Antioxidant and Antimicrobial Effects of Baby Leaves of Amaranthus tricolor L. Harvested as Vegetable in Correlation with Their Phytochemical Composition
Abstract
:1. Introduction
2. Results and Discussion
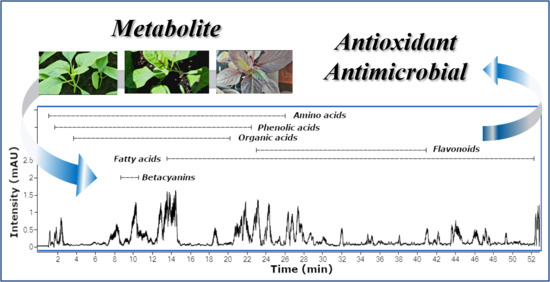
2.1. HPLC-DAD-ESI/HRMS Metabolite Profile
2.1.1. Amino Acids
2.1.2. Betacyanins
2.1.3. Fatty Acids
2.1.4. Flavonoids
2.1.5. Organic Acids
2.1.6. Phenolic Acids
2.2. Antioxidant Assays
Correlation between Antioxidant Activity and Phytochemical Composition
2.3. Antimicrobial Activity
Correlation between Antimicrobial Activity and Phytochemical Composition
3. Materials and Methods
3.1. Reference Compounds and Reagents
3.2. Plant Material and Sample Preparation
3.3. HPLC-DAD-ESI/HRMS Analysis
3.4. Antioxidant Activity
3.4.1. ABTS Radical Scavenging Assay
3.4.2. DPPH Radical Scavenging Assay
3.4.3. FRAP—Ferric Reducing Antioxidant Power Assay
3.5. Antimicrobial Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miguel, M.G. Betalains in some species of the Amaranthaceae family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef]
- Haber, T.; Obiedziński, M.; Waszkiewicz-Robak, B.; Biller, E.; Achremowicz, B.; Ceglińska, A. Pseudocereals and the possibilities of their application in food technology Amaranth and Quinoa application in food processing. Pol. J. Appl. Sci. 2017, 3, 57–65. [Google Scholar]
- Rahman, A.H.M.M.; Gulshana, M.I.A. Taxonomy and medicinal uses on Amaranthaceae family of Rajshahi, Bangladesh. Appl. Ecol. Environ. Sci. 2014, 2, 54–59. [Google Scholar]
- Nahar, K.; Kabir, F.; Islam, P.; Rahman, M.M.; Al Mamun, M.A.; Faruk, M.; Subhan, N.; Rahman, G.M.S.; Reza, H.M.; Alam, M.A. Cardioprotective effect of Amaranthus tricolor extract in isoprenaline induced myocardial damage in ovariectomized rats. Biomed. Pharmacother. 2018, 103, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Samsul, A.; Krupanidhi, K.; Sambasiva Rao, K.R.S. Evaluation of in-vitro antioxidant activity of Amaranthus tricolor Linn. Asian J. Pharmacol. Toxicol. 2013, 1, 12–16. [Google Scholar]
- Angerhofer, C.K.; Maes, D.; Giacomoni, P.U. The Use of Natural Compounds and Botanicals in the Development of Anti-Aging Skin Care Products. In Skin Aging Handbook; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 205–263. [Google Scholar]
- Hunyadi, A. Themechanism(s) of action of antioxidants:from scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [PubMed]
- Motyleva, S.; Gins, M.; Gins, V.; Tetyannikov, N.; Kulikov, I.; Kabashnikova, L.; Panischeva, D.; Mertvischeva, M.; Domanskaya, I. Metabolite profile of Amaranthus tricolor L. and Amaranthus cruentus L. in Adaptation to Drought; IntechOpen: Moscow, Russia, 2022; pp. 1–17. [Google Scholar]
- Castrillón-Arbeláez, P.A.; Frier, J.P.D. Secondary Metabolism in Amaranthus spp.-A Genomic Approach to Understand its Diversity and Responsiveness to Stress in Marginally Studied Crops with Highagronomic Potential; IntechOpen: Rijeka, Croatia, 2016; pp. 185–227. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Kumorkiewicz, A.; Szmyr, N.; Szneler, E.; Wybraniec, S. Separation of betacyanins from flowers of Amaranthus cruentus L. in a polar solvent system by high-speed counter-current chromatography. J. Sep. Sci. 2019, 42, 1676–1685. [Google Scholar] [CrossRef]
- Jeong, W.T.; Bang, J.H.; Han, S.; Hyun, T.K.; Cho, H.; Lim, H.B.; Chung, J.W. Establishment of a UPLC-PDA/ESI-Q-TOF/MS-based approach for the simultaneous analysis of multiple phenolic compounds in Amaranth (A. cruentus and A. tricolor). Molecules 2020, 25, 5674. [Google Scholar] [CrossRef]
- Sugimoto, H.; Kakehi, M.; Jinno, F. Bioanalytical Method for the Simultaneous Determination of D- and L-serine in human plasma by LC/MS/MS. Anal. Biochem. 2015, 487, 38–44. [Google Scholar] [CrossRef]
- Harder, U.; Koletzko, B.; Peissner, W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC–MS/MS. J. Chromatogr. B Biomed. Appl. 2011, 879, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, G.; Yang, J.; Yang, C.; Guo, M. Screening and characterisation of potential antioxidant, hypoglycemic and hypolipidemic components revealed in Portulaca oleracea via multi-target affinity ultrafiltration LC–MS and molecular docking. Phytochem Anal. 2021, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Yang, Z.; Lv, B.; Xiang, L. Rapid screening for cyclo-dopa and diketopiperazine alkaloids in crude extracts of Portulaca oleracea L. using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-basedmetabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Barkociová, M.; Tóth, J.; Sutor, K.; Drobnicka, N.; Wybraniec, S.; Dudík, B.; Bilková, A.; Czigle, S.; Braca, A.; De Leo, M. Betalains in edible fruits of three cactaceae taxa-Epiphyllum, Hylocereus, and Opuntia-their LC-MS/MS and FTIR identification and biological activities. Plants 2021, 10, 2669. [Google Scholar] [CrossRef]
- Gök, H.N.; Luca, S.V.; Ay, S.T.; Komsta, Ł.; Salmas, R.E.; Orhan, I.E.; Skalicka-Woźniak, K. Profiling the annual change of the neurobiological and antioxidant effects of five Origanum species in correlation with their phytochemical composition. Food Chem. 2022, 368, 130775. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Arráez-Román, D.; Segura-Carretero, A. Fernández-Gutiérrez, A. Characterization of phenolic and other polar compounds in a lemon verbena extract by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Sep. Sci. 2010, 33, 2818–2827. [Google Scholar] [CrossRef]
- Zengin, G.; Ferrante, C.; Orlando, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Recinella, L.; Chiavaroli, A.; Leone, A.; Brunetti, L.; Aumeeruddy, M.Z.; et al. Chemical profiling and pharmaco-toxicological activity of Origanum sipyleum extracts: Exploring for novel sources for potential therapeutic agents. J. Food Biochem. 2019, 43, e13003. [Google Scholar] [CrossRef]
- Farag, M.A.; Shakour, Z.T.A. Metabolomics driven analysis of 11 Portulaca leaf taxa as analysed via UPLCESI- MS/MS and chemometrics. Phytochemistry 2019, 161, 117–129. [Google Scholar] [CrossRef]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carretero, A. Comparative assessment of phytochemical profiles of comfrey (Symphytum officinale L.) root extracts obtained by different extraction techniques. Molecules 2020, 25, 837. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wang, L.; Jin, J.; Chang, S.; Xiao, X.; Feng, B.; Zhu, H. Simultaneous determination of calycosin-7-O-β-D-glucoside,cinnamic acid, paeoniflorin and albiflorin in rat plasma by UHPLC-MS/MS and its application to a pharmacokinetic study of Huangqi guizhi Wuwu decoction. J. Pharm. Biomed. Anal. 2019, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.K.; Arya, P. Amaranthus grain nutritional benefits: A review. J. Pharmacogn. Phytochem. 2018, 7, 2258–2262. [Google Scholar]
- Stintzing, F.C.; Kammerer, D.; Schieber, A.; Adama, H.; Nacoulma, O.G.; Carle, R. Betacyanins and phenolic compounds from Amaranthus spinosus L. and Boerhavia erecta L. Z. Naturforsch. C 2004, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, A.; Millan-Linares, M.C. Rodriguez-Martin, N.M.; Millan, F.; Montserrat-de la Paz, S. Nutraceutical value of Kiwicha (Amaranthus caudatus L.). J. Funct. Foods. 2020, 65, 103735. [Google Scholar] [CrossRef]
- Popa, I.; Solgadi, A.; Pin, D.; Watson, A.L.; Haftek, M.; Portoukalian, J. The linoleic acid content of the stratum corneum of ichthyotic golden retriever dogs is reduced as compared to healthy dogs and a significant part is oxidized in both free and esterified forms. Metabolites 2021, 11, 803. [Google Scholar] [CrossRef]
- Friedrich, W. Handbuch Der Vitamine C; Urban und Schwarzenberg: München, Germany; Wien, Austria; Baaltimore, MD, USA, 1987; p. 596. [Google Scholar]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef]
- Orabi, S.A.; Hussein, M.M.; Abd El-Motty, E.Z.; El-Faham, S.Y. Effect of alpha-tochopherol and glutamic acid on total phenols, antioxidant activity, yield and fruit properties of mango trees. Science 2018, 8, 1229–1239. [Google Scholar]
- Gülçin, I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Winkler-Moser, J.K.; Doll, K.M.; Gadgil, M.; Liu, S.X. Factors affecting antioxidant activity of amino acids in soybean oil at frying temperatures. Eur. J. Lipid Sci. Technol. 2019, 121, 1900091. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty acid, carotenoid and tocopherol compositions of 20 canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Yang, R.; Kramer, J.K.; Hernandez, M. Fatty acid profiles, tocopherol contents, and antioxidant activities of Heartnut (Juglans ailanthifolia var. Cordiformis) and Persian walnut (Juglans regia L.). J. Agric. Food Chem. 2007, 55, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Samolińska, W.; Kiczorowska, B.; Klebaniuk, R.; Kiczorowski, P. Content of minerals and fatty acids and their correlation with phytochemical compounds and antioxidant activity of Leguminous seeds. Biol. Trace Elem. Res. 2017, 180, 338–348. [Google Scholar] [CrossRef]
- Abdoulaye, T.; Claude, K.A.L.; Constant, A.A.R.; Faustin, K.A.; Marcelline, A.N.D.; Barthélémy, A.K. Antibacterial and acute toxicity studies of culinary leaves from Adansonia digitata L. (Malvaceae) and Amaranthus cruentus L. (Amaranthaceae) growing in Côte d’Ivoire. European J. Biotechnol. Biosci. 2018, 6, 24–29. [Google Scholar]
- Marsot, A.; Boulamery, A.; Bruguerolle, B.; Simon, N. Vancomycin. Clin. Pharmacokinet. 2012, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Markham, A.; Balfour, J.A. Ciprofloxacin. Drugs 1996, 51, 1019–1074. [Google Scholar] [CrossRef]
- Goa, K.L.; Barradell, L.B. Fluconazole. Drugs 1995, 50, 658–690. [Google Scholar] [CrossRef]
- EUCAST. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Inf. Dis. 2003, 40, 1–7. [Google Scholar]




| No. | Metabolite | Molecular Formula | tR (min) | m/z (M − H)−exp | m/z (M + H)+exp | m/z (M ± H)± calc | Δ (ppm) | m/z from MS2 of (M − H)− | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Amino acids | |||||||||
| 1 | Serine | C3H7NO3 | 1.6 | 104.0356 | 104.0353 | −2.70 | 60.0441 | [13] | |
| 2 | Aspartic acid | C4H7NO4 | 1.7 | 132.0301 | 132.0302 | 0.99 | 88.0422 | [14] | |
| 3 | Tyrosine | C9H11NO3 | 12.9 | 180.0662 | 180.0666 | 2.30 | 119.0348 | [14] | |
| 4 | N-benzoylaspartic acid | C11H11NO5 | 13.1 | 236.0564 | 236.0564 | 0.19 | 192.0695; 174.9560 | [15] | |
| 5 | Glutamic acid | C5H9NO4 | 13.4 | 146.0460 | 146.0459 | −0.81 | 102.0559 | [14] | |
| 6 | Isoleucine | C6H13NO2 | 13.7 | 130.0875 | 130.0874 | −1.13 | 69.0693 | [14] | |
| 7 | N-(carboxyacetyl) phenylalanine | C12H13NO5 | 14.2 | 250.0720 | 250.0721 | 0.38 | 206.0824 | [15] | |
| 8 | Dopa | C9H11NO4 | 14.7 | 196.0624 | 196.0615 | −4.41 | 179.0022; 135.0557 | [16] | |
| 9 | Leucine | C6H13NO2 | 18.1 | 130.0880 | 130.0874 | −4.94 | 69.0686 | [14] | |
| 10 | Phenylalanine | C9H11NO2 | 18.3 | 164.0710 | 164.0717 | 4.25 | 147.0432 | [14] | |
| 11 | Tryptophan | C11H12N2O2 | 22.3 | 203.0826 | 203.0826 | 0.01 | 142.0651 | [17] | |
| 12 | Norleucine | C6H13NO2 | 26.2 | 130.0873 | 130.0874 | 0.40 | 69.0689 | [14] | |
| Betacyanins | |||||||||
| 13 | Betanidin-5-O-ß-glucuronosylglucoside (Amaranthin) | C30H34N2O19 | 8.5 | 727.1826 | 727.1829 | 0.35 | 551.1512; 389.1637 | [28] | |
| 14 | Betanidin-5-O-ß-glucoside (Betanin) | C24H26N2O13 | 9.9 | 551.1508 | 551.1508 | −0.06 | 389.1640 | [18] | |
| 15 | Isobetanidin-5-O-ß-glucuronosylglucoside (Isoamaranthin) | C30H34N2O19 | 10.6 | 727.1832 | 727.1829 | −0.48 | 551.1507; 389.1632 | [28] | |
| 16 | Isobetanidin-5-O-ß-glucoside (Isobetanin) | C24H26N2O13 | 10.8 | 551.1516 | 551.1508 | −1.52 | 389.1634 | [18] | |
| Fatty acids | |||||||||
| 17 | Tuberonic acid hexoside | C18H28O9 | 13.7 | 387.1673 | 387.1661 | −3.20 | 225.1132; 207.0976; 163.0400 | [19] | |
| 18 | Tuberonic acid | C12H18O4 | 16.0 | 225.1127 | 225.1132 | 2.36 | 163.0425; | [19] | |
| 19 | Trihydroxyoctadecadienoic acid | C18H32O5 | 32.0 | 327.2181 | 327.2177 | −1.23 | 299.0780; 271.1568; 229.1437; 171.1008 | [19] | |
| 20 | Hydroperoxyoctadecadienoic acid | C18H32O4 | 44.5 | 311.2233 | 311.2228 | −1.66 | 293.1811; 161.0768 | [19] | |
| 21 | Hydroxyoctadecatrienoic acid I | C18H30O3 | 46.0 | 293.2129 | 293.2122 | −2.32 | 275.2002; 183.1435; 171.0994; 121.1026 | [19] | |
| 22 | Hydroxyoctadecatrienoic acid II | C18H30O3 | 46.8 | 293.2123 | 293.2122 | −0.28 | 275.2002; 183.1435; 171.0992; 121.1021 | [19] | |
| 23 | Hydroxyoctadecatrienoic acid III | C18H30O3 | 47.1 | 293.2128 | 293.2122 | −1.98 | 275.1915; 183.1427; 171.0987; 121.1018 | [19] | |
| 24 | Hydroxyoctadecadienoic acid I | C18H32O3 | 49.4 | 295.2279 | 295.2279 | −0.11 | 277.2162; 181.0419; 171.9492 | [19] | |
| 25 | Hydroxyoctadecadienoic acid II | C18H32O3 | 52.0 | 295.2280 | 295.2279 | −0.44 | 277.2162; 181.0410; 171.9496 | [19] | |
| Flavonoids | |||||||||
| 26 | Quercetin-O-hexoside | C21H20O12 | 23.7 | 463.0886 | 463.0882 | −0.86 | 301.0333 | [19] | |
| 27 | Luteolin-O-rhamnosylhexoside | C27H30O15 | 24.7 | 593.1514 | 593.1512 | −0.35 | 447.0893; 285.0180 | [19] | |
| 28 | Luteolin-6-C-hexoside (homoorientin) | C21H20O11 | 25.4 | 447.0944 | 447.0933 | −2.49 | 429.1788; 357.1467; 327.1175; 297.1072; 285.1535; 213.0244; 133.0106 | [21] | |
| 29 | Phlorizin | C21H24O10 | 28.8 | 435.1301 | 435.1297 | −0.98 | 329.1624; 273.1210; 167.0340 | [21] | |
| 30 | Luteolin-7-O-glucoside | C21H20O11 | 41.4 | 447.0933 | 447.0933 | −0.03 | 285.0174; 243.0144; 199.8430; 175.9898 | [21] | |
| Organic acids | |||||||||
| 31 | Citric acid | C6H8O7 | 1.9 | 191.0199 | 191.0197 | −0.91 | 111.0072 | [22,23] | |
| 32 | Mannonic acid | C6H12O7 | 1.9 | 195.0510 | 195.0510 | 0.13 | 159.0855 | [9] | |
| 33 | Malic acid | C4H6O5 | 2.1 | 133.0134 | 133.0142 | 6.32 | 115.0027 | [15] | |
| 34 | Maleic acid | C4H4O4 | 2.1 | 115.0037 | 115.0037 | −0.15 | 71.0156 | [9] | |
| 35 | Succinic acid | C4H6O4 | 2.5 | 117.0193 | 117.0193 | 0.27 | 73.0036 | [9,24] | |
| 36 | Glyceric acid | C3H6O4 | 2.9 | 105.0192 | 105.0193 | 1.25 | 73.0005 | [9] | |
| 37 | 2-Oxopentanoic acid | C5H8O3 | 9.2 | 115.0402 | 115.0401 | −1.14 | 71.1022 | [9] | |
| 38 | Fumaric acid | C4H4O4 | 23.4 | 115.0037 | 115.0037 | −0.15 | 71.1020 | [9,24] | |
| Phenolic acids | |||||||||
| 39 | Gallic acid | C7H6O5 | 3.7 | 169.0152 | 169.0142 | −5.61 | 125.0247 | [25] | |
| 40 | Gallic acid-O-hexoside | C13H16O16 | 5.7 | 331.0667 | 331.0671 | 1.12 | 237.0881; 169.0247; 151.0382; 125.0247; 123.0442; 115.0385 | [21] | |
| 41 | Vanillic acid-O-hexoside | C14H18O9 | 6.1 | 329.0878 | 329.0879 | −0.29 | 167.0345; 123.0442 | [19] | |
| 42 | Vanillic acid | C8H8O4 | 6.4 | 167.0345 | 167.0350 | 2.87 | 152.9122; 123.0442; 108.0206 | [21] | |
| 43 | Protocatechuic acid-O-hexoside | C13H16O9 | 7.4 | 315.0729 | 315.0722 | −2.35 | 153.0197; 109.0304 | [21] | |
| 44 | Dihydroxybenzoic acid | C7H6O4 | 7.4 | 153.0195 | 153.0193 | −1.09 | 121.0298; 77.1045 | [19] | |
| 45 | Hydroxybenzoic acid | C7H6O3 | 9.9 | 137.0244 | 137.0244 | 0.13 | 121.0298; 77.1045 | [19] | |
| 46 | Benzoic acid | C7H6O2 | 13.5 | 121.0298 | 121.0295 | −2.43 | 77.1045 | [19] | |
| 47 | Ferulic acid | C10H10O4 | 13.5 | 193.0503 | 193.0506 | 1.71 | 178.0280; 149.0626 | [15] | |
| 48 | p-Coumaric acid | C9H8O3 | 13.8 | 163.0401 | 163.0401 | −0.20 | 119.0500 | [15] | |
| 49 | o-Coumaric acid | C9H8O3 | 14.2 | 163.0401 | 163.0401 | −0.20 | 135.0453; 119.0502 | [21] | |
| 50 | Syringic acid | C9H10O5 | 17.1 | 197.0457 | 197.0455 | −0.77 | 153.1034; 179.0488; 135.0312 | [17] | |
| 51 | Cinnamic acid | C9H8O2 | 18.4 | 147.0452 | 147.0452 | −0.32 | 103.0530 | [26] | |
| 52 | Caffeic acid | C9H8O4 | 19.8 | 179.0350 | 179.0350 | −0.10 | 135.0430 | [21] |
| Antioxidant Activity | ABTS | FRAP | DPPH | |||||
|---|---|---|---|---|---|---|---|---|
| [mmol TE/g DE] * | ||||||||
| Red | H2O | 0.060 h | ±0.012 | 0.053 h | ±0.028 | 0.021 h | ±0.027 | |
| EtOH | 0.035 d | ±0.011 | 0.030 f | ±0.033 | 0.019 f | ±0.024 | ||
| Ac | 0.020 c | ±0.008 | 0.017 d | ±0.010 | 0.017 e | ±0.011 | ||
| Passion | H2O | 0.056 f | ±0.018 | 0.023 e | ±0.011 | 0.016 d | ±0.024 | |
| EtOH | 0.048 e | ±0.006 | 0.015 c | ±0.009 | 0.014 c | ±0.012 | ||
| Ac | 0.018 b | ±0.010 | 0.013 b | ±0.029 | 0.011 b | ±0.015 | ||
| Green | H2O | 0.058 g | ±0.012 | 0.035 g | ±0.014 | 0.020 g | ±0.049 | |
| EtOH | 0.018 b | ±0.010 | 0.015 c | ±0.008 | 0.014 c | ±0.059 | ||
| Ac | 0.015 a | ±0.015 | 0.009 a | ±0.019 | 0.009 a | ±0.051 | ||
| Ascorbic acid | 7.8 | ±0.035 | 4.6 | ±0.010 | 8.1 | ±0.040 | ||
| Fisher’ LSD | 0.003 | 0.002 | 0.002 | |||||
| Correlation | ||||||||
| No. | Amino acids | |||||||
| 1 | Serine | −0.373 | −0.412 | −0.430 | ||||
| 2 | Aspartic acid | −0.452 | −0.500 | −0.509 | ||||
| 3 | Tyrosine | −0.050 | −0.156 | 0.001 | ||||
| 4 | N-benzoylaspartic acid | 0.095 | −0.160 | −0.223 | ||||
| 5 | Glutamic acid | 0.200 | 0.539 | 0.524 | ||||
| 6 | Isoleucine | 0.314 | 0.234 | 0.035 | ||||
| 7 | N-(carboxyacetyl) phenylalanine | 0.161 | 0.407 | 0.285 | ||||
| 8 | Dopa | 0.550 | 0.690 | 0.640 | ||||
| 9 | Leucine | 0.109 | 0.0004 | −0.122 | ||||
| 10 | Phenylalanine | 0.012 | −0.314 | −0.302 | ||||
| 11 | Tryptophan | 0.005 | 0.353 | 0.179 | ||||
| 12 | Norleucine | 0.322 | 0.595 | 0.430 | ||||
| Betacyanins | ||||||||
| 13 | Betanidin-5-O-ß-glucuronosylglucoside (Amaranthin) | −0.019 | −0.382 | −0.353 | ||||
| 14 | Betanidin-5-O-ß-glucoside (Betanin) | −0.184 | −0.471 | −0.539 | ||||
| 15 | Isobetanidin-5-O-ß-glucuronosylglucoside (Isoamaranthin) | −0.529 | −0.585 | −0.671 | ||||
| 16 | Isobetanidin-5-O-ß-glucoside (Isobetanin) | −0.425 | −0.487 | −0.583 | ||||
| Fatty acids | ||||||||
| 17 | Tuberonic acid hexoside | 0.487 | 0.755 | 0.678 | ||||
| 18 | Tuberonic acid | −0.183 | −0.047 | −0.103 | ||||
| 19 | Trihydroxyoctadecadienoic acid | −0.267 | 0.068 | 0.239 | ||||
| 20 | Hydroperoxyoctadecadienoic acid | −0.172 | −0.432 | −0.296 | ||||
| 21 | Hydroxyoctadecatrienoic acid I | −0.092 | −0.202 | −0.037 | ||||
| 22 | Hydroxyoctadecatrienoic acid II | −0.217 | −0.118 | 0.103 | ||||
| 23 | Hydroxyoctadecatrienoic acid III | −0.162 | −0.213 | 0.008 | ||||
| 24 | Hydroxyoctadecadienoic acid I | −0.440 | −0.416 | −0.152 | ||||
| 25 | Hydroxyoctadecadienoic acid II | −0.633 | −0.663 | −0.681 | ||||
| Flavonoids | ||||||||
| 26 | Quercetin-O-hexoside | 0.590 | 0.775 | 0.500 | ||||
| 27 | Luteolin-O-rhamnosylhexoside | 0.464 | 0.474 | 0.277 | ||||
| 28 | Luteolin-6-C-hexoside (homoorientin) | 0.765 | 0.885 | 0.703 | ||||
| 29 | Phlorizin | 0.304 | 0.392 | 0.364 | ||||
| 30 | Luteolin-7-O-glucoside | 0.180 | −0.086 | −0.140 | ||||
| Organicacids | ||||||||
| 31 | Citric acid | −0.199 | −0.300 | −0.223 | ||||
| 32 | Mannonic acid | −0.394 | −0.593 | −0.625 | ||||
| 33 | Malic acid | −0.722 | −0.669 | −0.509 | ||||
| 34 | Maleic acid | −0.704 | −0.497 | −0.489 | ||||
| 35 | Succinic acid | 0.241 | 0.374 | 0.351 | ||||
| 36 | Glyceric acid | −0.408 | −0.340 | −0.245 | ||||
| 37 | 2-Oxopentanoic acid | 0.124 | −0.083 | −0.102 | ||||
| 38 | Fumaric acid | −0.296 | −0.031 | 0.056 | ||||
| Phenolicacids | ||||||||
| 39 | Gallic acid | −0.020 | −0.037 | −0.167 | ||||
| 40 | Gallic acid-O-hexoside | 0.629 | 0.649 | 0.541 | ||||
| 41 | Vanillic acid-O-hexoside | 0.069 | −0.140 | −0.041 | ||||
| 42 | Vanillic acid | 0.549 | 0.412 | 0.332 | ||||
| 43 | Protocatechuic acid-O-hexoside | 0.138 | 0.056 | 0.002 | ||||
| 44 | Dihydroxybenzoic acid | 0.535 | 0.427 | 0.339 | ||||
| 45 | Hydroxybenzoic acid | 0.708 | 0.772 | 0.546 | ||||
| 46 | Benzoic acid | 0.261 | −0.100 | −0.192 | ||||
| 47 | Ferulic acid | −0.107 | −0.085 | −0.061 | ||||
| 48 | p-Coumaric acid | 0.196 | 0.502 | 0.465 | ||||
| 49 | o-Coumaric acid | 0.167 | 0.439 | 0.427 | ||||
| 50 | Syringic acid | 0.209 | 0.156 | 0.115 | ||||
| 51 | Cinnamic acid | −0.024 | −0.083 | −0.105 | ||||
| 52 | Caffeic acid | 0.362 | 0.423 | 0.299 | ||||
| Gram-Positive Bacteria | Gram-Negative Bacteria | Fungal Strains | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microrganism/ Metabolite | S. aureus ATCC 29213 | S. aureus ATCC 25923 | S. aureus ATCC 6538 | S. aureus ATCC BAA-1707 | S. epidermidis ATCC 12228 | M. luteus ATCC 10240 | B. subtilis ATCC 6633 | B. cereus ATCC 10876 | E. coli ATCC 25922 | S. Typhimurium ATCC 14028 | P. aeruginosa ATCC 27853 | B. bronchiseptica ATCC 4617 | K. pneumoniae ATCC 13883 | C. albicans ATCC 10231 | C. glabrata ATCC 90030 | C. krusei ATCC 14243 | |
| MIC; MBC (MCB/MIC) | MIC; MBC (MCB/MIC) | MIC; MFC (MFC/MIC) | |||||||||||||||
| Red | H2O | 32; 32 (1) | 16; 32 (2) | 32; 32 (1) | 32; 32 (1) | 16; 32 (2) | 8; 32 (4) | 16; 16 (1) | 16; 16 (1) | 16; 16 (1) | 16; 32 (2) | 8; 16 (2) | 4; 16 (4) | 16; 32 (2) | 16; 16 (1) | 16; 32 (2) | 16; 6 (1) |
| EtOH | 16; 16 (1) | 32; 32 (1) | 16; 32 (2) | 32; 32 (1) | 32; 32 (1) | 8; 32 (4) | 16; 16 (1) | 16; 16 (1) | 16; 16 (1) | 16; 32 (2) | 8; 16 (2) | 4; 8 (2) | 16; 32 (2) | 8; 8 (1) | 16; 16 (1) | 8; 8 (1) | |
| Ac | 4; 32 (8) | 8; 16 (2) | 4; 32 (8) | 32; 32 (1) | 32; 32 (1) | 8; 16 (2) | 8; 16 (2) | 4; 16 (4) | 16; 16 (1) | 32; 32 (1) | 16; 16 (1) | 4; 8 (2) | 16; 32 (2) | 16; 16 (1) | 16; 16 (1) | 8; 8 (1) | |
| Passion | H2O | 16; 32 (2) | 16; 32 (2) | 16; 32 (2) | 16; 32 (2) | 16; 32 (2) | 4; 32 (8) | 8; 32 (4) | 16; 32 (2) | 16; 32 (2) | 16; 32 (2) | 16; 32 (2) | 8; 32 (4) | 16; 32 (2) | 16; 16 (1) | 16; 32 (2) | 16; 32 (2) |
| EtOH | 32; 32 (1) | 8; 32 (4) | 32; 32 (1) | 32; 32 (1) | 32; 32 (1) | 8; 32 (4) | 16; 16 (1) | 8; 16 (2) | 16; 16 (1) | 32; 32 (1) | 8; 16 (2) | 8; 8 (1) | 32; 32 (1) | 8; 8 (1) | 8; 16 (2) | 8; 8 (1) | |
| Ac | 16; 16 (1) | 16; 32 (2) | 32; 32 (1) | 16; 16 (1) | 32; 32 (1) | 32; 32 (1) | 16; 16 (1) | 4; 16 (4) | 16; 16 (1) | 32; 32 (1) | 16; 16 (1) | 8; 16 (2) | 32; 32 (1) | 8; 8 (1) | 8; 16 (2) | 8; 16 (2) | |
| Green | H2O | 32; 32 (1) | 32; 32 (1) | 32; 32 (1) | 16; 32 (2) | 16; 32 (2) | 8; 32 (4) | 16; 16 (1) | 16; 16 (1) | 16; 16 (1) | 16; 32 (2) | 8; 16 (2) | 4; 16 (4) | 32; 32 (1) | 8; 16 (2) | 8; 16 (2) | 8; 16 (2) |
| EtOH | 16; 16 (1) | 16; 16 (1) | 16; 32 (2) | 16; 16 (1) | 16; 16 (1) | 8; 32 (4) | 16; 16 (1) | 16; 16 (1) | 16; 16 (1) | 16; 32 (2) | 8; 16 (2) | 4; 16 (4) | 16; 32 (2) | 8; 8 (1) | 8; 16 (2) | 8; 16 (2) | |
| Ac | 16; 16 (1) | 8; 32 (4) | 8; 32 (4) | 8; 16 (2) | 8; 32 (4) | 32; 32 (1) | 8; 16 (2) | 8; 16 (2) | 16; 16 (1) | 16; 32 (2) | 8; 16 (2) | 16; 16 (1) | 16; 32 (2) | 8; 8 (1) | 8; 16 (2) | 16; 16 (1) | |
| Correlation | |||||||||||||||||
| Amino acids | |||||||||||||||||
| Serine | 0.096 | 0.367 | 0.348 | 0.595 | 0.524 | −0.449 | −0.117 | −0.167 | 0.199 | 0.473 | 0.199 | 0.387 | 0.061 | 0.472 | −0.383 | −0.270 | |
| Aspartic acid | 0.047 | 0.461 | 0.328 | 0.547 | 0.502 | −0.401 | −0.138 | −0.160 | 0.263 | 0.456 | 0.263 | 0.260 | 0.031 | 0.454 | −0.510 | −0.404 | |
| Tyrosine | 0.317 | 0.350 | −0.017 | −0.302 | 0.440 | 0.291 | 0.280 | −0.069 | 0.359 | −0.053 | 0.359 | −0.184 | −0.201 | −0.053 | −0.376 | 0.093 | |
| N-benzoylaspartic acid | −0.431 | 0.068 | 0.082 | −0.304 | −0.319 | 0.199 | −0.141 | 0.281 | 0.037 | −0.055 | 0.037 | −0.285 | 0.071 | 0.016 | −0.072 | −0.389 | |
| Glutamic acid | −0.252 | −0.151 | −0.109 | −0.250 | 0.065 | −0.107 | 0.576 | −0.060 | −0.716 | 0.127 | −0.716 | 0.127 | −0.375 | −0.387 | 0.741 | 0.603 | |
| Isoleucine | −0.331 | −0.331 | 0.180 | 0.142 | −0.547 | −0.125 | −0.296 | 0.326 | −0.376 | −0.030 | −0.376 | 0.090 | 0.271 | 0.150 | 0.391 | −0.133 | |
| N-(carboxyacetyl) phenylalanine | −0.424 | −0.524 | −0.295 | −0.071 | −0.259 | −0.110 | 0.242 | −0.154 | −0.650 | −0.047 | −0.650 | 0.237 | 0.027 | −0.544 | 0.839 | 0.601 | |
| Dopa | 0.425 | −0.125 | 0.074 | −0.235 | −0.442 | 0.372 | −0.015 | 0.324 | −0.371 | −0.462 | −0.371 | −0.193 | −0.185 | −0.065 | 0.320 | 0.264 | |
| Leucine | 0.185 | −0.332 | −0.075 | 0.272 | −0.684 | 0.144 | −0.675 | 0.206 | 0.211 | −0.456 | 0.211 | −0.231 | 0.502 | 0.127 | −0.220 | −0.361 | |
| Phenylalanine | −0.555 | −0.384 | −0.070 | −0.211 | −0.139 | −0.078 | −0.227 | −0.122 | 0.165 | 0.200 | 0.165 | 0.490 | 0.143 | −0.015 | 0.385 | −0.036 | |
| Tryptophan | −0.296 | −0.368 | −0.321 | 0.180 | −0.358 | −0.128 | 0.141 | −0.061 | −0.587 | −0.178 | −0.587 | −0.178 | 0.194 | −0.495 | 0.422 | 0.321 | |
| Norleucine | −0.082 | −0.159 | 0.012 | 0.002 | −0.399 | 0.030 | 0.144 | 0.242 | −0.685 | −0.202 | −0.685 | −0.202 | −0.047 | −0.214 | 0.450 | 0.242 | |
| Betacyanins | |||||||||||||||||
| Betanidin-5-O-ß-glucuronosylglucoside (Amaranthin) | −0.404 | 0.331 | 0.439 | 0.055 | 0.429 | −0.377 | 0.221 | 0.377 | 0.203 | 0.608 | 0.203 | −0.132 | 0.190 | 0.611 | −0.383 | −0.491 | |
| Betanidin-5-O-ß-glucoside (Betanin) | −0.285 | 0.304 | 0.499 | 0.516 | 0.509 | −0.570 | 0.020 | 0.171 | 0.162 | 0.659 | 0.162 | 0.201 | 0.344 | 0.654 | −0.387 | −0.423 | |
| Isobetanidin-5-O-ß-glucuronosylglucoside (Isoamaranthin) | −0.179 | 0.272 | 0.138 | 0.491 | 0.054 | −0.249 | −0.476 | −0.233 | 0.259 | 0.223 | 0.259 | 0.223 | 0.118 | 0.223 | −0.414 | −0.557 | |
| Isobetanidin-5-O-ß-glucoside (Isobetanin) | −0.103 | 0.202 | 0.318 | 0.752 | 0.307 | −0.558 | −0.329 | −0.170 | 0.157 | 0.477 | 0.157 | 0.450 | 0.249 | 0.477 | −0.314 | −0.397 | |
| Fatty acids | |||||||||||||||||
| Tuberonic acid hexoside | 0.483 | 0.029 | 0.049 | −0.175 | −0.324 | 0.382 | 0.178 | 0.316 | −0.493 | −0.494 | −0.493 | −0.392 | −0.213 | −0.185 | 0.211 | 0.341 | |
| Tuberonic acid | −0.155 | −0.660 | −0.466 | 0.347 | −0.504 | −0.221 | −0.398 | −0.200 | 0.066 | −0.202 | 0.066 | 0.009 | 0.493 | −0.210 | 0.136 | −0.027 | |
| Trihydroxyoctadecadienoic acid | 0.011 | −0.487 | −0.606 | 0.034 | 0.004 | −0.249 | 0.163 | −0.401 | 0.079 | −0.006 | 0.079 | 0.004 | 0.025 | −0.340 | 0.195 | 0.308 | |
| Hydroperoxyoctadecadienoic acid | −0.381 | 0.196 | 0.039 | −0.249 | 0.259 | −0.115 | 0.164 | 0.112 | 0.372 | 0.347 | 0.372 | −0.228 | 0.016 | 0.220 | −0.343 | −0.407 | |
| Hydroxyoctadecatrienoic acid I | 0.115 | 0.363 | −0.050 | −0.357 | 0.336 | 0.227 | 0.308 | 0.061 | 0.364 | 0.001 | 0.364 | −0.444 | −0.155 | −0.008 | −0.477 | −0.131 | |
| Hydroxyoctadecatrienoic acid II | 0.302 | 0.202 | −0.319 | −0.270 | 0.377 | 0.188 | 0.372 | −0.179 | 0.376 | −0.101 | 0.376 | −0.417 | −0.170 | −0.221 | −0.424 | 0.110 | |
| Hydroxyoctadecatrienoic acid III | 0.176 | 0.118 | −0.300 | −0.349 | 0.321 | 0.211 | 0.294 | −0.132 | 0.469 | −0.078 | 0.469 | −0.359 | −0.077 | −0.175 | −0.391 | 0.050 | |
| Hydroxyoctadecadienoic acid I | 0.062 | 0.430 | −0.045 | −0.161 | 0.643 | −0.127 | 0.284 | −0.291 | 0.442 | 0.384 | 0.442 | 0.019 | −0.404 | 0.125 | −0.411 | −0.171 | |
| Hydroxyoctadecatdienoic acid II | −0.078 | 0.352 | −0.017 | 0.462 | 0.079 | −0.199 | −0.376 | −0.145 | 0.480 | 0.119 | 0.480 | −0.272 | 0.242 | 0.210 | −0.818 | −0.729 | |
| Flavonoids | |||||||||||||||||
| Quercetin-O-hexoside | −0.053 | 0.190 | 0.346 | −0.179 | −0.243 | 0.289 | 0.372 | 0.421 | −0.996 | −0.235 | −0.996 | −0.172 | −0.253 | −0.264 | 0.568 | 0.439 | |
| Luteolin-O-rhamnosylhexoside | −0.060 | −0.071 | 0.336 | −0.033 | −0.116 | 0.082 | 0.074 | 0.135 | −0.660 | −0.018 | −0.660 | 0.476 | −0.160 | −0.071 | 0.706 | 0.461 | |
| Luteolin-6-C-hexoside (homoorientin) | 0.306 | 0.265 | 0.482 | −0.306 | −0.359 | 0.440 | 0.200 | 0.615 | −0.788 | −0.354 | −0.788 | −0.268 | −0.392 | 0.037 | 0.403 | 0.242 | |
| Phlorizin | −0.311 | 0.048 | 0.249 | −0.326 | 0.023 | −0.059 | 0.324 | 0.123 | −0.596 | 0.262 | −0.596 | 0.316 | −0.483 | −0.015 | 0.678 | 0.266 | |
| Luteolin-7-O-glucoside | 0.106 | −0.090 | 0.344 | 0.167 | 0.050 | −0.062 | −0.342 | −0.019 | 0.088 | 0.138 | 0.088 | 0.694 | 0.064 | 0.343 | 0.222 | 0.046 | |
| Organic acids | |||||||||||||||||
| Citric acid | −0.121 | 0.420 | 0.022 | −0.206 | 0.185 | 0.109 | 0.164 | 0.125 | 0.270 | 0.073 | 0.270 | −0.509 | −0.077 | 0.058 | −0.534 | −0.396 | |
| Mannonic acid | −0.169 | 0.351 | 0.383 | 0.487 | 0.342 | −0.422 | −0.303 | −0.078 | 0.309 | 0.509 | 0.309 | 0.351 | 0.116 | 0.552 | −0.424 | −0.559 | |
| Malic acid | 0.142 | 0.042 | −0.297 | 0.486 | 0.473 | −0.384 | −0.142 | −0.524 | 0.677 | 0.255 | 0.677 | 0.118 | 0.236 | 0.094 | −0.620 | −0.231 | |
| Maleic acid | −0.130 | −0.437 | −0.615 | 0.660 | 0.017 | −0.455 | −0.258 | −0.575 | 0.372 | 0.026 | 0.372 | 0.030 | 0.590 | −0.277 | −0.299 | −0.058 | |
| Succinic acid | −0.170 | −0.622 | −0.255 | −0.130 | −0.321 | −0.047 | −0.007 | −0.161 | −0.364 | −0.093 | −0.364 | 0.433 | −0.023 | −0.319 | 0.811 | 0.517 | |
| Glyceric acid | −0.274 | −0.366 | −0.127 | 0.397 | 0.301 | −0.661 | −0.142 | −0.536 | 0.135 | 0.567 | 0.135 | 0.879 | 0.032 | 0.150 | 0.346 | 0.100 | |
| 2-Oxopentanoic acid | −0.004 | 0.148 | 0.562 | 0.232 | 0.190 | −0.320 | −0.211 | 0.149 | −0.002 | 0.451 | −0.002 | 0.588 | −0.095 | 0.659 | 0.106 | −0.231 | |
| Fumaric acid | 0.033 | 0.195 | 0.115 | 0.302 | 0.624 | −0.505 | 0.334 | −0.338 | −0.168 | 0.547 | −0.168 | 0.494 | −0.331 | 0.145 | 0.145 | 0.222 | |
| Phenolic acids | |||||||||||||||||
| Gallic acid | 0.150 | −0.223 | −0.071 | 0.399 | −0.615 | 0.021 | −0.609 | 0.208 | 0.173 | −0.374 | 0.173 | −0.365 | 0.507 | 0.159 | −0.357 | −0.466 | |
| Gallic acid-O-hexoside | 0.122 | 0.003 | 0.363 | −0.249 | −0.413 | 0.227 | 0.004 | 0.505 | −0.532 | −0.191 | −0.532 | −0.071 | −0.243 | 0.167 | 0.431 | 0.081 | |
| Vanillic acid-O-hexoside | 0.119 | −0.378 | 0.055 | 0.076 | −0.081 | −0.151 | −0.444 | −0.139 | 0.344 | 0.128 | 0.344 | 0.665 | 0.067 | 0.347 | 0.228 | −0.036 | |
| Vanillic acid | 0.078 | −0.143 | 0.411 | −0.087 | −0.179 | 0.027 | −0.096 | 0.304 | −0.340 | 0.041 | −0.340 | 0.432 | −0.111 | 0.303 | 0.532 | 0.185 | |
| Protocatechuic acid-O-hexoside | −0.192 | −0.329 | 0.097 | 0.065 | 0.080 | −0.186 | −0.060 | −0.225 | −0.222 | 0.229 | −0.222 | 0.837 | −0.026 | −0.022 | 0.668 | 0.418 | |
| Dihydroxybenzoic acid | 0.225 | −0.225 | 0.268 | −0.119 | −0.361 | 0.221 | −0.268 | 0.213 | −0.259 | −0.214 | −0.259 | 0.398 | −0.094 | 0.141 | 0.509 | 0.223 | |
| Hydroxybenzoic acid | 0.108 | 0.012 | 0.389 | −0.202 | −0.364 | 0.323 | 0.109 | 0.420 | −0.806 | −0.279 | −0.806 | 0.086 | −0.236 | −0.082 | 0.654 | 0.410 | |
| Benzoic acid | −0.249 | 0.272 | 0.581 | −0.092 | −0.129 | 0.012 | −0.249 | 0.442 | −0.031 | 0.223 | −0.031 | 0.116 | −0.051 | 0.538 | −0.028 | −0.492 | |
| Ferulic acid | −0.356 | −0.260 | 0.052 | 0.131 | 0.243 | −0.454 | 0.034 | −0.337 | −0.184 | 0.506 | −0.184 | 0.859 | −0.145 | 0.066 | 0.607 | 0.267 | |
| p-Coumaric acid | −0.137 | −0.098 | −0.014 | −0.141 | 0.217 | −0.114 | 0.553 | −0.154 | −0.708 | 0.164 | −0.708 | 0.366 | −0.382 | −0.357 | 0.769 | 0.727 | |
| o-Coumaric acid | −0.208 | −0.185 | −0.047 | −0.149 | 0.213 | −0.175 | 0.508 | −0.201 | −0.648 | 0.227 | −0.648 | 0.469 | −0.363 | −0.331 | 0.824 | 0.710 | |
| Syringic acid | 0.429 | 0.037 | 0.190 | 0.147 | −0.466 | 0.173 | −0.479 | 0.378 | 0.162 | −0.335 | 0.162 | −0.334 | 0.140 | 0.388 | −0.350 | −0.448 | |
| Cinnamic acid | −0.660 | −0.206 | −0.004 | 0.104 | 0.104 | −0.532 | 0.340 | 0.224 | −0.173 | 0.476 | −0.173 | −0.185 | 0.342 | 0.157 | 0.098 | −0.134 | |
| Caffeic acid | −0.021 | −0.077 | 0.269 | −0.030 | 0.061 | −0.010 | 0.168 | −0.006 | −0.585 | 0.092 | −0.585 | 0.586 | −0.242 | −0.077 | 0.715 | 0.543 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spórna-Kucab, A.; Tekieli, A.; Kisiel, A.; Grzegorczyk, A.; Skalicka-Woźniak, K.; Starzak, K.; Wybraniec, S. Antioxidant and Antimicrobial Effects of Baby Leaves of Amaranthus tricolor L. Harvested as Vegetable in Correlation with Their Phytochemical Composition. Molecules 2023, 28, 1463. https://doi.org/10.3390/molecules28031463
Spórna-Kucab A, Tekieli A, Kisiel A, Grzegorczyk A, Skalicka-Woźniak K, Starzak K, Wybraniec S. Antioxidant and Antimicrobial Effects of Baby Leaves of Amaranthus tricolor L. Harvested as Vegetable in Correlation with Their Phytochemical Composition. Molecules. 2023; 28(3):1463. https://doi.org/10.3390/molecules28031463
Chicago/Turabian StyleSpórna-Kucab, Aneta, Anna Tekieli, Aneta Kisiel, Agnieszka Grzegorczyk, Krystyna Skalicka-Woźniak, Karolina Starzak, and Sławomir Wybraniec. 2023. "Antioxidant and Antimicrobial Effects of Baby Leaves of Amaranthus tricolor L. Harvested as Vegetable in Correlation with Their Phytochemical Composition" Molecules 28, no. 3: 1463. https://doi.org/10.3390/molecules28031463