CdS Deposited In Situ on g-C3N4 via a Modified Chemical Bath Deposition Method to Improve Photocatalytic Hydrogen Production
Abstract
:1. Introduction
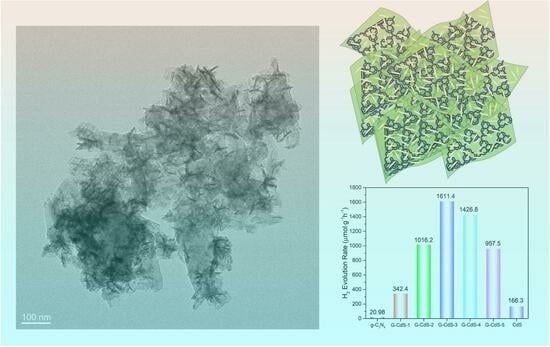
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Synthesis of g-C3N4 Nanosheets
3.3. Synthesis of G-CdS Heterojunction
3.4. Characterization
3.5. Evaluation of Photocatalytic H2 Production Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dillon, M.E.; Wang, G.; Huey, R.B. Global metabolic impacts of recent climate warming. Nature 2010, 467, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.J.; Yu, H.G.; Yu, J.G. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Shi, R.; Waterhouse, I.; Tang, J.; Zhang, T. Two-dimensional photocatalyst design: A critical review of recent experimental and computational advances. Mater. Today 2020, 34, 78–91. [Google Scholar] [CrossRef]
- Ma, L.; Ai, X.; Yang, X.; Cao, X.; Han, D.; Song, X.; Jiang, H.; Yang, W.; Yan, S.; Wu, X. Cd (II)-based metal-organic framework-derived CdS photocatalysts for enhancement of photocatalytic activity. J. Mater. Sci. 2021, 56, 8643–8657. [Google Scholar] [CrossRef]
- Ma, L.; Ai, X.; Jiang, W.; Liu, P.; Chen, Y.; Lu, K.; Song, X.; Wu, X. Zn/Ce metal-organic framework-derived ZnO@CeO2 nano-heterojunction for enhanced photocatalytic activity. Colloid Interface Sci. Commun. 2022, 49, 100636. [Google Scholar] [CrossRef]
- Ma, L.; Ai, X.; Chen, Y.; Liu, P.; Lin, C.; Lu, K.; Jiang, W.; Wu, J.; Song, X. Improved photocatalytic activity via N-Type ZnO/p-Type NiO heterojunctions. Nanomaterials 2022, 12, 3665. [Google Scholar] [CrossRef]
- Xiao, N.; Li, S.; Li, X.; Ge, L.; Gao, Y.; Li, N. The roles and mechanism of cocatalysts in photocatalytic water splitting to produce hydrogen. Chin. J. Catal. 2020, 41, 642–671. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Dutta, S.; Chowdhury, B.; Bhaumik, A. Catalytic reduction of CO2 into fuels and fine chemicals. Green Chem. 2020, 22, 4002–4033. [Google Scholar] [CrossRef]
- Song, Y.; Johnson, D.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.; Huang, J.; Yang, F.; Zhang, F.; Qiao, R.; et al. A physical catalyst for the electrolysis of nitrogen to ammonia. Sci. Adv. 2018, 4, e1700336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In situ irradiated XPS investigation on S-scheme TiO2@ ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Yang, G.; Li, S.; Xiao, N.; Li, N.; Gao, Y.; Lv, D.; Ge, L. Novel dual co-catalysts decorated Au@ HCS@ PdS hybrids with spatially separated charge carriers and enhanced photocatalytic hydrogen evolution activity. Chem. Eng. J. 2020, 379, 122350. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal sulfide photocatalysts for hydrogen generation: A review of recent advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Lu, M.; Pei, Z.; Weng, S.; Feng, W.; Fang, Z.; Zheng, Z.; Huang, M.; Liu, P. Constructing atomic layer g-C3N4-CdS nanoheterojunctions with efficiently enhanced visible light photocatalytic activity. Phys. Chem. Chem. Phys. 2014, 16, 21280–21288. [Google Scholar] [CrossRef]
- Soheila, A.; Aziz, H. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review. J. Clean. Prod. 2020, 276, 124319. [Google Scholar]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Liu, Y.; Zhao, Y.; Xie, C.; Song, Y. Crystallinity and phase controlling of g-C3N4/CdS hetrostructures towards high efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 2019, 44, 30151–30159. [Google Scholar] [CrossRef]
- Ghosh, U.; Majumdar, A.; Pal, A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures: Recent progress and prospects. J. Environ. Chem. Eng. 2021, 9, 104631. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.; Ismail, A.; Bahnemann, D. Soft and hard templates assisted synthesis mesoporous CuO/g-C3N4 heterostructures for highly enhanced and accelerated Hg (II) photoreduction under visible light. J. Colloid Interface Sci. 2020, 580, 223–233. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Z.; Qiu, B.; Xing, M.; Zhang, J. Emerging cocatalysts on g-C3N4 for photocatalytic hydrogen evolution. Small 2021, 17, 2101070. [Google Scholar] [CrossRef]
- Ran, Y.; Cui, Y.; Zhang, Y.; Fang, Y.; Zhang, W.; Yu, X.; Lan, H.; An, X. Assembly-synthesis of puff pastry-like g-C3N4/CdS heterostructure as S-junctions for efficient photocatalytic water splitting. Chem. Eng. J. 2022, 431, 133348. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Sun, Y.; Yin, X.; Chen, R.; Yin, G.; Sun, M.; Liu, B. The 2D porous g-C3N4/CdS heterostructural nanocomposites with enhanced visible-light-driven photocatalytic activity. J. Nanosci. Nanotechnol. 2020, 20, 1098–1108. [Google Scholar] [CrossRef]
- Liu, J. Origin of high photocatalytic efficiency in monolayer g-C3N4/CdS heterostructure: A hybrid DFT study. J. Phys. Chem. C 2015, 119, 28417–28423. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Zhao, H.; Yan, J.; Zhu, X.; Yang, D. Fabrication of single-layer graphitic carbon nitride and coupled systems for the photocatalytic degradation of dyes under visible-light irradiation. Eur. J. Inorg. Chem. 2015, 2015, 1359–1367. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.; Huang, Y.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z.; et al. Carbon quantum dot implanted graphite carbon nitride nanotubes: Excellent charge separation and enhanced photocatalytic hydrogen evolution. Angew. Chem. 2018, 130, 5867–5873. [Google Scholar] [CrossRef]
- Tan, M.; Ma, Y.; Yu, C.; Luan, Q.; Li, J.; Liu, C.; Dong, W.; Su, Y.; Qiao, L.; Gao, L.; et al. Boosting photocatalytic hydrogen production via interfacial engineering on 2D ultrathin Z-scheme ZnIn2S4/g-C3N4 heterojunction. Adv. Funct. Mater. 2022, 32, 2111740. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Feng, Y.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 277, 119254. [Google Scholar] [CrossRef]
- Gao, B.; Liu, L.; Liu, J.; Yang, F. Photocatalytic degradation of 2, 4, 6-tribromophenol over Fe-doped ZnIn2S4: Stable activity and enhanced debromination. Appl. Catal. B Environ. 2013, 129, 89–97. [Google Scholar] [CrossRef]
- Devamani, R.H.P.; Kiruthika, R.; Mahadevi, P.; Sagithapriya, S. Synthesis and characterization of cadmium sulfide nanoparticles. Int. J. Innov. Sci. Eng. Technol. 2017, 4, 181–185. [Google Scholar]
- Dastan, D.; Panahi, S.L.; Chaure, N.B. Characterization of titania thin films grown by dip-coating technique. J. Mater. Sci. Mater. Electron. 2016, 27, 12291–12296. [Google Scholar] [CrossRef]
- Dastan, D.; Chaure, N.; Kartha, M. Surfactants assisted solvothermal derived titania nanoparticles: Synthesis and simulation. J. Mater. Sci. Mater. Electron. 2017, 28, 7784–7796. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zhu, X.; Ai, C.; Zeng, Y.; Shi, W.; Zhang, X.; Zhang, H.; Si, H.; Li, J.; et al. Photodepositing CdS on the active cyano groups decorated g-C3N4 in Z-scheme manner promotes visible-light-driven hydrogen evolution. Small 2021, 17, 2102699. [Google Scholar] [CrossRef]
- Li, H.; An, M.; Zhao, Y.; Pi, S.; Li, C.; Sun, W.; Wang, H. Co nanoparticles encapsulated in N-doped carbon nanofibers as bifunctional catalysts for rechargeable Zn-air battery. Appl. Surf. Sci. 2019, 478, 560–566. [Google Scholar] [CrossRef]
- Madhurima, V.; Kumari, K.; Jain, P. A facile single-step approach to achieve in situ expanded g-C3N4 for improved photodegradation performance. Polym. Adv. Technol. 2023, 34, 578–586. [Google Scholar] [CrossRef]
- Ma, L.; Liu, W.; Cai, H.; Zhang, F.; Wu, X. Catalyst-and template-free low-temperature in situ growth of n-type CdS nanowire on p-type CdTe film and p-n heterojunction properties. Sci. Rep. 2016, 6, 38858. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xiu, Z.; Xie, Z.; Li, H.; Zhao, G.; Wang, W.; Wu, Y.; Hao, X. Reduced graphene oxide-CdS nanocomposites with enhanced visible-light photoactivity synthesized using ionic-liquid precursors. New J. Chem. 2014, 38, 4312–4320. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Güy, N. Directional transfer of photocarriers on CdS/g-C3N4 heterojunction modified with Pd as a cocatalyst for synergistically enhanced photocatalytic hydrogen production. Appl. Surf. Sci. 2020, 522, 146442. [Google Scholar] [CrossRef]
- Jiang, W.; Zong, X.; An, L.; Hua, S.; Miao, X.; Luan, S.; Wen, Y.; Tao, F.; Sun, Z. Consciously constructing heterojunction or direct Z-scheme photocatalysts by regulating electron flow direction. ACS Catal. 2018, 8, 2209–2217. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Zhao, J.; Zhu, Y.; Li, Y.; Deng, W.; Wang, C. Enhanced photocatalytic H2 evolution over CdS/Au/g-C3N4 composite photocatalyst under visible-light irradiation. APL Mater. 2015, 3, 104410. [Google Scholar] [CrossRef]
- Ji, C.; Du, C.; Steinkruger, J.; Zhou, C.; Yang, S. In-situ hydrothermal fabrication of CdS/g-C3N4 nanocomposites for enhanced photocatalytic water splitting. Mater. Lett. 2019, 240, 128–131. [Google Scholar] [CrossRef]
- Wu, H.; Meng, S.; Zhang, J.; Zhang, X.; Wang, Y.; Chen, S.; Qi, G.; Fu, X. Construction of two-dimensionally relative p-n heterojunction for efficient photocatalytic redox reactions under visible light. Appl. Surf. Sci. 2020, 505, 144638. [Google Scholar] [CrossRef]
- Ma, L.; Ai, X.; Wu, X. Effect of substrate and Zn doping on the structural, optical and electrical properties of CdS thin films prepared by CBD method. J. Alloys Compd. 2017, 691, 399–406. [Google Scholar] [CrossRef]












| Catalyst | Synthesis Method | Dosage (mg) | Type of Light Source | Sacrificial Reagent | H2 Evolution Rate (μmol·g−1·h−1) | Refs. |
|---|---|---|---|---|---|---|
| Pd-CdS/g-C3N4 | hydrothermal method and borohydride reduction method | 50 | 300 W Xe arc Lamp (λ > 400 nm) | 0.5 M Na2S and 0.5 M Na2SO3 | 293.0 | [44] |
| CdS/g-C3N4 | photodeposited method | 10 | 300 W Xe arc Lamp (λ > 420 nm) | 0.5 M Na2S and 0.5 M Na2SO3 | 56.9 | [45] |
| CdS/Au/g-C3N4 | photodeposited method | 100 | Xenon Lamp (λ > 420 nm) | 10 mL methanol | 19.02 | [46] |
| CdS/g-C3N4 | hydrothermal synthesis | 10 | 300 W Xe arc Lamp (λ > 400 nm) | 20 mL triethanolamine | 216.48 | [47] |
| G-CdS | modified CBD method | 50 | 300 W Xe arc Lamp (λ > 420 nm) | 20% lactic acid aqueous solution | 1611.4 μmol·g−1·h−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Jiang, W.; Lin, C.; Xu, L.; Zhu, T.; Ai, X. CdS Deposited In Situ on g-C3N4 via a Modified Chemical Bath Deposition Method to Improve Photocatalytic Hydrogen Production. Molecules 2023, 28, 7846. https://doi.org/10.3390/molecules28237846
Ma L, Jiang W, Lin C, Xu L, Zhu T, Ai X. CdS Deposited In Situ on g-C3N4 via a Modified Chemical Bath Deposition Method to Improve Photocatalytic Hydrogen Production. Molecules. 2023; 28(23):7846. https://doi.org/10.3390/molecules28237846
Chicago/Turabian StyleMa, Ligang, Wenjun Jiang, Chao Lin, Le Xu, Tianyu Zhu, and Xiaoqian Ai. 2023. "CdS Deposited In Situ on g-C3N4 via a Modified Chemical Bath Deposition Method to Improve Photocatalytic Hydrogen Production" Molecules 28, no. 23: 7846. https://doi.org/10.3390/molecules28237846