Hybrid Zn-β-Aminoporphyrin–Carbon Nanotubes: Pyrrolidine and Direct Covalent Linkage Recognition, and Multiple-Photo Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. Functionalization of Carbon Nanotubes
2.2. Physicochemical Characterization
2.2.1. XPS Data and Analysis
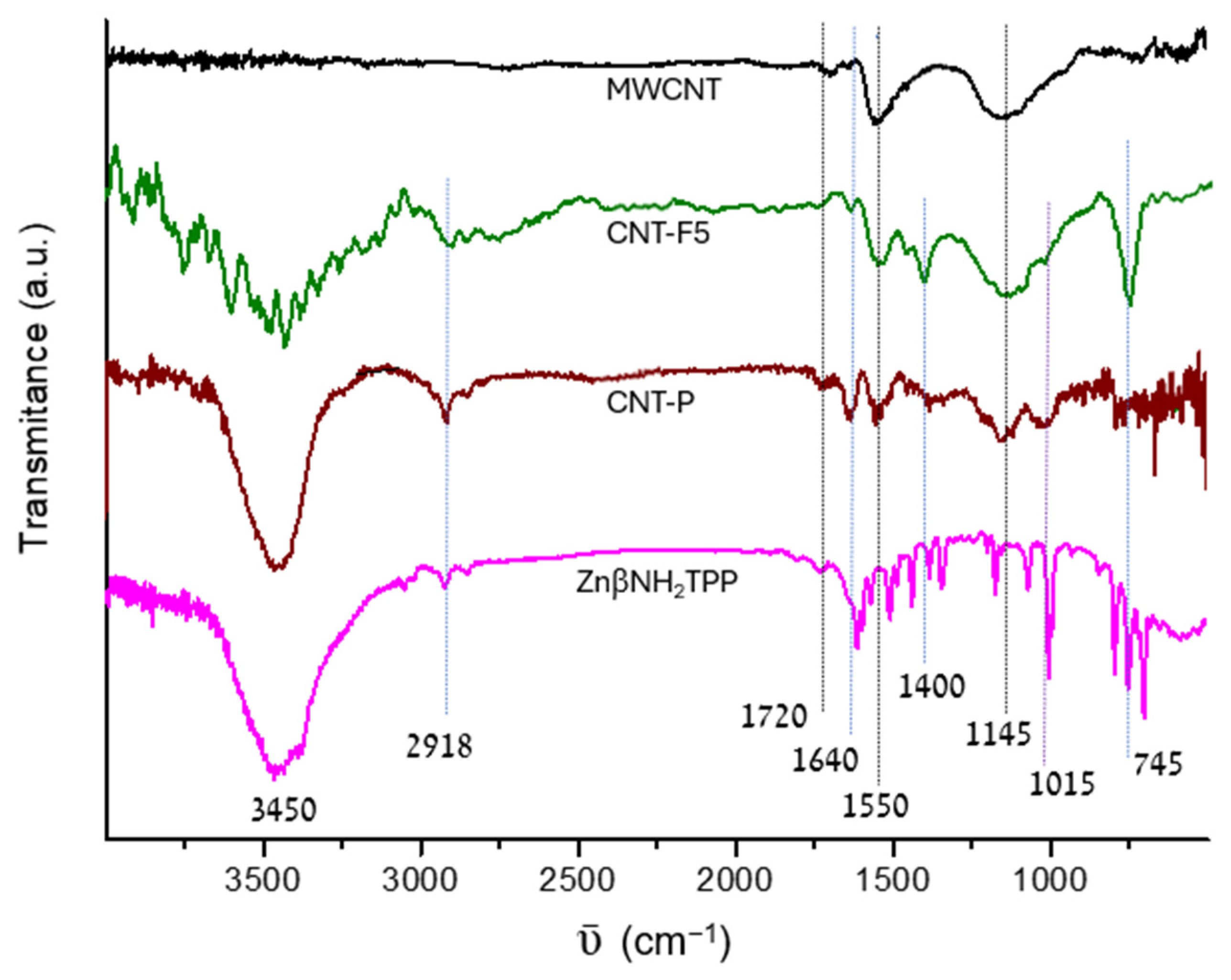
2.2.2. FTIR Data and Analysis
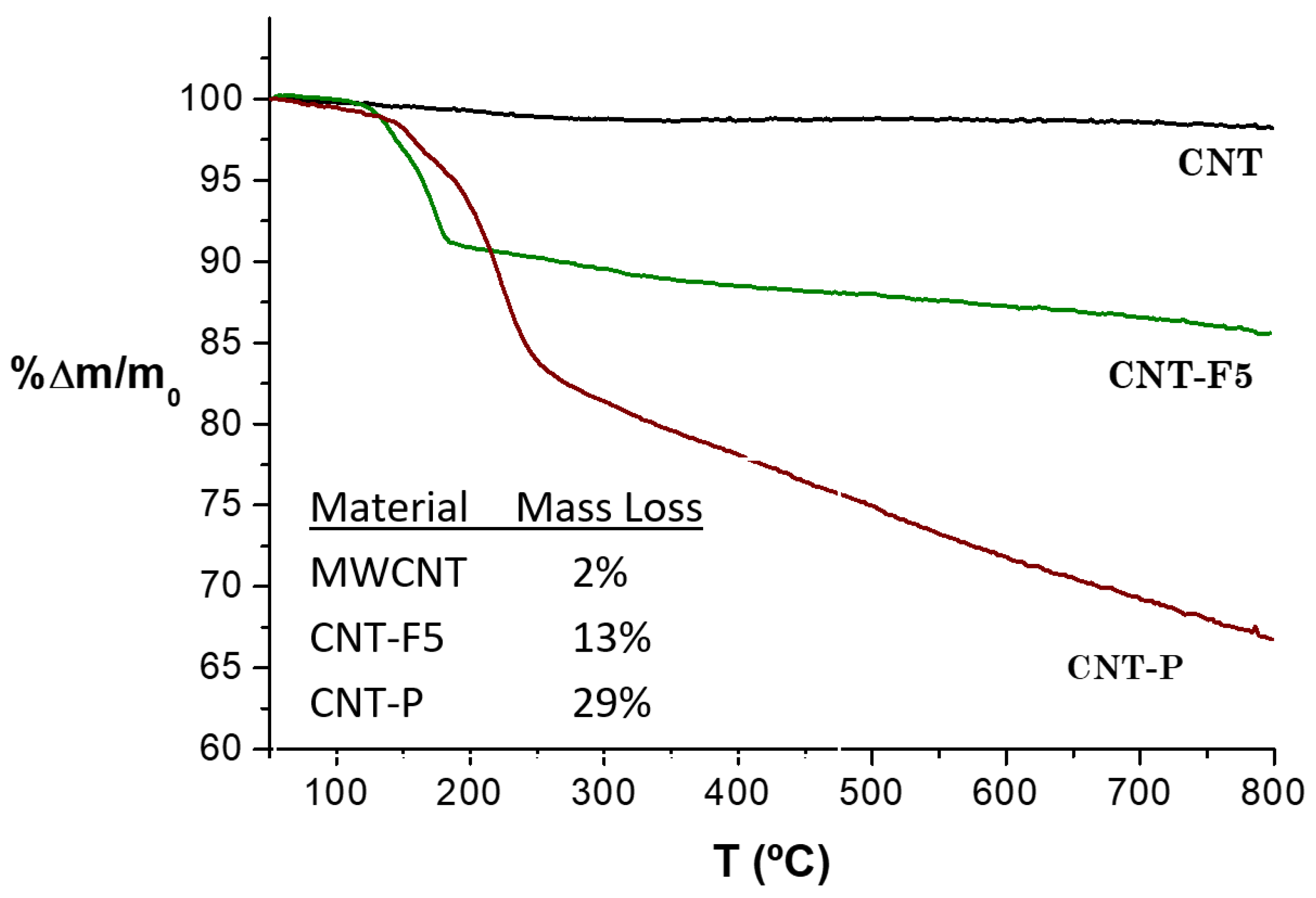
2.2.3. Thermogravimetric Analysis
2.2.4. Raman Spectroscopy
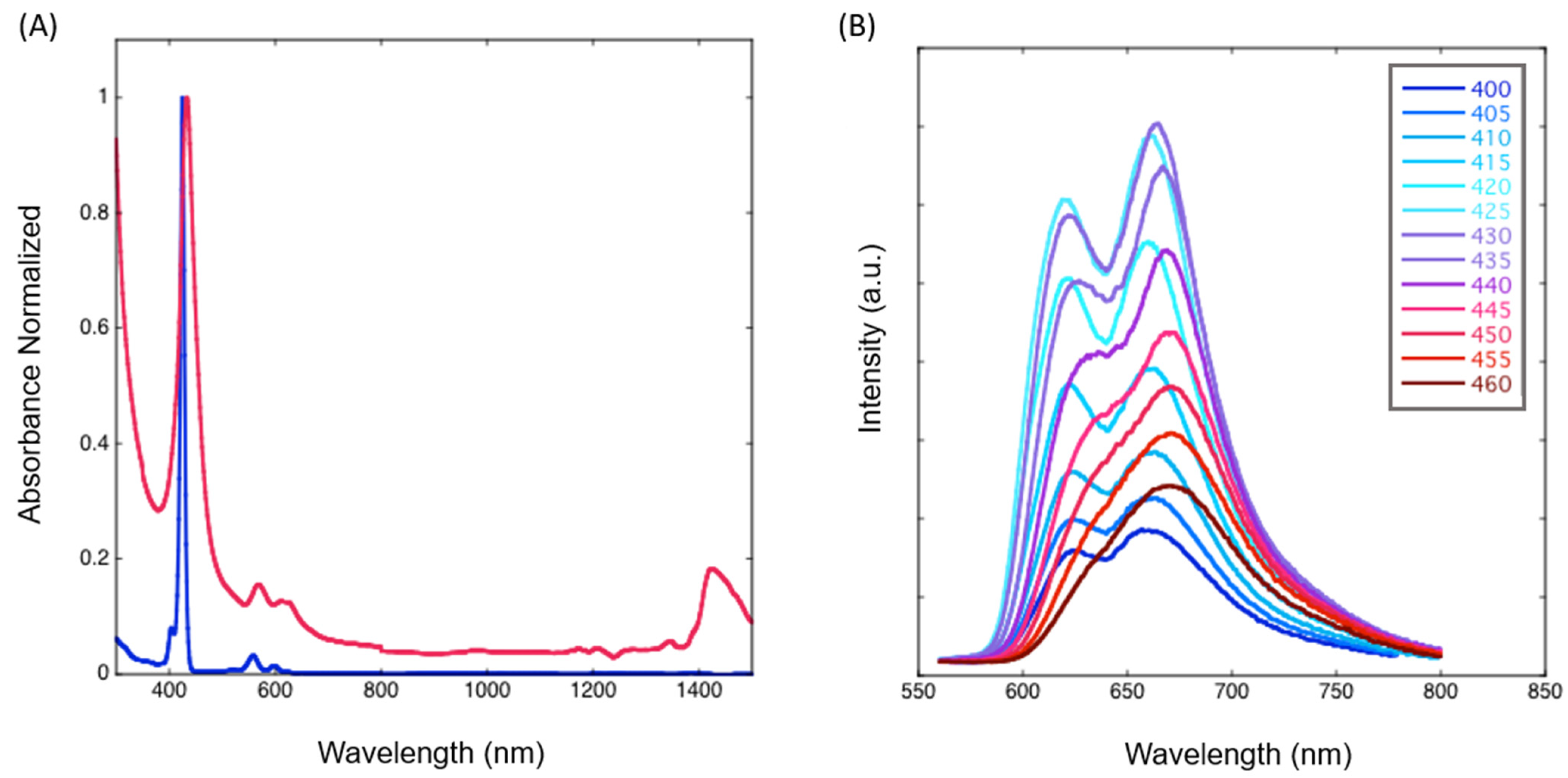
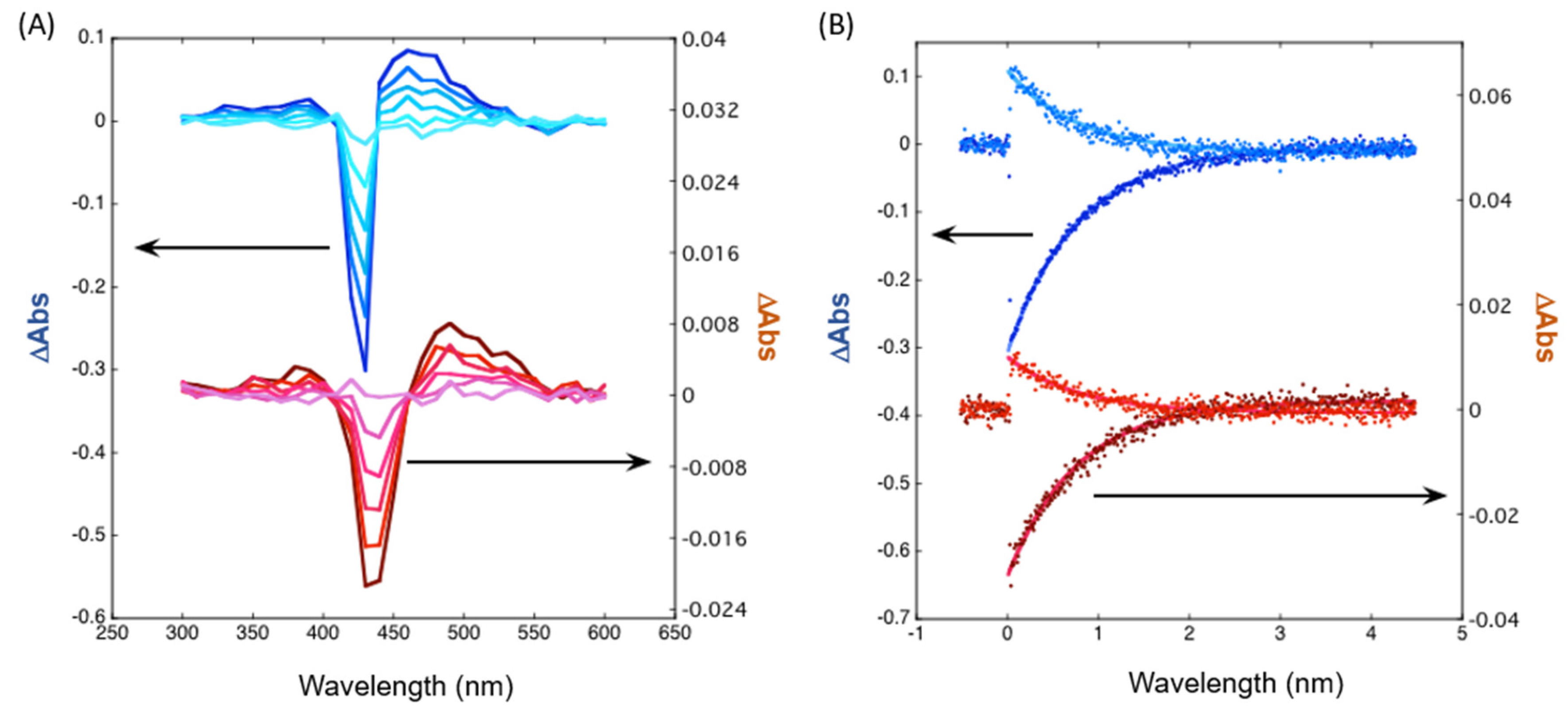
2.3. Photophysical Studies
2.4. Parallel Functionalization
3. Materials and Methods
3.1. Chemicals
3.2. Functionalization of CNTs
3.2.1. Pentafluorophenyl-N-Methylpyrrolidine Carbon Nanotubes (CNT-F5)
3.2.2. Preparation of Nanohybrid (CNT-P)
3.3. Physico-Chemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, A.; Münich, P.W.; Wagner, P.; Officer, D.L.; Guldi, D.M. Amphiphilic zinc porphyrin single-walled carbon nanotube hybrids: Efficient formation and excited state charge transfer studies. Small 2021, 17, 2005648. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiao, J.; Zhang, X.; Yang, Y.; Li, B.; Wu, L.; Liu, W.; Zeng, Z.; Liu, J. Noncovalent self-assembly of a minuscule amount of nickel porphyrin on carbon nanotube for high-performance electromagnetic wave absorbing. Compos. Part A Appl. Sci. Manuf. 2023, 164, 107281. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, M.; Yang, X.-Y.; Xu, W.-J.; Lei, Q.-J.; Zhang, J.; Wen, J.-X.; Gu, J.-X.; Chao, Z.-S.; Jin, H.-G. Metal-porphyrin frameworks supported by carbon nanotubes: Efficient polysulfide electrocatalysts for lithium-sulfur batteries. Chem. Eng. J. 2022, 437, 135150. [Google Scholar] [CrossRef]
- Groves, J.T. Cytochrome P450 enzymes: Understanding the biochemical hieroglyphs. F1000Research 2015, 4, 178. [Google Scholar] [CrossRef]
- Arellano, L.M.; Gobeze, H.B.; Gómez-Escalonilla, M.J.; Fierro, J.L.G.; D’Souza, F.; Langa, F. Triplet photosensitizer-nanotube conjugates. Nanoscale 2020, 12, 9890–9898. [Google Scholar] [CrossRef] [PubMed]
- Calvete, M.J.F.; Piñeiro, M.; Dias, L.D.; Pereira, M.M. Hydrogen Peroxide and Metalloporphyrins in Oxidation Catalysis: Old Dogs with Some New Tricks. ChemCatChem 2018, 10, 3615–3635. [Google Scholar] [CrossRef]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Wu, N.; Zhang, Q.-X.; Xu, Y.-J.; Zheng, H.; Xu, F.-C.; Tang, D.-L. Covalent linking of the porphyrin light harvester to the multiwalled-carbon-nanotube-based polypyridineruthenium(II) complex for boosting the electrocatalytic oxygen evolution reaction. ACS Appl. Energy Mater. 2023, 6, 6580–6592. [Google Scholar] [CrossRef]
- D’Souza, F.; Osamu, I. Supramolecular donor–acceptor hybrids of porphyrins/phthalocyanines with fullerenes/carbon nanotubes: Electron transfer, sensing, switching, and catalytic applications. Chem. Commun. 2009, 33, 4913–4928. [Google Scholar] [CrossRef]
- Li, J.; Ren, Q.; Liu, L.; Sun, K.; Gu, X. Non-covalent conjugation of sulfonated porphyrins to polyethylenimine-grafted multiwalled carbon nanotubes as efficient recyclable heterogeneous catalysts for dihydroxynaphthalenes photooxidation. Mol. Catal. 2019, 470, 97–103. [Google Scholar] [CrossRef]
- Monteiro, C.S.; Ferreira, D.C.; Sáfar, G.A.M.; Gontijo, R.N.; Fantini, C.; Martins, D.C.S.; Idemori, Y.M.; Pinheiro, M.V.B.; Krambrock, K. Unravelling the mechanisms of reactive oxygen species formation in nanohybrid systems of porphyrins and enriched (6,5) single-walled carbon nanotubes for photosensitization. Phys. Chem. Chem. Phys. 2016, 18, 20459–20465. [Google Scholar] [CrossRef] [PubMed]
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial photodynamic therapy: Single-walled carbon nanotube (SWCNT)-Porphyrin conjugate for visible light mediated inactivation of Staphylococcus aureus. Colloid Surf. B-Biointerfaces 2018, 162, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Tasleem, M.; Yadav, M.; Ganesan, V.; Sankar, M. Co(II) Porphyrin-MWCNT Nanoconjugate as an Efficient and Durable Electrocatalyst for Oxygen Reduction Reaction. Langmuir 2023, 39, 8075–8082. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Marianov, A.N.; Jiang, Y. Covalent grafting of cobalt aminoporphyrin-based electrocatalyst onto carbon nanotubes for excellent activity in CO2 reduction. Appl. Catal. B Environ. 2022, 300, 120750. [Google Scholar] [CrossRef]
- Bai, Y.; Miao, J.; Bian, X.; Wang, Q.; Gao, W.; Xue, Y.; Yang, G.; Zhu, P.; Yu, J. In situ growth of a cobalt porphyrin-based covalent organic framework on multi-walled carbon nanotubes for ultrasensitive real-time monitoring of living cell-released nitric oxide. Analyst 2023, 148, 4219–4226. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Wu, L.; Liu, L.; Hu, J.; Hou, H.; Liang, S.; Yang, J. Generation of high-valent iron-oxo porphyrin cation radicals on hemin loaded carbon nanotubes for efficient degradation of sulfathiazole. J. Hazard. Mater. 2023, 444, 130402. [Google Scholar] [CrossRef]
- Wan, L.-S.; Ke, B.-B.; Wu, J.; Xu, Z.-K. Catalase immobilization on electrospun nanofibers: effects of porphyrin pendants and carbon nanotubes. J. Phys. Chem. C 2007, 111, 14091–14097. [Google Scholar] [CrossRef]
- Malhi, H.S.; Zhang, Z.; Shi, Y.; Gao, X.; Liu, W.; Tu, W.; Han, Y.-F. The promotional effects of carbon nanotube on Fe5C2-ZnO catalysts for CO2 hydrogenation to heavy olefins. Fuel 2023, 127, 127267. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. A review on novel composites of MWCNTs mediated semiconducting materials as photocatalysts in water treatment. Sci. Total Environ. 2019, 646, 1398–1412. [Google Scholar] [CrossRef]
- He, D.; Peng, Y.; Yang, H.; Ma, D.; Wang, Y.; Chen, K.; Chen, P.; Shi, J. Single-wall carbon nanotubes covalently linked with zinc (II) phthalocyanine bearing poly (aryl benzyl ether) dendritic substituents. Dye Pigment. 2013, 99, 395–401. [Google Scholar] [CrossRef]
- Campbell, W.M.; Burrell, A.K.; Officer, D.L.; Jolley, K.W. Porphyrins as light harvesters in the dye-sensitised TiO2 solar cell. Coord. Chem. Rev. 2004, 248, 1363–1379. [Google Scholar] [CrossRef]
- Lipińska, M.E.; Rebelo, S.L.H.; Pereira, M.F.R.; Figueiredo, J.L.; Freire, C. Photoactive Zn(II)porphyrin–multi-walled carbon nanotubes nanohybrids through covalent β-linkages. Mater. Chem. Phys. 2013, 143, 296–304. [Google Scholar] [CrossRef]
- Garrido, M.; Gualandi, L.; Di Noja, S.; Filippini, G.; Bosi, S.; Prato, M. Synthesis and applications of amino-functionalized carbon nanomaterials. Chem. Commun. 2020, 56, 12698–12716. [Google Scholar] [CrossRef]
- Worsley, K.A.; Moonoosawmy, K.R.; Kruse, P. Long-range periodicity in carbon nanotube sidewall functionalization. Nano Lett. 2004, 4, 1541–1546. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, M.E.; Rebelo, S.L.H.; Pereira, M.F.R.; Gomes, J.A.N.F.; Freire, C.; Figueiredo, J.L. New insights into the functionalization of multi-walled carbon nanotubes with aniline derivatives. Carbon 2012, 50, 3280–3294. [Google Scholar] [CrossRef]
- Enes, R.F.; Cid, J.J.; Hausmann, A.; Trukhina, O.; Gouloumis, A.; Vázquez, P.; Cavaleiro, J.A.S.; Tomé, A.C.; Guldi, D.M.; Torres, T. Synthesis and Photophysical Properties of Fullerene–Phthalocyanine–Porphyrin Triads and Pentads. Chem. Eur. J. 2012, 18, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, M.E.; Novais, J.P.; Rebelo, S.L.H.; Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Microwave-assisted silylation of graphite oxide and iron(III) porphyrin intercalation. Polyhedron 2014, 81, 475–484. [Google Scholar] [CrossRef]
- Wang, J.; Chu, H.; Li, Y. Why single-walled carbon nanotubes can be dispersed in imidazolium-based ionic liquids. ACS Nano 2008, 2, 2540–2546. [Google Scholar] [CrossRef]
- Ganji, M.D. Density functional theory based treatment of amino acids adsorption on single-walled carbon nanotubes. Diam. Relat. Mater. 2009, 18, 662–668. [Google Scholar] [CrossRef]
- Corrêa, G.A.; de Castro, B.; Rebelo, S.L.H. Binuclear Mn(III) and Fe(III) porphyrin nanostructured materials in catalytic reduction of 4-nitrophenol. Catal. Today 2023, 418, 114149. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Fujimoto, A.; Yamada, Y.; Koinuma, M.; Sato, S. Origins of sp3C peaks in C1s X-ray Photoelectron Spectra of Carbon Materials. Anal. Chem. 2016, 88, 6110–6114. [Google Scholar] [CrossRef] [PubMed]
- Gengenbach, T.R.; Major, G.H.; Linford, M.R.; Easton, C.D. Practical guides for X-ray photoelectron spectroscopy (XPS): Interpreting the carbon 1s spectrum. J. Vac. Sci. Technol. A 2021, 39, 013204. [Google Scholar] [CrossRef]
- Lipińska, M.E.; Rebelo, S.L.H.; Teixeira, D.M.D.; Laia, C.A.T.; Silva, A.M.G.; Freire, C. β-Functionalized zinc(II)aminoporphyrins by direct catalytic hydrogenation. Tetrahedron Lett. 2013, 54, 110–113. [Google Scholar] [CrossRef]

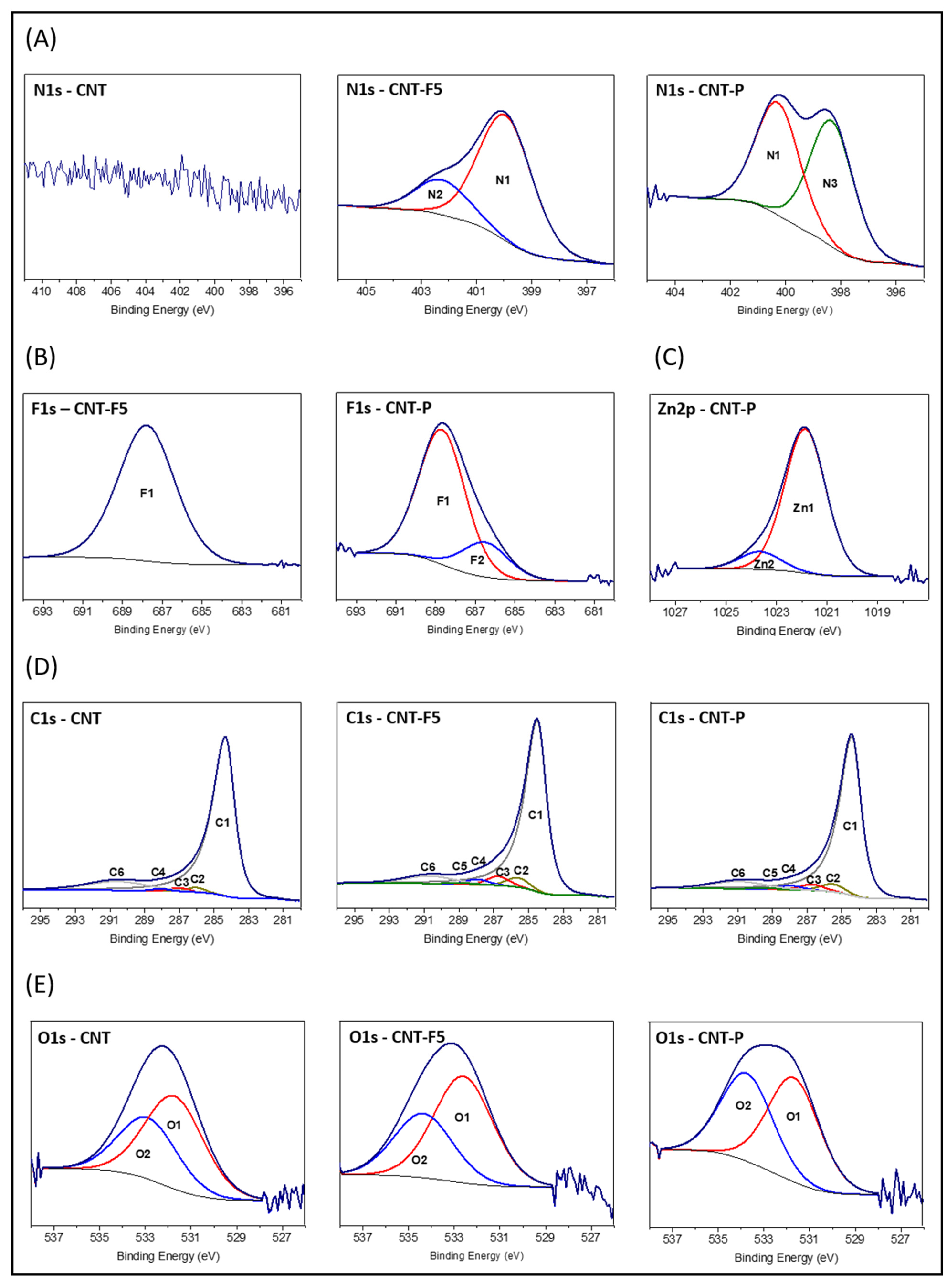


| At % | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Material | C1s | N1s | O1s | F1s | Zn2p3 | F/N |
| 1 | CNT | 98.0 | <DL a | 2.0 | - | - | - |
| 2 | CNT-F5 | 92.4 | 1.2 | 1.5 | 4.9 | - | 4.1 |
| 3 | CNT-F5DMF b | 93.4 | 1.5 | 2.0 | 3.1 | - | 2.1 |
| 4 | CNT-P | 95.5 | 1.0 | 1.25 | 2.0 | 0.2 | 2.0 |
| Material | BE, eV (Relative Area, %) a | |||||||
| C1s | O1s | |||||||
| C1 | C2 | C3 | C4 | C5 | C6 | O1 | O2 | |
| CNT | 284.4 (85.9) | 286.1 (1.6) | 287.1 (1.3) | 288.2 (0.8) | - | 290.7 (10.5) | 531.7 (60.2) | 532.9 (39.2) |
| CNT-F5 | 284.5(82.6) | 285.6 (3.5) | 286.7 (4.3) | 288.0 (2.2) | 289.0 (0.5) | 290.7 (6.8) | 532.6 (62.4) | 534.0 (37.6) |
| CNT-P | 284.4(83.5) | 285.5 (3.7) | 286.7 (2.7) | 288.0 (1.9) | 289.2 (0.6) | 290.9 (7.6) | 531.7 (50.5) | 533.8 (49.5) |
| C=C | C-C, C-H | C-O, C-N, C-F | C=O, C=N, C-N+, (C-F)n | COO, C=N+ | π to π b | C=O, (C=O)-O | C-OH (C=O)-O | |
| Material | BE, eV (Relative Area, %) a | |||||||
| N1s | F1s | Zn2p | ||||||
| N1 | N2 | N3 | F1 | F2 | Zn1 | Zn2 | ||
| CNT | - | - | - | - | - | - | - | |
| CNT-F5 | 400.1 (72.7) | 402.2 (27.3) | - | 687.8 (100) | - | - | - | |
| CNT-P | 400.3 (47.2) | - | 398.3 (52.8) | 688.7 (79.5) | 686.5 (20.5) | 1021.9 (89) | 1023.7 (11) | |
| N-H | N+-C | N=C | Ar-F | F− | Zn-P1 | Zn-P2 | ||
| Material | Dl+Dr/D | Dl+Dr/Gl | Dl+Dr/G |
|---|---|---|---|
| MWCNT | 0.12 | 0.8 | 0.21 |
| CNT-F5 | 0.39 | 2.6 | 0.72 |
| CNT-P | 0.46 | 3.1 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebelo, S.L.H.; Laia, C.A.T.; Szefczyk, M.; Guedes, A.; Silva, A.M.G.; Freire, C. Hybrid Zn-β-Aminoporphyrin–Carbon Nanotubes: Pyrrolidine and Direct Covalent Linkage Recognition, and Multiple-Photo Response. Molecules 2023, 28, 7438. https://doi.org/10.3390/molecules28217438
Rebelo SLH, Laia CAT, Szefczyk M, Guedes A, Silva AMG, Freire C. Hybrid Zn-β-Aminoporphyrin–Carbon Nanotubes: Pyrrolidine and Direct Covalent Linkage Recognition, and Multiple-Photo Response. Molecules. 2023; 28(21):7438. https://doi.org/10.3390/molecules28217438
Chicago/Turabian StyleRebelo, Susana L. H., César A. T. Laia, Monika Szefczyk, Alexandra Guedes, Ana M. G. Silva, and Cristina Freire. 2023. "Hybrid Zn-β-Aminoporphyrin–Carbon Nanotubes: Pyrrolidine and Direct Covalent Linkage Recognition, and Multiple-Photo Response" Molecules 28, no. 21: 7438. https://doi.org/10.3390/molecules28217438






