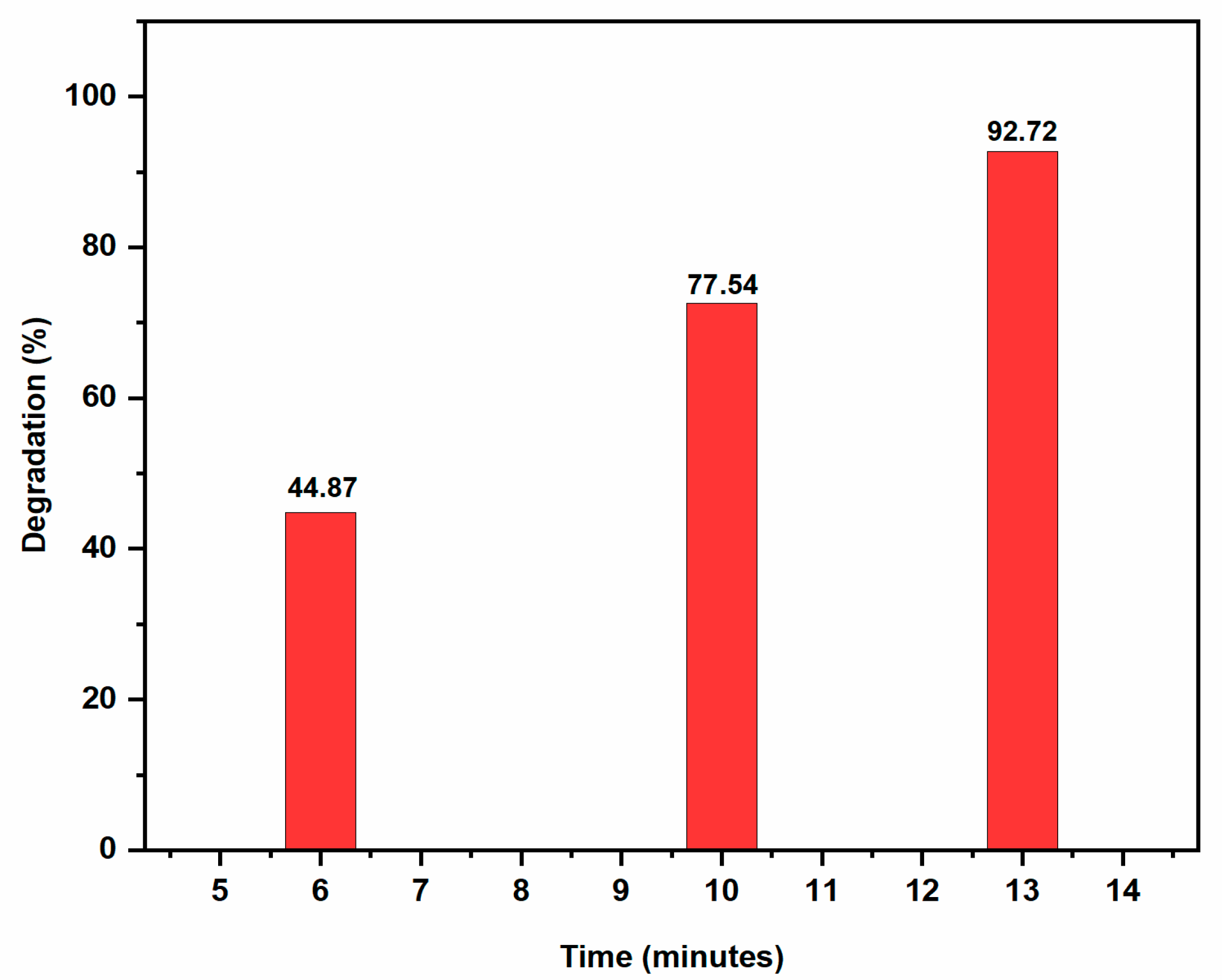
2.3.3. Degradation of Rhodamine B
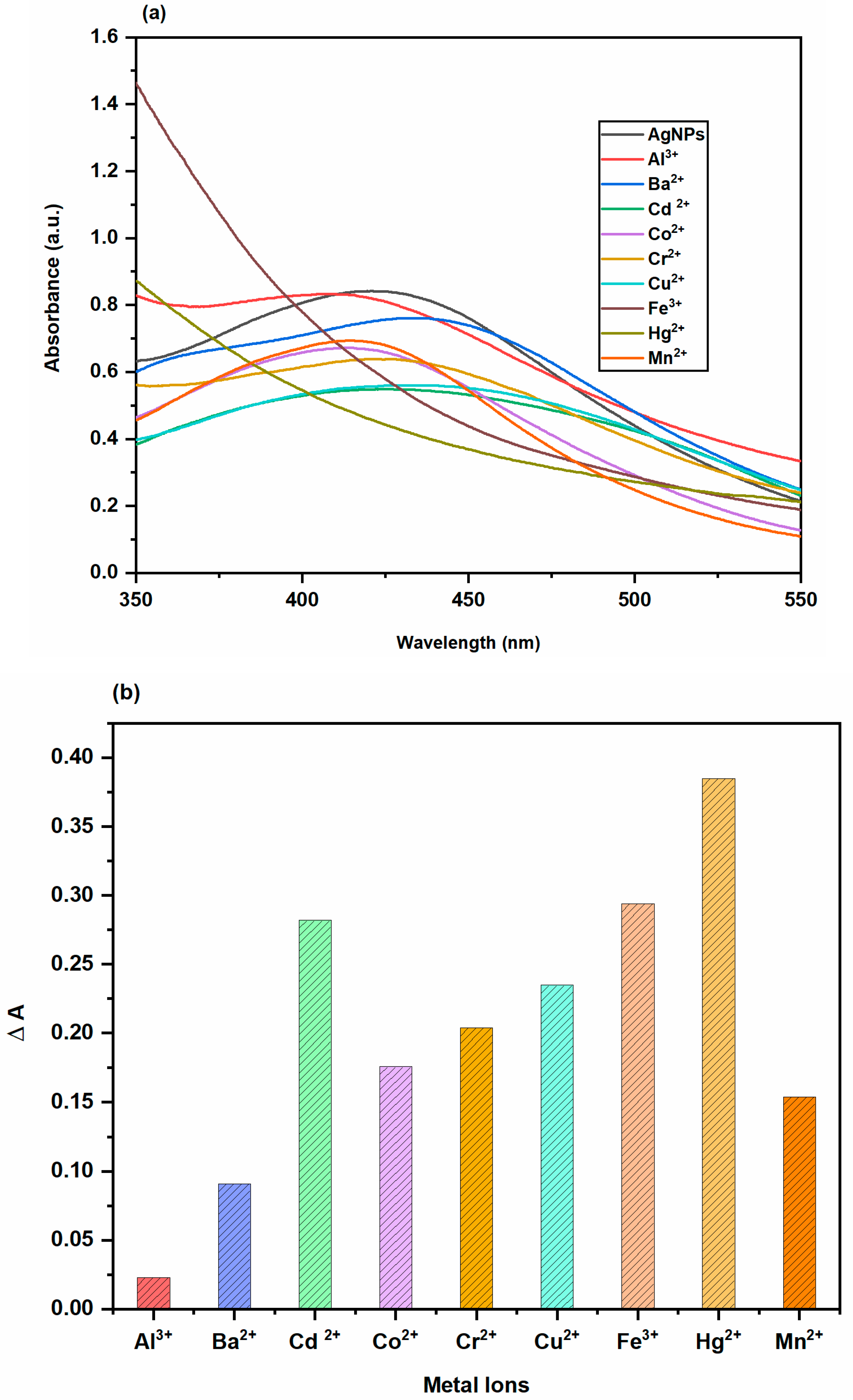
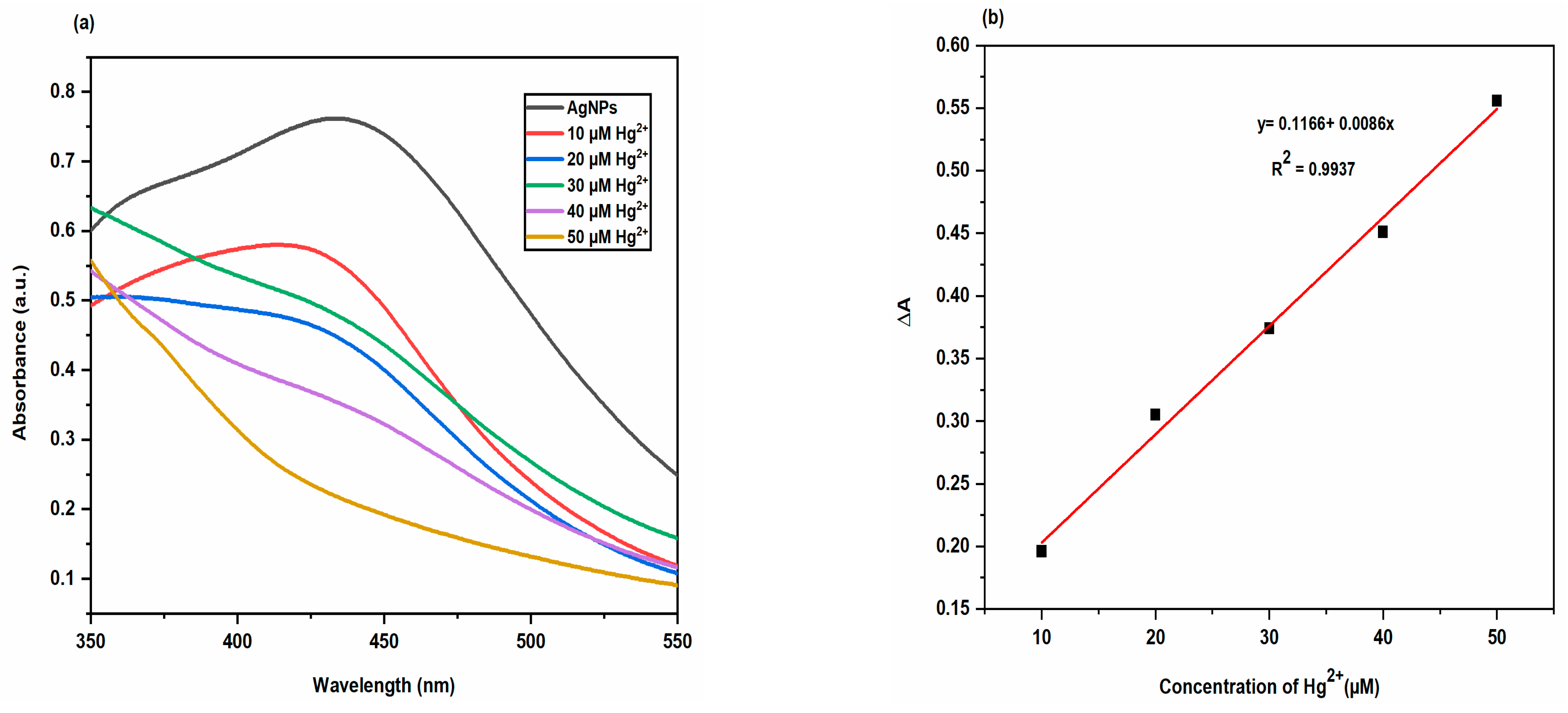
2.3.4. Colorimetric Sensing of Metal Ions
3. Materials and Methods
3.1. Chemicals and Collection of Plant Materials
3.2. Preparation of Plant Extract
3.3. Synthesis of AgNPs
3.4. Characterization of AgNPs
3.5. Catalytic Activity
3.6. Colorimetric Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, X.Y.; Gao, Z.W.; Yang, K.F.; Zhang, W.Q.; Xu, L.W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Wei, H. Plasmonic silver nanoparticles for energy and optoelectronic applications. Adv. Nanomater. Nanostructures 2011, 229, 171–184. [Google Scholar]
- Meheretu, G.M.; Cialla, D.; Popp, J. Surface enhanced raman spectroscopy on silver nanoparticles. Int. J. Biochem. Biophys. 2014, 2, 63–67. [Google Scholar] [CrossRef]
- Thanh, N.T.; Green, L.A. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J. Nanobiotechnology 2021, 19, 256. [Google Scholar] [CrossRef]
- Singh, S.; Sangwan, S.; Sharma, P.; Devi, P.; Moond, M. Nanotechnology for sustainable agriculture: An emerging perspective. J. Nanosci. Nanotechnol. 2021, 21, 3453–3465. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- Abboud, Y.; Eddahbi, A.; El Bouari, A.; Aitenneite, H.; Brouzi, K.; Mouslim, J. Microwave-assisted approach for rapid and green phytosynthesis of silver nanoparticles using aqueous onion (Allium cepa) extract and their antibacterial activity. J. Nanostructure Chem. 2013, 3, 84. [Google Scholar] [CrossRef]
- Joseph, S.; Mathew, B. Microwave-assisted green synthesis of silver nanoparticles and the study on catalytic activity in the degradation of dyes. J. Mol. Liq. 2015, 204, 184–191. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Dalal, R.; Singh, S.; Sangwan, S.; Moond, M.; Beniwal, R. Biochemical and Molecular Mechanism of Plant-mediated Synthesis of Silver Nanoparticles–A Review. Mini-Rev. Org. Chem. 2022, 19, 939–954. [Google Scholar]
- Naseem, K.; Begum, R.; Wu, W.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Catalytic reduction of toxic dyes in the presence of silver nanoparticles impregnated core-shell composite microgels. J. Clean. Prod. 2019, 211, 855–864. [Google Scholar] [CrossRef]
- Moond, M.; Singh, S.; Sangwan, S.; Devi, R.; Beniwal, R. Green Synthesis and Applications of Silver Nanoparticles: A Systematic Review. AATCC J. Res. 2022, 9, 272–285. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Ghosh, M.K.; Lal, M.; Ghorai, T.K.; Shukla, R. Colorimetric sensing of Fe3+ and Hg2+ and photocatalytic activity of green synthesized silver nanoparticles from the leaf extract of Sonchu sarvensis L. New J. Chem. 2019, 43, 18175–18183. [Google Scholar] [CrossRef]
- Samari, F.; Salehipoor, H.; Eftekhar, E.; Yousefinejad, S. Low-temperature biosynthesis of silver nanoparticles using mango leaf extract: Catalytic effect, antioxidant properties, anticancer activity and application for colorimetric sensing. New J. Chem. 2018, 42, 15905–15916. [Google Scholar] [CrossRef]
- Sooraj, M.P.; Nair, A.S.; Vineetha, D.J.C.P. Sunlight-mediated green synthesis of silver nanoparticles using Sida retusa leaf extract and assessment of its antimicrobial and catalytic activities. Chem. Pap. 2021, 75, 351–363. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Bhawal, P.; Remanan, S.; Mondal, S.; Das, N.C. Mussel inspired green synthesis of silver nanoparticles-decorated halloysite nanotube using dopamine: Characterization and evaluation of its catalytic activity. Appl. Nanosci. 2018, 8, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Review nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and Hg2+ biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef] [Green Version]
- Das, T.K.; Remanan, S.; Ghosh, S.; Ghosh, S.K.; Das, N.C. Efficient synthesis of catalytic active silver nanoparticles illuminated cerium oxide nanotube: A mussel inspired approach. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100411. [Google Scholar] [CrossRef]
- Aromal, S.A.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Part A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arshad, H.; Sami, M.A.; Sadaf, S.; Hassan, U. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci. Rep. 2021, 11, 5996. [Google Scholar] [CrossRef] [PubMed]
- Alamier, W.M.; Hasan, N.; Ali, S.K.; Oteef, M.D. Biosynthesis of Ag Nanoparticles Using Caralluma acutangula Extract and Its Catalytic Functionality towards Degradation of Hazardous Dye Pollutants. Crystals 2022, 12, 1069. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 60. [Google Scholar] [CrossRef] [Green Version]
- Indana, M.K.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Guttena, V. A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red dyes. J. Anal. Sci. Technol. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Saha, J.; Begum, A.; Mukherjee, A.; Kumar, S. A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain. Environ. Res. 2017, 27, 245–250. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Genet. Eng. Biotechnol. 2016, 14, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Shahwan, T.; Sirriah, S.A.; Nairat, M.; Boyacı, E.; Eroğlu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 1, 258–266. [Google Scholar] [CrossRef]
- Isa, N.; Lockman, Z. Methylene blue dye removal on silver nanoparticles reduced by Kyllinga brevifolia. Environ. Sci. Pollut. Res. 2019, 26, 11482–11495. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 1, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environ. Sci. Pollut. Res. 2017, 24, 17347–17357. [Google Scholar] [CrossRef] [PubMed]
- Varadavenkatesan, T.; Selvaraj, R.; Vinayagam, R. Phyto-synthesis of silver nanoparticles from Mussaenda erythrophylla leaf extract and their application in catalytic degradation of methyl orange dye. J. Mol. Liq. 2016, 221, 1063–1070. [Google Scholar] [CrossRef]
- Wilhelm, P.; Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@ TiO2 nano-spheres. J. Photochem. Photobiol. A Chem. 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Joseph, S.; Mathew, B. Facile synthesis of silver nanoparticles and their application in dye degradation. Mater. Sci. Eng. B 2015, 195, 90–97. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sailaja, V.; Kennedy, L.J.; Vijaya, J.J. Photocatalytic degradation of rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: Kinetics and mechanism. Ceram. Int. 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Awad, M.A.; Hendi, A.A.; Ortashi, K.M.; Alzahrani, B.; Soliman, D.; Alanazi, A.; Alenazi, W.; Taha, R.M.; Ramadan, R.; El-Tohamy, M.; et al. Biogenic synthesis of silver nanoparticles using Trigonella foenum-graecum seed extract: Characterization, photocatalytic and antibacterial activities. Sens. Actuators A Phys. 2021, 323, 112670. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Fitriani, I.; Wyantuti, S.; Hartati, Y.W.; Khaydarov, R.; Mcalister, J.A.; Obata, H.; Gamo, T. Colorimetric detection of mercury (II) ion in aqueous solution using silver nanoparticles. Anal. Sci. 2017, 33, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Zou, R.; Guo, X.; Yang, J.; Li, D.; Peng, F.; Zhang, L.; Wang, H.; Yu, H. Selective etching of gold nanorods by ferric chloride at room temperature. Cryst. Eng. Comm. 2009, 11, 2797–2803. [Google Scholar] [CrossRef]
- Liu, J.M.; Jiao, L.; Cui, M.L.; Lin, L.P.; Wang, X.X.; Zheng, Z.Y.; Zhang, L.H.; Jiang, S.L. A highly sensitive non-aggregation colorimetric sensor for the determination of I− based on its catalytic effect on Fe3+ etching gold nanorods. Sens. Actuators B Chem. 2013, 1, 644–650. [Google Scholar] [CrossRef]
- Tanvir, F.; Yaqub, A.; Tanvir, S.; An, R.; Anderson, W.A. Colorimetric detection of mercury ions in water with capped silver nanoprisms. Materials 2019, 12, 1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firdaus, M.; Andriana, S.; Alwi, W.; Swistoro, E.; Ruyani, A.; Sundaryono, A. Green synthesis of silver nanoparticles using Carica Papaya fruit extract under sunlight irradiation and their colorimetric detection of mercury ions. J. Phys. Conf. Ser. 2017, 817, 012029. [Google Scholar] [CrossRef]
- Karthiga, D.; Anthony, S.P. Selective colorimetric sensing of toxic metal cations by green synthesized silver nanoparticles over a wide pH range. RSC Adv. 2013, 3, 16765–16774. [Google Scholar] [CrossRef]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Rapid colorimetric detection of Hg2+ ion by green silver nanoparticles synthesized using Dahlia pinnata leaf extract. Green Process. Synth. 2015, 4, 455–461. [Google Scholar] [CrossRef]
- Kalam, A.; Al-Sehemi, A.G.; Alrumman, S.; Assiri, M.; Moustafa, M.F.M.; Yadav, P.; Pannipara, M. Colorimetric optical chemosensor of toxic metal ion (Hg2+) and biological activity using green synthesized AgNPs. Green Chem. Lett. Rev. 2018, 11, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Uzunoğlu, D.; Ergüt, M.; Kodaman, C.G.; Özer, A. Biosynthesized silver nanoparticles for colorimetric detection of Fe3+ ions. Arab. J. Sci. Eng. 2020, 1–12. [Google Scholar] [CrossRef]


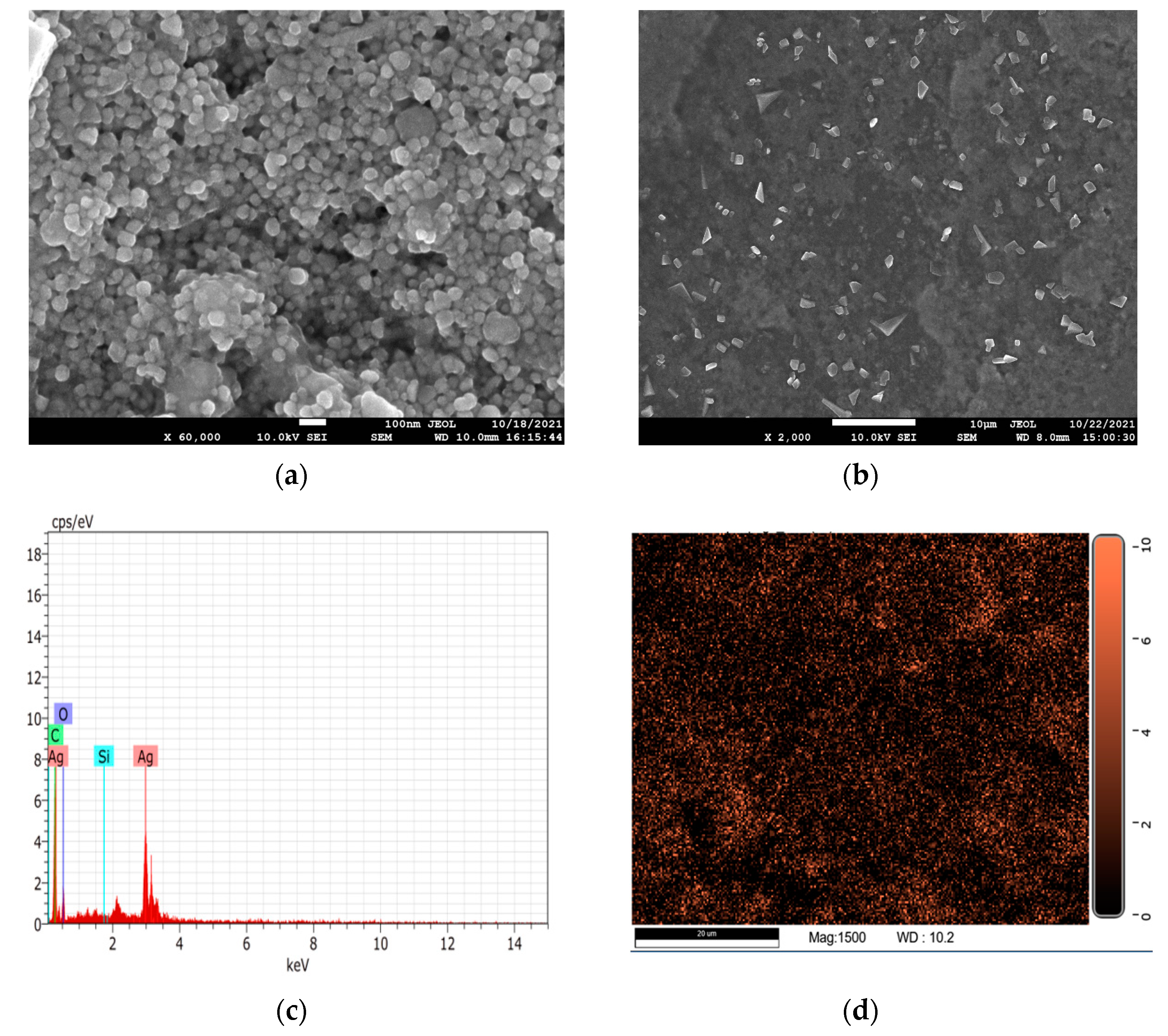






| Element | Weight% | Atomic% |
|---|---|---|
| Carbon | 27.65 | 57.79 |
| Oxygen | 18.93 | 29.71 |
| Silver | 53.33 | 12.41 |
| Silicon | 0.10 | 0.09 |
| S.No. | FTIR Peaks (cm−1) | Assignment of Peaks | Range (cm−1) |
|---|---|---|---|
| 1 | 3326.65, 3337.18 | -OH or –NH stretching vibrations | 3200–3500 |
| 2 | 2835.90, 2832.32, 2947.70, 2838.78, 2926.24 | C-H stretching vibration of -CH3 groups, aldehydes H-C=O | 2700–2950 |
| 3 | 1651.61, 1646.78 | -C=O Stretching of amide groups, N–H of the proteins | 1650–1850 |
| 4 | 1450.60, 1449.69 | stretching vibration of alkenes C=C in aromatic rings | 1380–1465 |
| 5 | 1113.63, 1111.45, 1113.78, 1017.12, 1016.75 | -C-O-C or C-N stretching | 1016–1250 |
| Dyes (10 mL of 4 × 10−5 M) | Volume of AgNPs | Volume of 0.05 M NaBH4 | Rate Constant (min−1) | R2 | Degradation (%) | Reduction Time (min) |
|---|---|---|---|---|---|---|
| Methylene Blue (MB) | 0.5 mL | 0.5 mL | 0.1665 ± 0.03 | 0.9366 | 96.57 | 20 |
| Methyl Orange (MO) | 100 μL | 100 μL | 0.1054 ± 0.002 | 0.9653 | 71.45 | 30 |
| Rhodamine B (RhB) | 0.2 mL | 0.2 mL | 0.2004 ± 0.01 | 0.9838 | 92.72 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moond, M.; Singh, S.; Sangwan, S.; Devi, P.; Beniwal, A.; Rani, J.; Kumari, A.; Rani, S. Biosynthesis of Silver Nanoparticles Utilizing Leaf Extract of Trigonella foenum-graecum L. for Catalytic Dyes Degradation and Colorimetric Sensing of Fe3+/Hg2+. Molecules 2023, 28, 951. https://doi.org/10.3390/molecules28030951
Moond M, Singh S, Sangwan S, Devi P, Beniwal A, Rani J, Kumari A, Rani S. Biosynthesis of Silver Nanoparticles Utilizing Leaf Extract of Trigonella foenum-graecum L. for Catalytic Dyes Degradation and Colorimetric Sensing of Fe3+/Hg2+. Molecules. 2023; 28(3):951. https://doi.org/10.3390/molecules28030951
Chicago/Turabian StyleMoond, Monika, Sushila Singh, Seema Sangwan, Parvesh Devi, Anuradha Beniwal, Jyoti Rani, Anita Kumari, and Savita Rani. 2023. "Biosynthesis of Silver Nanoparticles Utilizing Leaf Extract of Trigonella foenum-graecum L. for Catalytic Dyes Degradation and Colorimetric Sensing of Fe3+/Hg2+" Molecules 28, no. 3: 951. https://doi.org/10.3390/molecules28030951






