Effects of the Supercritical Fluid Extract of Magnolia figo on Inducing the Apoptosis of Human Non-Small-Cell Lung Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of FMO on A549 Cell Viability
2.2. TUNEL Staining Revealed Apoptosis in A549 Cells Induced by FMO
2.3. Annexin V Assay Revealed FMO-Induced Apoptosis in A549 Cells
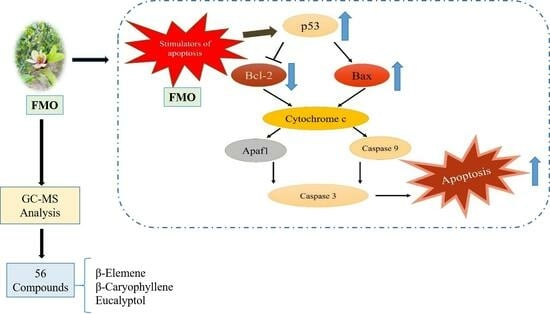
2.4. Effects of FMO on A549 Cell Apoptosis-Related Proteins
2.5. FMO GC-MS Composition Analysis
3. Discussion
4. Materials and Methods
4.1. FMO Extraction and Preparation Methods
4.2. Cell Culturing
4.3. Cell Viability Analysis
4.4. Annexin V Apoptosis Assay (Cell Surface Phosphatidylserine Analysis)
4.5. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay
4.6. Western Blotting
4.7. Gas Chromatography/Mass Spectrometry Composition Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, A.K.; Husain, N.; Shukla, S.; Pandey, R.K.; Kant, S.; Bala, L. Impact of glutathione S transferases P1 (Ile105Val) variants on the risk of GSTp, phosphorylated c-Jun kinase, and P53 phenotypic expression and their implications on overall survival outcomes in non-small cell lung cancer patients treated with chemotherapy. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2022, 824, 111775. [Google Scholar]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.C.; Mauer, A.M.; Vokes, E.E. Lung cancer. Lancet 2000, 355, 479–485. [Google Scholar] [CrossRef]
- Strasser, A.; Harris, A.W.; Huang, D.C.; Krammer, P.H.; Cory, S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995, 14, 6136–6147. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.L.; Strasser, A. The essential role of evasion from cell death in cancer. Adv. Cancer Res. 2011, 111, 39–96. [Google Scholar] [PubMed]
- Vaseva, A.V.; Marchenko, N.D.; Ji, K.; Tsirka, S.E.; Holzmann, S.; Moll, U.M. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 2012, 149, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Huai-zhou, Z.; Ying, W.; Ru-zhu, H. MS Analysis of the Volatile Oil Compositions of the Leaf and Flower of Michelia figo. J. Fujian For. Sci. Technol. 2011, 38, 5653–5671. [Google Scholar]
- Chericoni, S.; Testai, L.; Campeol, E.; Calderone, V.; Morelli, I.; Martinotti, E. Vasodilator activity of Michelia figo Spreng. (Magnoliaceae) by in vitro functional study. J. Ethnopharmacol. 2004, 91, 263–266. [Google Scholar] [CrossRef]
- Yao, T.; Song, J.; Yan, H.; Chen, S. Functionalized aqueous biphasic system coupled with HPLC for highly sensitive detection of quinolones in milk. LWT 2023, 173, 114398. [Google Scholar] [CrossRef]
- Yao, T.; Feng, C.; Chen, W.; Chen, S. Selective separation and simultaneous recoveries of amino acids by temperature-sensitive magnetic ionic liquid aqueous biphasic system. J. Mol. Liq. 2023, 371, 121099. [Google Scholar] [CrossRef]
- Yao, T.; Li, Q.; Li, H.; Peng, L.; Liu, Y.; Du, K. Extractive resolution of racemic phenylalanine and preparation of optically pure product by chiral magnetic ionic liquid aqueous two-phase system. Sep. Purif. Technol. 2021, 274, 119024. [Google Scholar] [CrossRef]
- Yao, T.; Li, H.; Ren, Y.; Feng, M.; Hu, Y.; Yan, H.; Peng, L. Extraction and recovery of phenolic compounds from aqueous solution by thermo-separating magnetic ionic liquid aqueous two-phase system. Sep. Purif. Technol. 2022, 282, 120034. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 Extraction and Purification of Compounds with Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef] [PubMed]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Mirgayazova, R.; Khadiullina, R.; Mingaleeva, R.; Chasov, V.; Gomzikova, M.; Garanina, E.; Rizvanov, A.; Bulatov, E. Novel Isatin-based activator of p53 transcriptional functions in tumor cells. Mol. Biol. Res. Commun. 2019, 8, 119–128. [Google Scholar]
- Basu, A.; Haldar, S. The relationship between BcI2, Bax and p53: Consequences for cell cycle progression and cell death. Mol. Hum. Reprod. 1998, 4, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar]
- Tan, T.; Li, J.; Luo, R.; Wang, R.; Yin, L.; Liu, M.; Zeng, Y.; Zeng, Z.; Xie, T. Recent Advances in Understanding the Mechanisms of Elemene in Reversing Drug Resistance in Tumor Cells: A Review. Molecules 2021, 26, 5792. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Zhang, N.; Han, X.; Li, Q.; Zhang, M.; Chen, X.; Li, G.; Zhang, R.; Chen, P.; Wang, W.; et al. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: A review. Biomed. Pharmacother. 2019, 114, 108812. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, L.; Qu, X.; Zhao, M.; Yu, P.; Kang, J.; Liu, Y.; Hu, X. Cbl-regulated Akt and ERK signals are involved in β-elemene-induced cell apoptosis in lung cancer cells. Mol. Med. Rep. 2011, 4, 1243–1246. [Google Scholar] [PubMed]
- Fang, Y.; Kang, Y.; Zou, H.; Cheng, X.; Xie, T.; Shi, L.; Zhang, H. β-elemene attenuates macrophage activation and proinflammatory factor production via crosstalk with Wnt/β-catenin signaling pathway. Fitoterapia 2018, 124, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Structure and function of SARS-CoV and SARS-CoV-2 main proteases and their inhibition: A comprehensive review. Eur. J. Med. Chem. 2023, 260, 115772. [Google Scholar] [CrossRef]
- Shabana, S.M.; Gad, N.S.; Othman, A.I.; Mohamed, A.F.; El-Missiry, M.A. β-caryophyllene oxide induces apoptosis and inhibits proliferation of A549 lung cancer cells. Med. Oncol. 2023, 40, 189. [Google Scholar] [CrossRef]








| No. | Retention Time | Formula | Type | Compound Name |
|---|---|---|---|---|
| 1 | 5.400 | C15H24 | Sesquiterpenes | β-Elemene |
| 2 | 5.620 | C15H24 | Sesquiterpenes | γ-Elemene |
| 3 | 6.716 | C15H24O | Sesquiterpenols | Caryophyllene Oxide |
| 4 | 6.910 | C15H24O | Sesquiterpenols | (1R,3E,7E,11R)-1,5,5,8-Tetramethyl-12-oxabicyclo[9.1.0]dodeca-3,7-diene |
| 5 | 6.974 | C15H24O | Sesquiterpenols | cis-α-Copaene-8-ol |
| 6 | 7.187 | C15H24O | Sesquiterpenols | Spathulenol |
| 7 | 7.761 | C15H24O | Sesquiterpenols | Ledene Oxide (II) |
| 8 | 8.128 | C15H24O | Sesquiterpenols | Ledene Alcohol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-S.; Chen, S.-Y.; Tsai, J.-C. Effects of the Supercritical Fluid Extract of Magnolia figo on Inducing the Apoptosis of Human Non-Small-Cell Lung Cancer Cells. Molecules 2023, 28, 7445. https://doi.org/10.3390/molecules28217445
Kuo C-S, Chen S-Y, Tsai J-C. Effects of the Supercritical Fluid Extract of Magnolia figo on Inducing the Apoptosis of Human Non-Small-Cell Lung Cancer Cells. Molecules. 2023; 28(21):7445. https://doi.org/10.3390/molecules28217445
Chicago/Turabian StyleKuo, Chun-Sheng, Shih-Yun Chen, and Jen-Chieh Tsai. 2023. "Effects of the Supercritical Fluid Extract of Magnolia figo on Inducing the Apoptosis of Human Non-Small-Cell Lung Cancer Cells" Molecules 28, no. 21: 7445. https://doi.org/10.3390/molecules28217445