Development of a Ratiometric Fluorescent Cu(II) Indicator Based on Poly(N-isopropylacrylamide) Thermal Phase Transition and an Aminopyridyl Cu(II) Ligand
Abstract
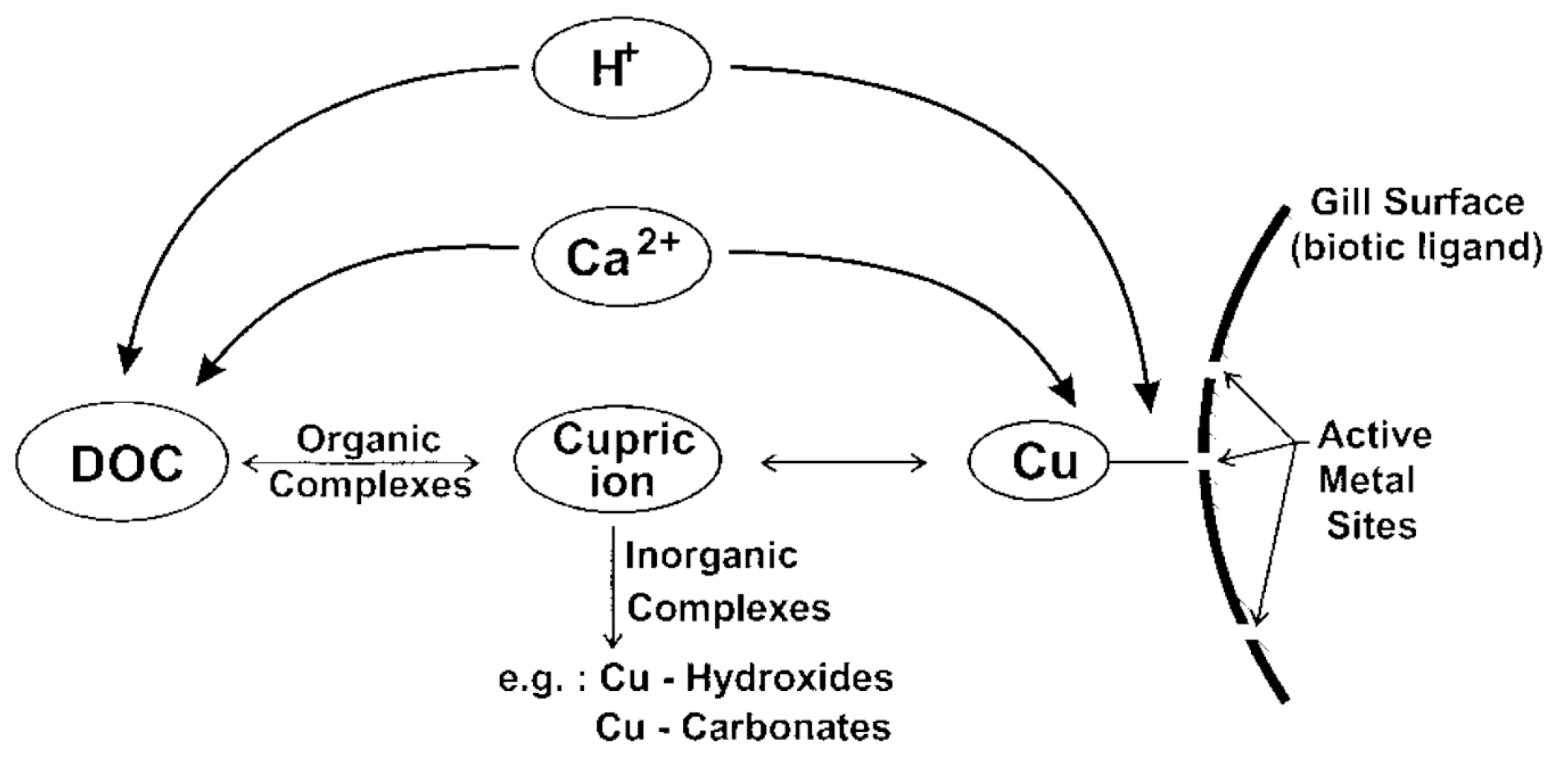
:1. Introduction
2. Results and Discussion
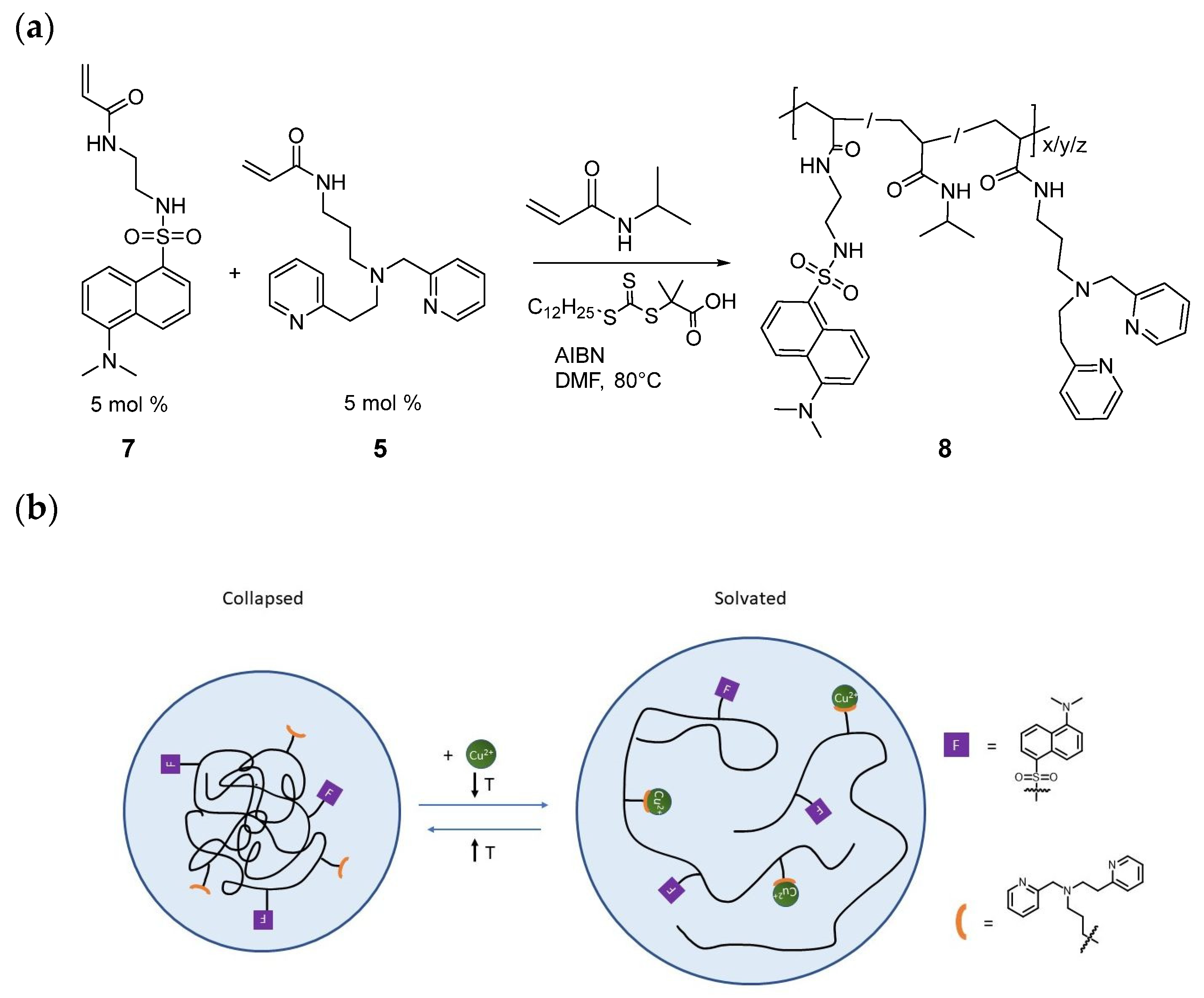
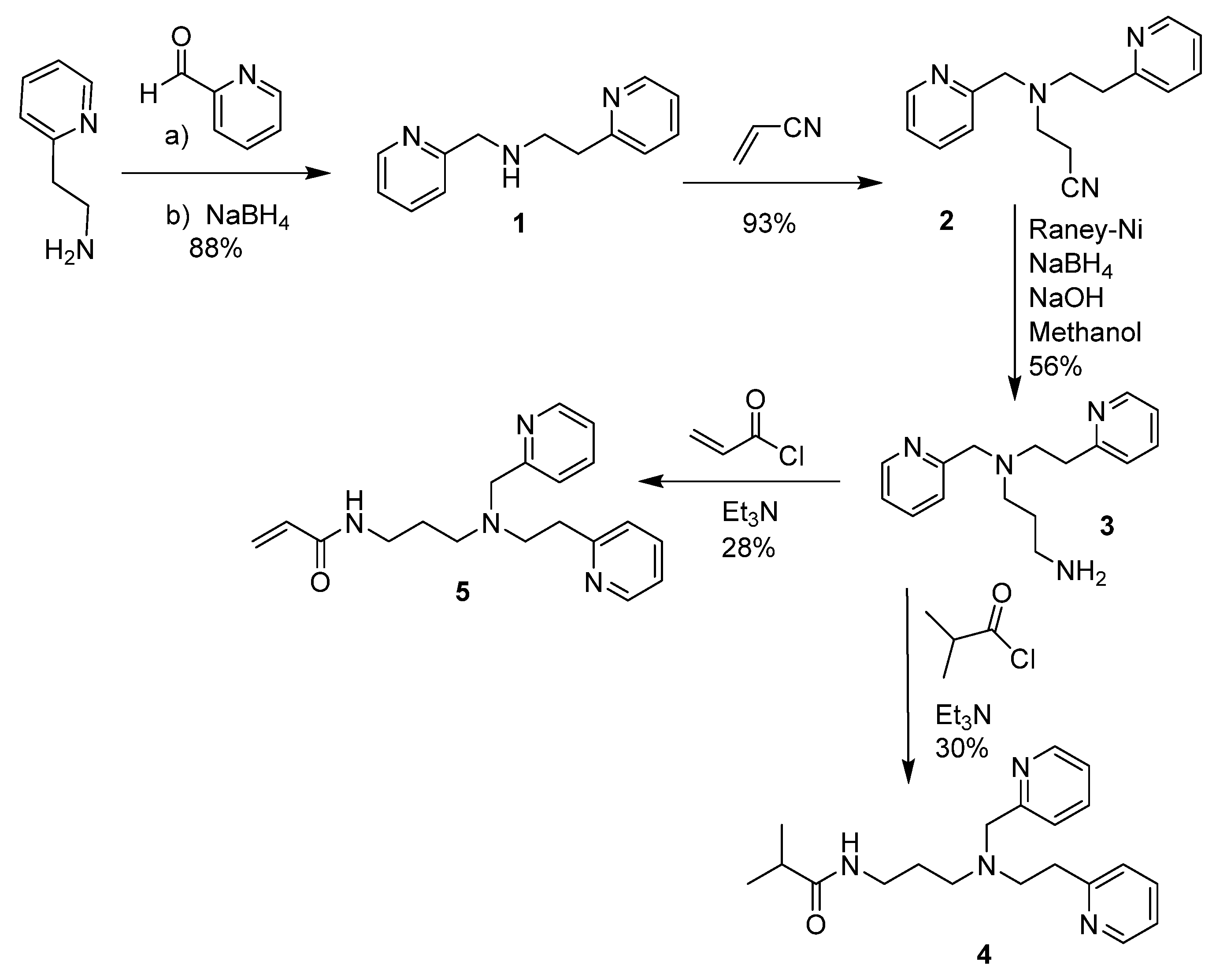
2.1. Synthesis and Characterization of PEPMA-C3-Acrylamide, the PEPMA-C3-Isobutyramide Model Ligand, and the Indicator Polymer 8
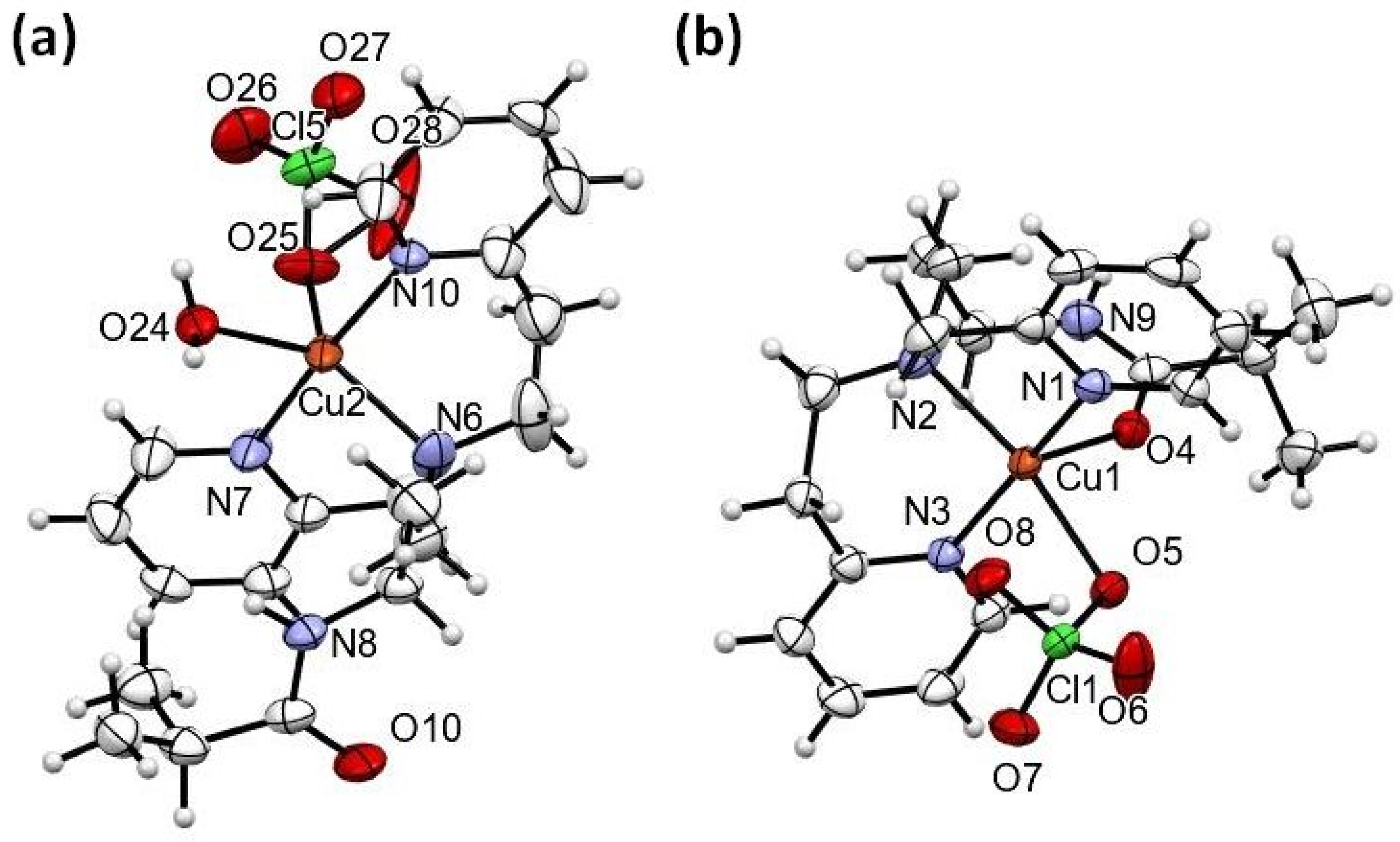
2.2. Structural Studies
2.3. Formation Constants of PEPMA-C3-Isobutyramide (4) with Cu2+ and Zn2+
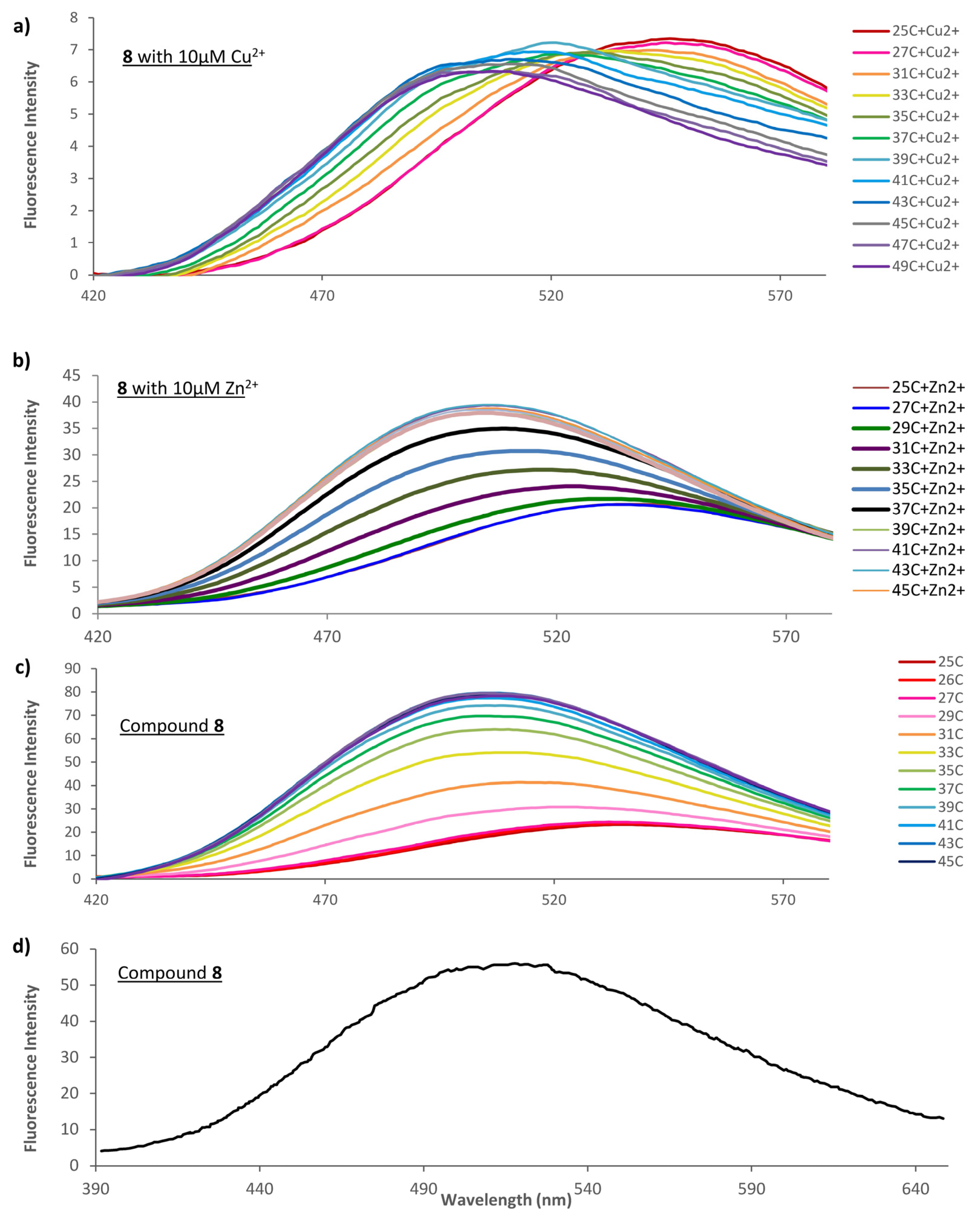
2.4. Thermally Dependent Fluorescence of Indicator 8 Alone and in Presence of Metal Ion
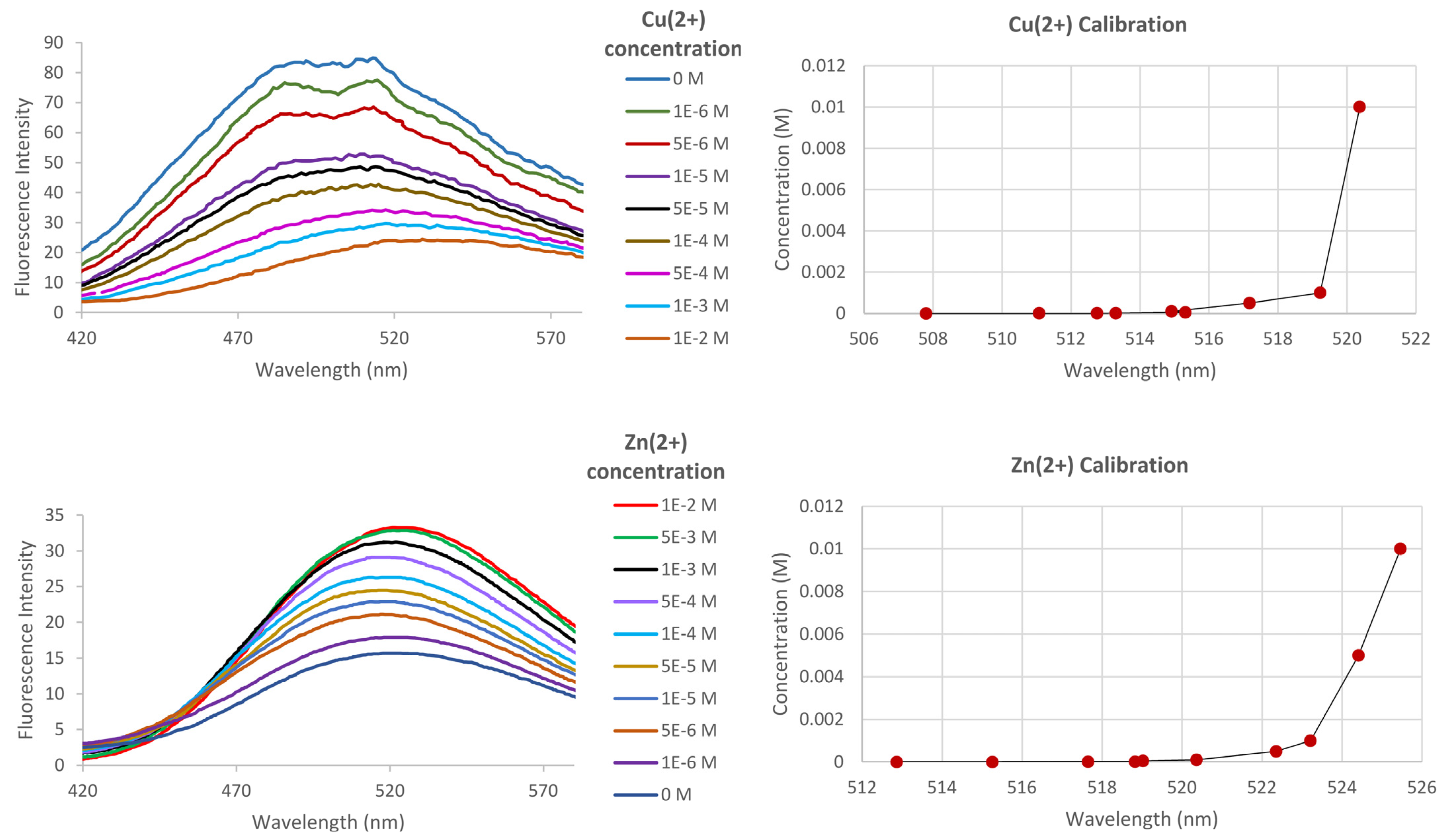
2.5. Indicator Response to Aqueous Cu2+and Zn2 at Fixed Temperature+
2.6. Selectivity of the Metal Indicator for Cu2+ Relative to Ni2+, Fe2+, and Zn2+
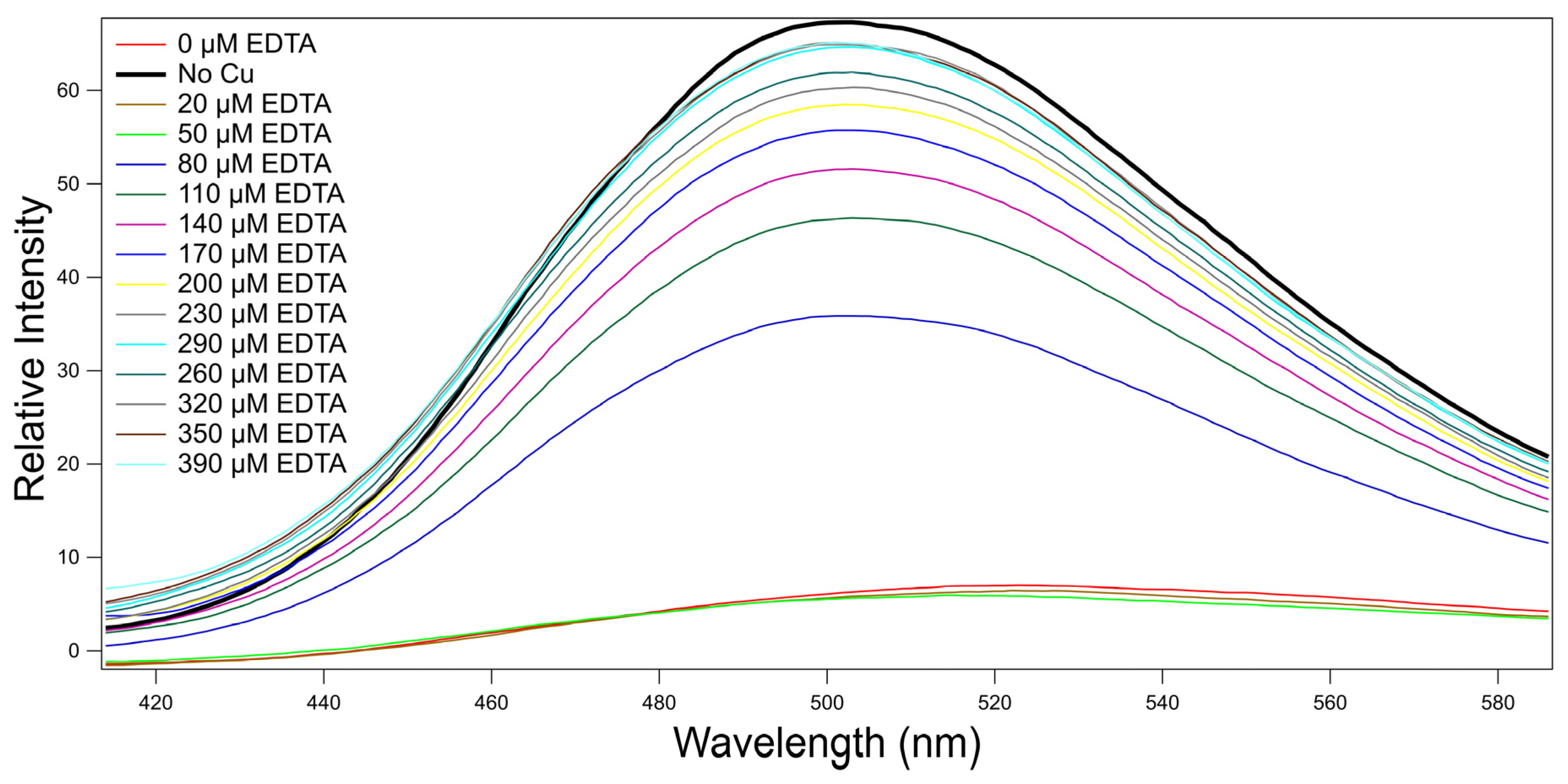
2.7. Reversibility of the Metal Indicator
3. Discussion and Conclusions
4. Materials and Methods
4.1. General
4.2. Synthesis of Compounds
4.2.1. N-(2-pyridinylethyl)-2-pyridinemethanamine (PEPMA) (1)
4.2.2. 3-((2-(pyridin-2-yl)ethyl)(pyridin-2-ylmethyl)amino)propionitrile (2)
4.2.3. N-(2-(pyridin-2-yl)ethyl)-N-(pyridin-2-ylmethyl)propane-1,3-diamine (3)
4.2.4. N-(3-((2-(pyridin-2-yl)ethyl)(pyridin-2-ylmethyl)aminopropylisobutyramide (4)
4.2.5. N-(3-((2-(pyridin-2-yl)ethyl)(pyridin-2-ylmethyl)aminopropylacrylamide (PEPMA-C3-acrylamide) (5)
4.2.6. N-(2-aminoethyl)-5-(dimethylamino)naphthalene-1-sulfonamide (6)
4.2.7. N-(2-(5-(dimethylamino)naphthalene-1-sulfonamido)ethyl)acrylamide (7)
4.2.8. [Cu(4)](ClO4)2•0.5 H2O
4.3. Polymer Synthesis
4.4. Structural Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Song, H.D.; Choi, I.; Lee, S.; Yang, Y.I.; Kang, T.; Yi, J. On-chip colorimetric detection of Cu2+ ions via density-controlled plasmonic core–satellites nanoassembly. Anal. Chem. 2013, 85, 7980–7986. [Google Scholar] [CrossRef] [PubMed]
- Besser, J.M.; Ivey, C.D.; Steevens, J.A.; Cleveland, D.; Soucek, D.; Dickinson, A.; Van Genderen, E.J.; Ryan, A.C.; Schlekat, C.E.; Garman, E.; et al. Modeling the Bioavailability of Nickel and Zinc to Ceriodaphnia dubia and Neocloeon triangulifer in Toxicity Tests with Natural Waters. Environ. Toxicol. Chem. 2021, 40, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R. Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function, 3rd ed.; Academic Press: London, UK, 2019. [Google Scholar]
- Knobeloch, L.; Ziarnik, M.; Howard, J.; Theis, B.; Farmer, D.; Anderson, H.; Proctor, M. Gastrointestinal upsets associated with ingestion of copper-contaminated water. Environ. Health Perspect. 1994, 102, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Santore, R.C.; Di Toro, D.M.; Paquin, P.R.; Allen, H.E.; Meyer, J.S. Biotic ligand model of the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish and Daphnia. Environ. Toxicol. Chem. 2001, 20, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Prodi, L.; Bolletta, F.; Montalti, M.; Zaccheroni, N. Luminescent chemosensors for transition metal ions. Coord. Chem. Rev. 2000, 205, 59–83. [Google Scholar] [CrossRef]
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Xie, J.; Ménand, M.; Maisonneuve, S.; Métivier, R. Synthesis of bispyrenyl sugar-aza-crown ethers as new fluorescent molecular sensors for Cu(II). J. Org. Chem. 2007, 72, 5980–5985. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Shao, S. A BODIPY based fluorescent chemosensor for Cu(II) ions and homocysteine/cysteine. Sens. Actuators B Chem. 2012, 171, 872–877. [Google Scholar] [CrossRef]
- Du, J.; Yao, S.; Seitz, W.R.; Bencivenga, N.E.; Massing, J.O.; Planalp, R.P.; Jackson, R.K.; Kennedy, D.P.; Burdette, S.C. A ratiometric fluorescent metal ion indicator based on dansyl labeled poly(N-isopropylacrylamide) responds to a quenching metal ion. Analyst 2011, 136, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Jones, A.M.; Du, J.; Jackson, R.K.; Massing, J.O.; Kennedy, D.P.; Bencivenga, N.E.; Planalp, R.P.; Burdette, S.C.; Seitz, W.R. Intermolecular approach to metal ion indicators based on polymer phase transitions coupled to fluorescence resonance energy transfer. Analyst 2012, 137, 4734–4741. [Google Scholar] [CrossRef]
- Osambo, J.; Seitz, W.; Kennedy, D.; Planalp, R.; Jones, A.; Jackson, R.; Burdette, S. Fluorescent Ratiometric Indicators Based on Cu(II)-Induced Changes in Poly(NIPAM) Microparticle Volume. Sensors 2013, 13, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Guan, X.; Wang, D.; Liu, S. Metal-Chelating and Dansyl-Labeled Poly(N-isopropylacrylamide) Microgels as Fluorescent Cu2+ Sensors with Thermo-Enhanced Detection Sensitivity. Langmuir 2009, 25, 11367–11374. [Google Scholar] [CrossRef] [PubMed]
- Nor, Y.M. Ecotoxicity of copper to aquatic biota: A review. Environ. Res. 1987, 43, 274–282. [Google Scholar] [CrossRef]
- Di Toro, D.M.; Allen, H.E.; Bergman, H.L.; Meyer, J.S.; Paquin, P.R.; Santore, R.C. Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ. Toxicol. Chem. 2001, 20, 2383–2396. [Google Scholar] [CrossRef]
- Allen, H.E.; Hall, R.H.; Brisbin, T.D. Metal speciation. Effects on aquatic toxicity. Environ. Sci. Technol. 1980, 14, 441–443. [Google Scholar] [CrossRef]
- Li, W.; Chung, H.; Daeffler, C.; Johnson, J.A.; Grubbs, R.H. Application of 1H DOSY for Facile Measurement of Polymer Molecular Weights. Macromolecules 2012, 45, 9595–9603. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Massing, J.O.; Planalp, R.P. Influence of PNIPAm on log Kf of a copolymerized 2,2′-bipyridine: Revised bifunctional ligand design for ratiometric metal-ion sensing. Dalton Trans. 2015, 44, 11887–11892. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Zhang, P.-L.; Shao, J.; Li, X.-T.; Chen, Q.-Y.; Qu, L.-L. A protein amantadine-BODIPY assembly as a turn-on sensor for free copper(ii). Anal. Methods 2019, 11, 827–831. [Google Scholar] [CrossRef]
- Silpcharu, K.; Soonthonhut, S.; Sukwattanasinitt, M.; Rashatasakhon, P. Fluorescent Sensor for Copper(II) and Cyanide Ions via the Complexation–Decomplexation Mechanism with Di(bissulfonamido)spirobifluorene. ACS Omega 2021, 6, 16696–16703. [Google Scholar] [CrossRef]
- Wan, J.; Duan, W.; Che~n, K.; Tao, Y.; Dang, J.; Zeng, K.; Ge, Y.; Wu, J.; Liu, D. Selective and sensitive detection of Zn(II) ion using a simple peptide-based sensor. Sens. Actuators B Chem. 2018, 255, 49–56. [Google Scholar] [CrossRef]
- Slocum, J.D.; Palmer, A.E.; Jimenez, R. Intramolecular Fluorescent Protein Association in a Class of Zinc FRET Sensors Leads to Increased Dynamic Range. J. Phys. Chem. B 2019, 123, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Chae, J.B.; So, H.; Lee, H.; Kim, K.-T.; Kim, C. Sensing of zinc ions and sulfide using a highly practical and water-soluble fluorescent sensor: Applications in test kits and zebrafish. New J. Chem. 2020, 44, 442–449. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Hyperquad2008-Equilibrium Constants from Potentiometric Data. Available online: http://www.hyperquad.co.uk/HQ2008.htm (accessed on 8 October 2023).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyiranshuti, L.; Andrews, E.R.; Povolotskiy, L.I.; Gomez, F.M.; Bartlett, N.R.; Royappa, A.T.; Rheingold, A.L.; Seitz, W.R.; Planalp, R.P. Development of a Ratiometric Fluorescent Cu(II) Indicator Based on Poly(N-isopropylacrylamide) Thermal Phase Transition and an Aminopyridyl Cu(II) Ligand. Molecules 2023, 28, 7097. https://doi.org/10.3390/molecules28207097
Nyiranshuti L, Andrews ER, Povolotskiy LI, Gomez FM, Bartlett NR, Royappa AT, Rheingold AL, Seitz WR, Planalp RP. Development of a Ratiometric Fluorescent Cu(II) Indicator Based on Poly(N-isopropylacrylamide) Thermal Phase Transition and an Aminopyridyl Cu(II) Ligand. Molecules. 2023; 28(20):7097. https://doi.org/10.3390/molecules28207097
Chicago/Turabian StyleNyiranshuti, Lea, Emily R. Andrews, Leonid I. Povolotskiy, Frances M. Gomez, Nathan R. Bartlett, Arun Timothy Royappa, Arnold L. Rheingold, William Rudolf Seitz, and Roy P. Planalp. 2023. "Development of a Ratiometric Fluorescent Cu(II) Indicator Based on Poly(N-isopropylacrylamide) Thermal Phase Transition and an Aminopyridyl Cu(II) Ligand" Molecules 28, no. 20: 7097. https://doi.org/10.3390/molecules28207097






