Design, Synthesis, In Vitro Biological Evaluation and In Silico Molecular Docking Study of Benzimidazole-Based Oxazole Analogues: A Promising Acetylcholinesterase and Butyrylcholinesterase Inhibitors
Abstract
:1. Introduction
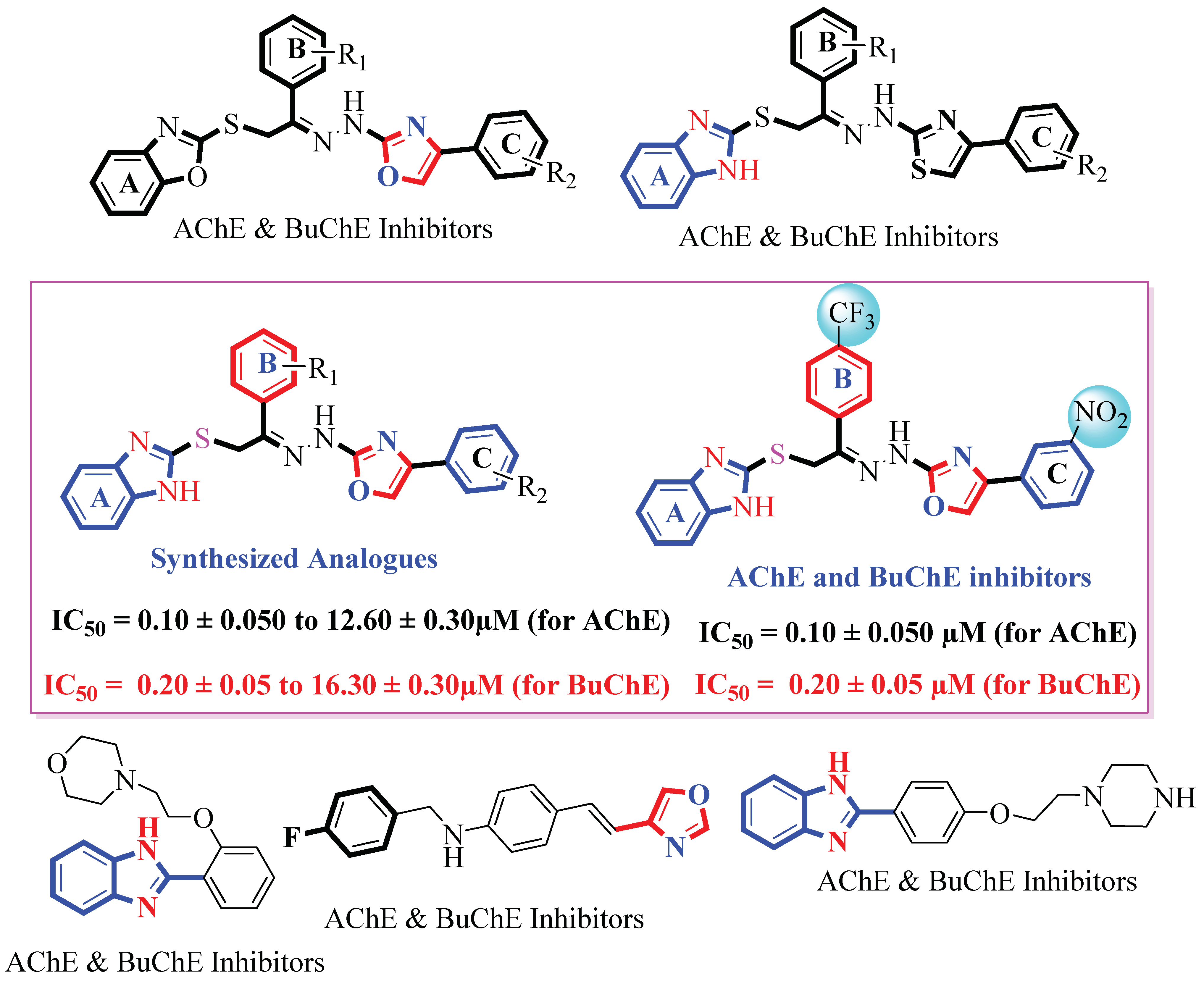
2. Results and Discussion
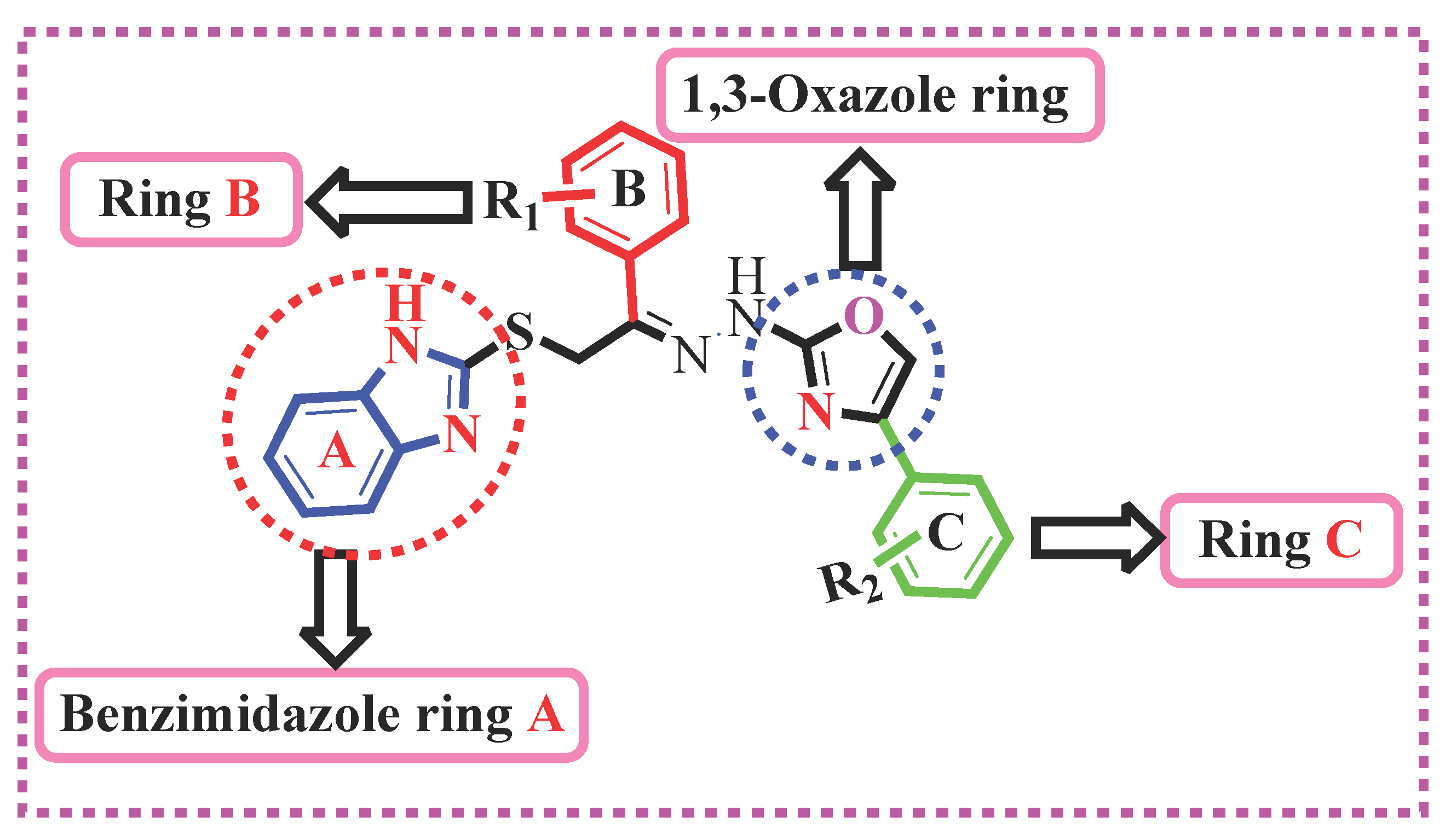
2.1. Chemistry
2.2. In Vitro Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activities
Structure Activity Relationship (SAR) for Acetylcholinesterase (AChE) and Butyrylcholinesterase (BuChE) Activities
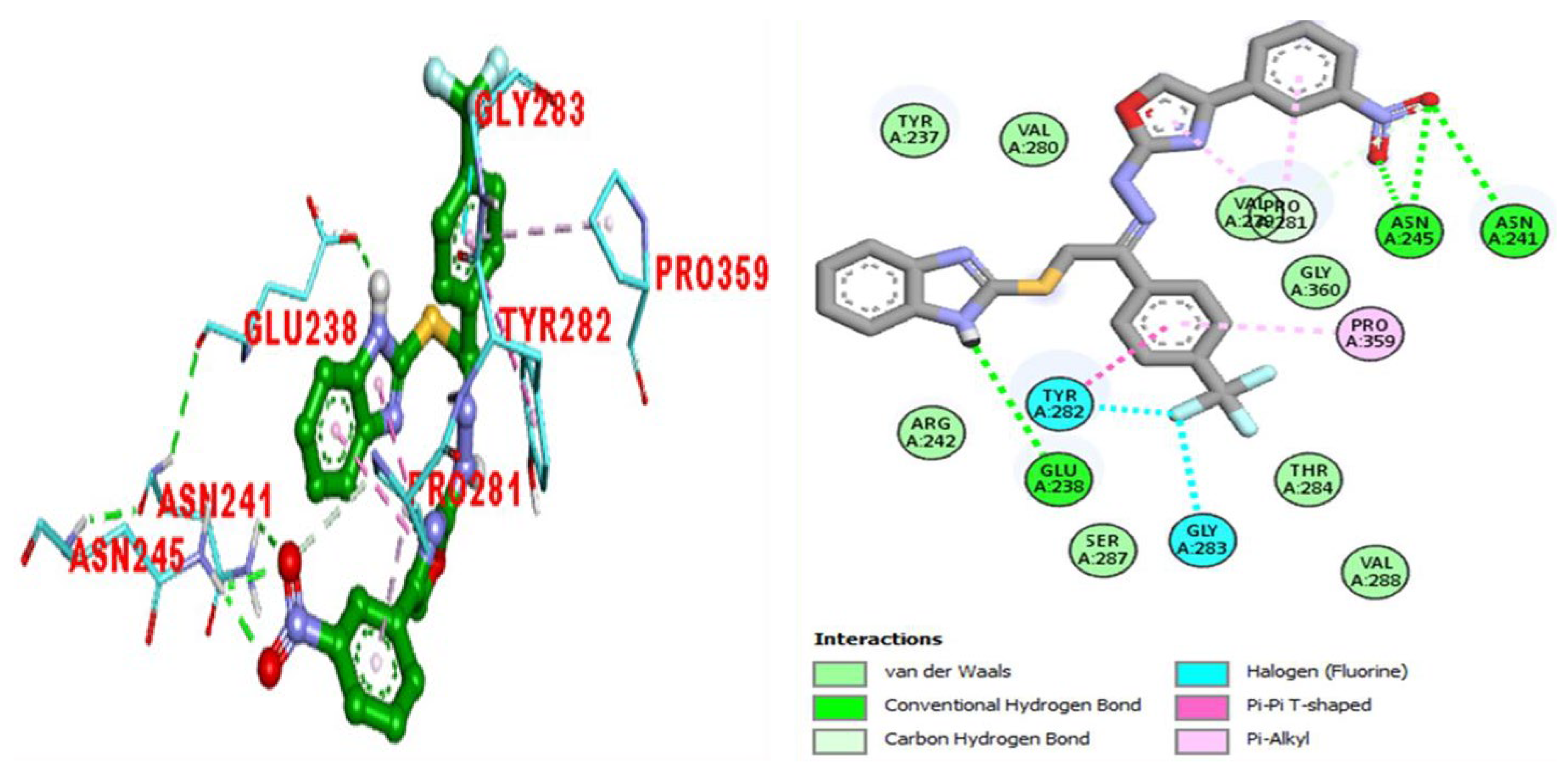
2.3. Molecular Docking Study
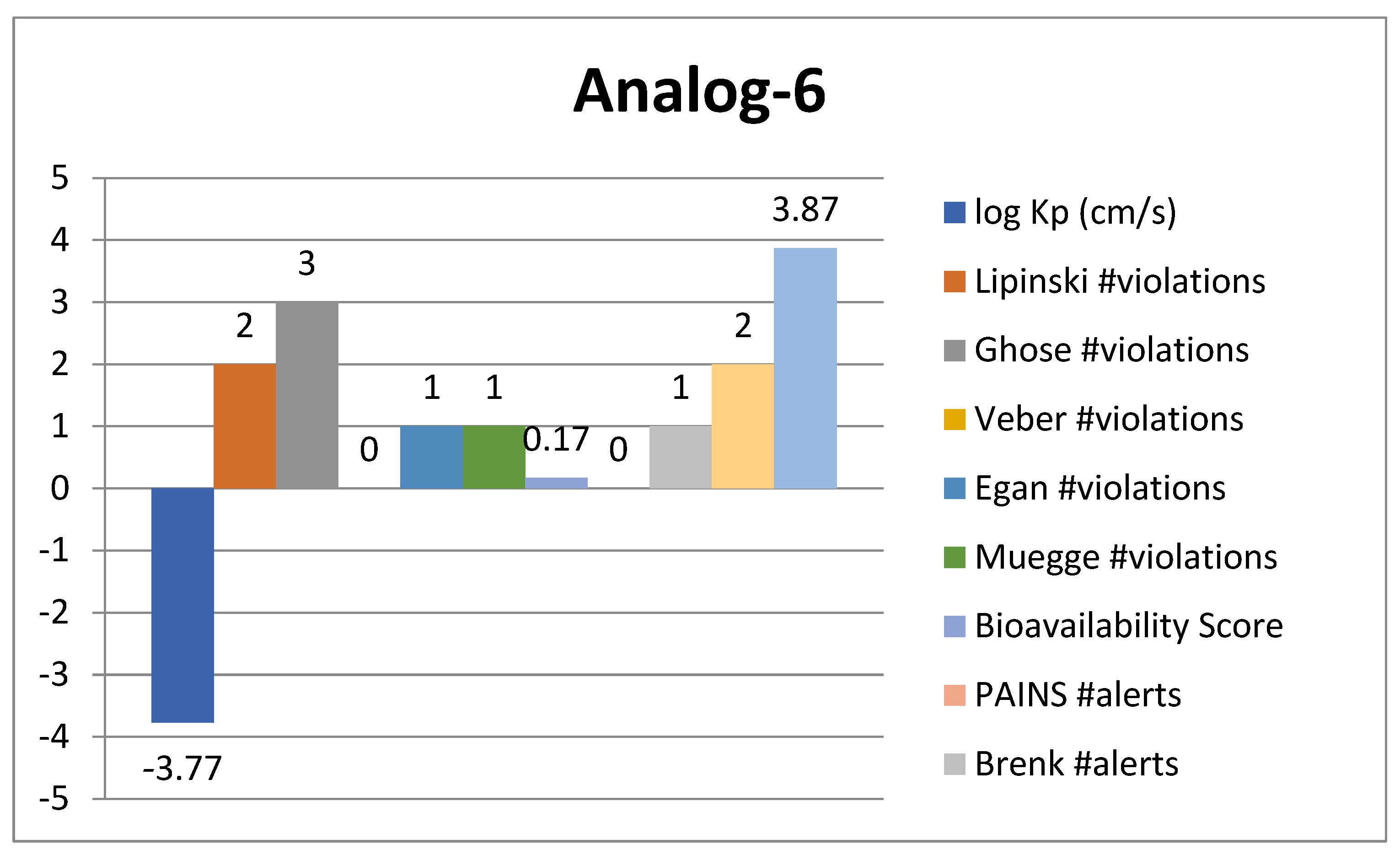
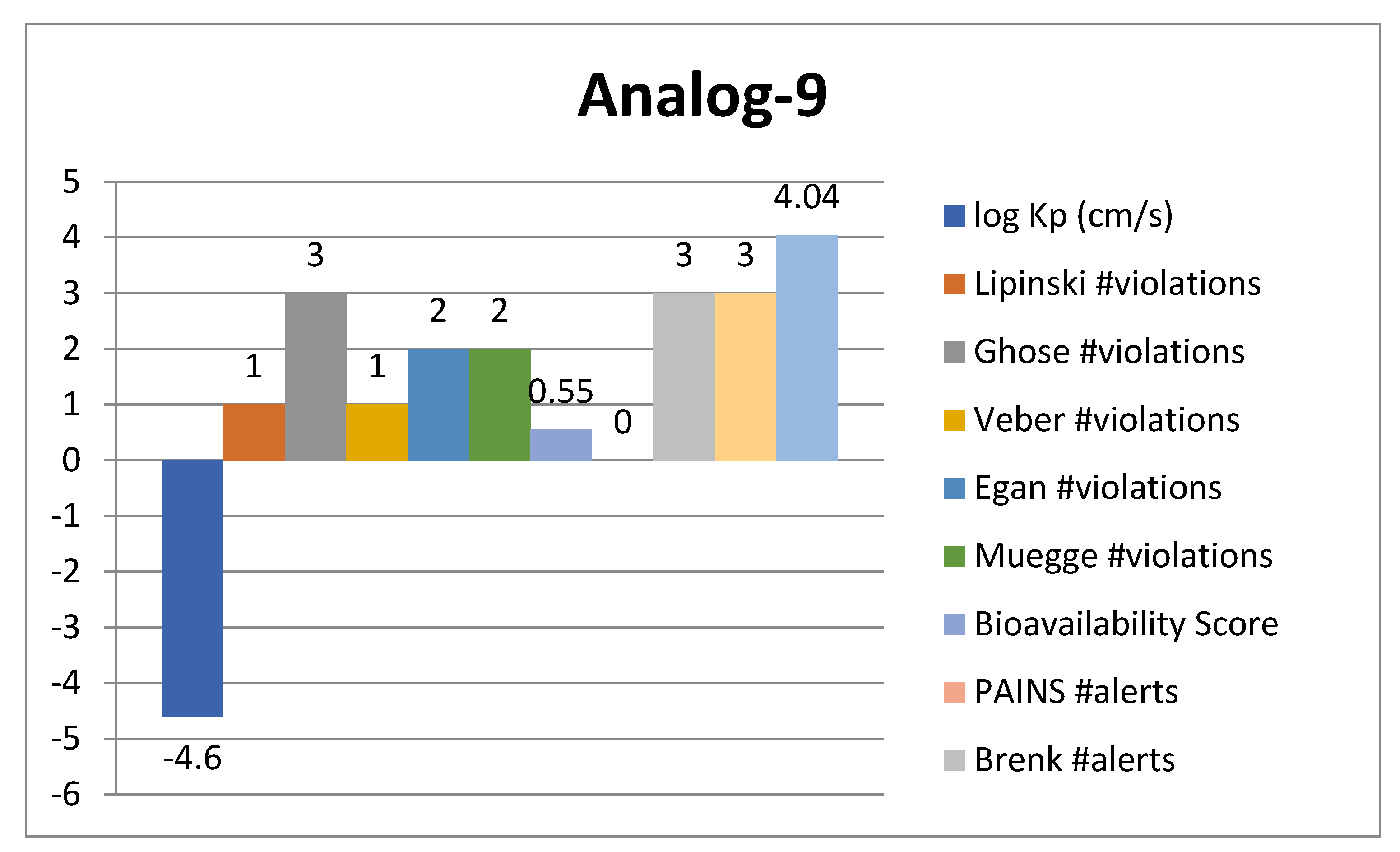
2.4. ADMET Analysis
3. Experiment
3.1. General Procedure for the Synthesis of Benzimidazole-Oxazole Hybrid Derivatives (1–19)
3.2. Spectral Analysis
3.2.1. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(4-methoxyphenyl)ethylidene)hydrazinyl)-4-(2-nitrophenyl)oxazole (1)
3.2.2. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(2-nitrophenyl)oxazole (2)
3.2.3. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(3-nitrophenyl)oxazole (3)
3.2.4. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(p-tolyl)oxazole (4)
3.2.5. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(3-methoxyphenyl)oxazole (5)
3.2.6. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(3,4-dichlorophenyl)oxazole (6)
3.2.7. (E)-4-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(2-(4-(4-bromophenyl)oxazol-2-yl)hydrazono)ethyl)-N,N-dimethylaniline (7)
3.2.8. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(2-methoxyphenyl)oxazole (8)
3.2.9. (E)-4-(2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl) Oxa-zol-4-yl)phenol (9)
3.2.10. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-phenylethylidene)hydrazinyl)-4-(3-nitrophenyl)oxazole (10)
3.2.11. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(4-chlorophenyl)ethylidene)hydrazi-nyl)-4-(3-nitrophenyl)oxazole (11)
3.2.12. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(4-chlorophenyl)oxazole (12)
3.2.13. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-phenyloxazole (13)
3.2.14. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(p-tolyl)ethylidene)hydrazinyl)-4-(2,4-dichlorophenyl)oxazole (14)
3.2.15. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-phenylethylidene)hydrazinyl)-4-(3-methoxyphenyl)oxazole (15)
3.2.16. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-([1,1′-biphenyl]-4-yl)ethylidene) Hydrazinyl)-4-(p-tolyl)oxazole (16)
3.2.17. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-phenylethylidene)hydrazinyl)-4-(2-nitrophenyl)oxazole (17)
3.2.18. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(4-chlorophenyl)ethylidene)hydrazinyl)-4-(2-nitrophenyl)oxazole (18)
3.2.19. (E)-2-(2-(2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(4-chlorophenyl)ethylidene)hydrazinyl)-4-(3-methoxyphenyl)oxazole (19)
3.3. Assay Protocol for Acetylcholinesterase and Butyrylcholinesterase Activities
3.4. Protocol for Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Millard, C.B.; Broomfield, C.A. Anticholinesterases: Medical application of neurochemical principles. J. Neurochem. 1995, 64, 1909–1918. [Google Scholar] [CrossRef]
- Milatovifil, D.; Dettbarn, W.D. The role of carboxylesterase in toxicity and tolerance to organophosphorus anticholinesterases paraoxon and DFP. Toxicol. Appl. Pharmacol. 1966, 136, 20–28. [Google Scholar]
- Weinstock, M.; Groner, E. Rational design of a drug for Alzheimer’s disease with cholinesterase inhibitory and neuroprotective activity. Chem.-Biol. Interact. 2008, 175, 216–221. [Google Scholar] [CrossRef]
- Jann, M.W. Preclinical pharmacology of metrifonate. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1998, 18, 55–67. [Google Scholar]
- Massoulié, J.; Pezzementi, L.; Bon, S.; Krejci, E.; Vallette, F.M. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993, 41, 31–91. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Davis, K.L. The search for disease-modifying treatment for Alzheimer’s disease. Neurology 1997, 48 (Suppl. 6), 35S–41S. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Ullah, H.; Bibi, U.; Hussain, A.; Sarfraz, M.; Rahim, F.; Hayat, S.; Zada, H.; Khan, F.; Wadood, A. Synthesis and Molecular Docking Study of Bis-Indolylmethane Thiourea Derivatives as Anti-Alzheimer Agents. Russ. J. Org. Chem. 2023, 59, 181–189. [Google Scholar] [CrossRef]
- Maruish, M.E.; Moses, J.A. Clinical Neuropsychology: Theoretical Foundations for Practitioners; Psychology Press: London, UK, 2013; p. 371. [Google Scholar]
- Schetinger, M.R.; Porto, N.M.; Moretto, M.B.; Morsch, V.M.; da Rocha, J.B.T.; Vieira, V.; Moro, F.; Neis, R.T.; Bittencourt, S.; Bonacorso, H.G.; et al. New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem. Res. 2000, 25, 949–955. [Google Scholar] [CrossRef]
- Prandota, J. Unexplained liver damage, cryptogenic liver cirrhosis, and steatohepatitis may be caused by latent chronic toxoplasmosis. Toxicol. Appl. Pharmacol. 1973, 24, 29–100. [Google Scholar] [CrossRef]
- Kubo, K.; Inada, Y.; Kohara, Y.; Sugiura, Y.; Ojima, M.; Itoh, K.; Furukawa, Y.; Nishikawa, K.; Naka, T. Nonpeptide angiotensin II receptor antagonists. Synthesis and biological activity of benzimidazoles. J. Med. Chem. 1993, 36, 1772–1784. [Google Scholar] [CrossRef]
- Sabat, M.; VanRens, J.C.; Laufersweiler, M.J.; Brugel, T.A.; Maier, J.; Golebiowski, A.; De, B.; Easwaran, V.; Hsieh, L.C.; Walter, R.L.; et al. The development of 2-benzimidazole substituted pyrimidine based inhibitors of lymphocyte specific kinase (Lck). Bioorganic Med. Chem. Lett. 2006, 16, 5973–5977. [Google Scholar] [CrossRef] [PubMed]
- Güven, Ö.Ö.; Erdoğan, T.; Göker, H.; Yıldız, S. Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers. Bioorganic Med. Chem. Lett. 2007, 17, 2233–2236. [Google Scholar] [CrossRef]
- Townsend, L.B.; Devivar, R.V.; Turk, S.R.; Nassiri, M.R.; Drach, J.C. Design, synthesis, and antiviral activity of certain 2,5,6-Trihalo-1-(.beta.-D-ribofuranosyl) benzimidazoles. J. Med. Chem. 1995, 38, 4098–4105. [Google Scholar] [CrossRef]
- Valdez, J.; Cedillo, R.; Hernández-Campos, A.; Yepez, L.; Hernández-Luis, F.; Navarrete-Vazquez, G.; Tapia, A.; Cortés, R.; Hernández, M.; Castillo, R. Synthesis and antiparasitic activity of 1H-benzimidazole derivatives. Bioorganic Med. Chem. Lett. 2002, 12, 2221–2224. [Google Scholar] [CrossRef]
- Kuş, C.; Ayhan-Kılcıgil, G.; Özbey, S.; Kaynak, F.B.; Kaya, M.; Çoban, T.; Can-Eke, B. Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3 (4H)-thione derivatives of benzimidazole class. Bioorganic Med. Chem. 2008, 16, 4294–4303. [Google Scholar] [CrossRef]
- Arnaiz, D.O.; Liang, A.; Trinh, L.; Whitlow, M.; Koovakkat, S.K.; Shaw, K.J. Design, synthesis, and in vitro biological activity of indole-based factor Xa inhibitors. Bioorganic Med. Chem. Lett. 2000, 10, 957–961. [Google Scholar] [CrossRef]
- Selvakumar, S.; Babu, I.S.; Chidambaranathan, N. In-Vivo Central Nervous System-Locomotor Activity of Some Synthesized 2-[(1-((phenyl amino) methyl) Substituted 1-benzoimidazol-2-yl) alkyl] Isoindoline-1,3-diones. Biosci. Biotech. Res. Asia 2013, 10, 937–943. [Google Scholar] [CrossRef]
- Schulz, W.G.; Islam, I.; Skibo, E.B. Pyrrolo [1,2-a] benzimidazole-based quinones and iminoquinones. The role of the 3-substituent on cytotoxicity. J. Med. Chem. 1995, 38, 109–118. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorganic Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Zhou, R.R.; Cai, Q.; Li, D.K.; Zhuang, S.Y.; Wu, Y.D.; Wu, A.X. Acid-Promoted Multicomponent Tandem Cyclization to Synthesize Fully Substituted Oxazoles via Robinson–Gabriel-Type Reaction. J. Org. Chem. 2017, 82, 6450–6456. [Google Scholar] [CrossRef]
- Taha, M.; Sadia, H.; Rahim, F.; Khan, M.I.; Hayat, S.; Iqbal, N.; Nawaz, F.; Ullah, H.; Zada, H.; Shah, S.A.A.; et al. Synthesis, biological evaluation and molecular docking study of oxindole based chalcone analogues as potent anti-Alzheimer agents. J. Mol. Struct. 2023, 1285, 135530. [Google Scholar] [CrossRef]
- Kakkar, S.; Kumar, S.; Lim, S.M.; Ramasamy, K.; Mani, V.; Shah, S.A.A.; Narasimhan, B. Design, synthesis and biological evaluation of 3-(2-aminooxazol-5-yl)-2H-chromen-2-one derivatives. Chem. Cent. J. 2018, 12, 130. [Google Scholar] [CrossRef]
- Khan, S.; Iqbal, S.; Khan, M.; Rehman, W.; Shah, M.; Hussain, R.; Rasheed, L.; Khan, Y.; Dera, A.A.; Pashameah, R.A.; et al. Design, synthesis, in silico testing, and in vitro evaluation of thiazolidinone-based benzothiazole derivatives as inhibitors of α-amylase and α-glucosidase. Pharmaceuticals 2022, 15, 1164. [Google Scholar] [CrossRef]
- Mumtaz, S.; Iqbal, S.; Shah, M.; Hussain, R.; Rahim, F.; Rehman, W.; Khan, S.; Abid, O.U.R.; Rasheed, L.; Dera, A.A.; et al. New triazinoindole bearing benzimidazole/benzoxazole hybrids analogs as potent inhibitors of urease: Synthesis, in vitro analysis and molecular docking studies. Molecules 2022, 27, 6580. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, H.; Taha, M.; Hussain, R.; Sarfraz, M.; Iqbal, R.; Iqbal, N.; Khan, S.; Ali Shah, S.A.; Albalawi, M.A.; et al. Synthesis of new triazole-based thiosemicarbazone derivatives as anti-Alzheimer’s disease candidates: Evidence-based in vitro study. Molecules 2022, 28, 21. [Google Scholar] [CrossRef]
- Alpan, A.S.; Parlar, S.; Carlino, L.; Tarikogullari, A.H.; Alptüzün, V.; Güneş, H.S. Synthesis, biological activity and molecular modeling studies on 1H-benzimidazole derivatives as acetylcholinesterase inhibitors. Bioorganic Med. Chem. 2013, 21, 4928–4937. [Google Scholar] [CrossRef]
- Hussain, R.; Ullah, H.; Rahim, F.; Sarfraz, M.; Taha, M.; Iqbal, R.; Rehman, W.; Khan, S.; Shah, S.A.A.; Hyder, S.; et al. Multipotent cholinesterase inhibitors for the treatment of Alzheimer’s disease: Synthesis, biological analysis and molecular docking study of benzimidazole-based thiazole derivatives. Molecules 2022, 27, 6087. [Google Scholar] [CrossRef] [PubMed]
- Coban, G.; Carlino, L.; Tarikogullari, A.H.; Parlar, S.; Sarıkaya, G.; Alptüzün, V.; Alpan, A.S.; Güneş, H.S.; Erciyas, E. 1H-benzimidazole derivatives as butyrylcholinesterase inhibitors: Synthesis and molecular modeling studies. Med. Chem. Res. 2016, 25, 2005–2014. [Google Scholar] [CrossRef]
- Šagud, I.; Maček Hrvat, N.; Grgičević, A.; Čadež, T.; Hodak, J.; Dragojević, M.; Lasić, K.; Kovarik, Z.; Škorić, I. Design, synthesis and cholinesterase inhibitory properties of new oxazole benzylamine derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Khan, S.; Ullah, H.; Ali, F.; Khan, Y.; Sardar, A.; Iqbal, R.; Ataya, F.S.; El-Sabbagh, N.M.; Batiha, G.E.S. Benzimidazole-Based Schiff Base Hybrid Scaffolds: A Promising Approach to Develop Multi-Target Drugs for Alzheimer’s Disease. Pharmaceuticals 2023, 16, 1278. [Google Scholar] [CrossRef] [PubMed]
- Chigurupati, S.; Selvaraj, M.; Mani, V.; Selvarajan, K.K.; Mohammad, J.I.; Kaveti, B.; Bera, H.; Palanimuthu, V.R.; Teh, L.K.; Salleh, M.Z. Identification of novel acetylcholinesterase inhibitors: Indolopyrazoline derivatives and molecular docking studies. Bioorganic Chem. 2016, 67, 9–17. [Google Scholar] [CrossRef]
- Chigurupati, S.; Selvaraj, M.; Mani, V.; Mohammad, J.I.; Selvarajan, K.K.; Akhtar, S.S.; Marikannan, M.; Raj, S.; Teh, L.K.; Salleh, M.Z. Synthesis of azomethines derived from cinnamaldehyde and vanillin: In vitro aetylcholinesterase inhibitory, antioxidant and insilico molecular docking studies. Med. Chem. Res. 2018, 27, 807–816. [Google Scholar] [CrossRef]
- Wadood, A.; Shareef, A.; Ur Rehman, A.; Muhammad, S.; Khurshid, B.; Khan, R.S.; Shams, S.; Afridi, S.G. In silico drug designing for ala438 deleted ribosomal protein S1 (RpsA) on the basis of the active compound Zrl15. ACS Omega 2021, 7, 397–408. [Google Scholar] [CrossRef]
- Rehman, A.U.; Zhen, G.; Zhong, B.; Ni, D.; Li, J.; Nasir, A.; Gabr, M.T.; Rafiq, H.; Wadood, A.; Lu, S.; et al. Mechanism of zinc ejection by disulfiram in nonstructural protein 5A. Phys. Chem. Chem. Phys. 2021, 23, 12204–12215. [Google Scholar] [CrossRef] [PubMed]





| S.NO | R1 | R2 | AChE IC50 ± SEM a (µM) | BuChE IC50 ± SEM a (µM) |
|---|---|---|---|---|
| 1 | 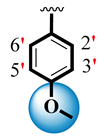 | 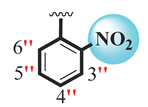 | 1.10 ± 0.10 | 1.80 ± 0.10 |
| 2 |  | 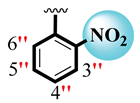 | 1.60 ± 0.010 | 1.90 ± 0.10 |
| 3 | 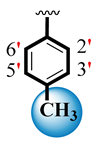 | 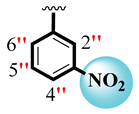 | 4.40 ± 0.10 | 5.80 ± 0.10 |
| 4 | 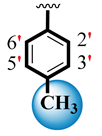 | 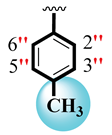 | 6.50 ± 0.20 | 8.30 ± 0.20 |
| 5 | 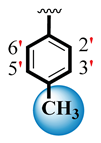 |  | 9.80 ± 0.20 | 11.50 ± 0.20 |
| 6 | 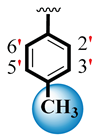 |  | 0.40 ± 0.05 | 1.10 ± 0.05 |
| 7 |  | 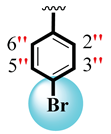 | 12.60 ± 0.30 | 16.30 ± 0.30 |
| 8 | 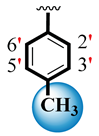 | 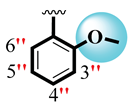 | 5.30 ± 0.20 | 7.20 ± 0.10 |
| 9 | 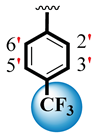 | 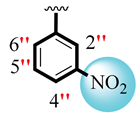 | 0.10 ± 0.050 | 0.20 ± 0.05 |
| 10 | 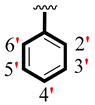 |  | 1.40 ± 0.10 | 2.10 ± 0.10 |
| 11 |  | 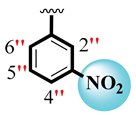 | 0.70 ± 0.050 | 1.20 ± 0.010 |
| 12 |  | 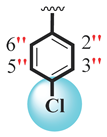 | 3.40 ± 0.10 | 5.70 ± 0.10 |
| 13 | 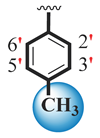 | 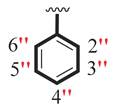 | 8.10 ± 0.10 | 9.70 ± 0.10 |
| 14 | 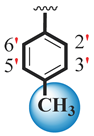 | 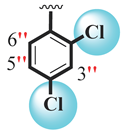 | 0.20 ± 0.050 | 0.30 ± 0.050 |
| 15 |  | 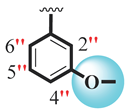 | 5.40 ± 0.10 | 7.20 ± 0.10 |
| 16 |  | 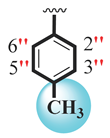 | 6.10 ± 0.10 | 9.30 ± 0.10 |
| 17 | 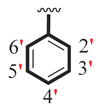 | 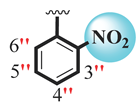 | 2.50 ± 0.10 | 3.50 ± 0.10 |
| 18 | 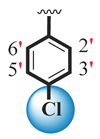 | 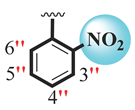 | 0.80 ± 0.10 | 1.70 ± 0.10 |
| 19 | 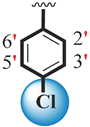 | 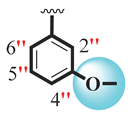 | 2.70 ± 0.20 | 3.70 ± 0.10 |
| Standard drug Donepezil | 2.16 ± 0.12 | 4.5 ± 0.11 | ||
| Active Analogues | Targeted Enzymes | Receptors | Interactions | Binding Affinities (kJ/mol) |
|---|---|---|---|---|
| Analogue-9 | AChE | GLY-117 | HB | −12.37 |
| TYR-130 | HB | |||
| GLU-198 | π-cation | |||
| TRP-84 | π-π T-shaped | |||
| PHE-330 | π-π T-shaped, π-alkyl and HB | |||
| TYR-221 | HB and Carbon HB | |||
| ASP-72 | π-anion | |||
| TYR-70 | π-sulphur | |||
| TYR-334 | π-π stacked and π-anion | |||
| TRP-279 | π-alkyl | |||
| SER-286 | Halogen (fluorine) | |||
| BuChE | GLU-238 | HB | −11.97 | |
| ASN-245 | HB | |||
| ASN-241 | HB | |||
| PRO-281 | π-alkyl | |||
| VAL-229 | π-alkyl | |||
| PRO-359 | π-alkyl | |||
| TYR-282 | π-π stacked and halogen (fluorine) | |||
| GLY-283 | halogen (fluorine) | |||
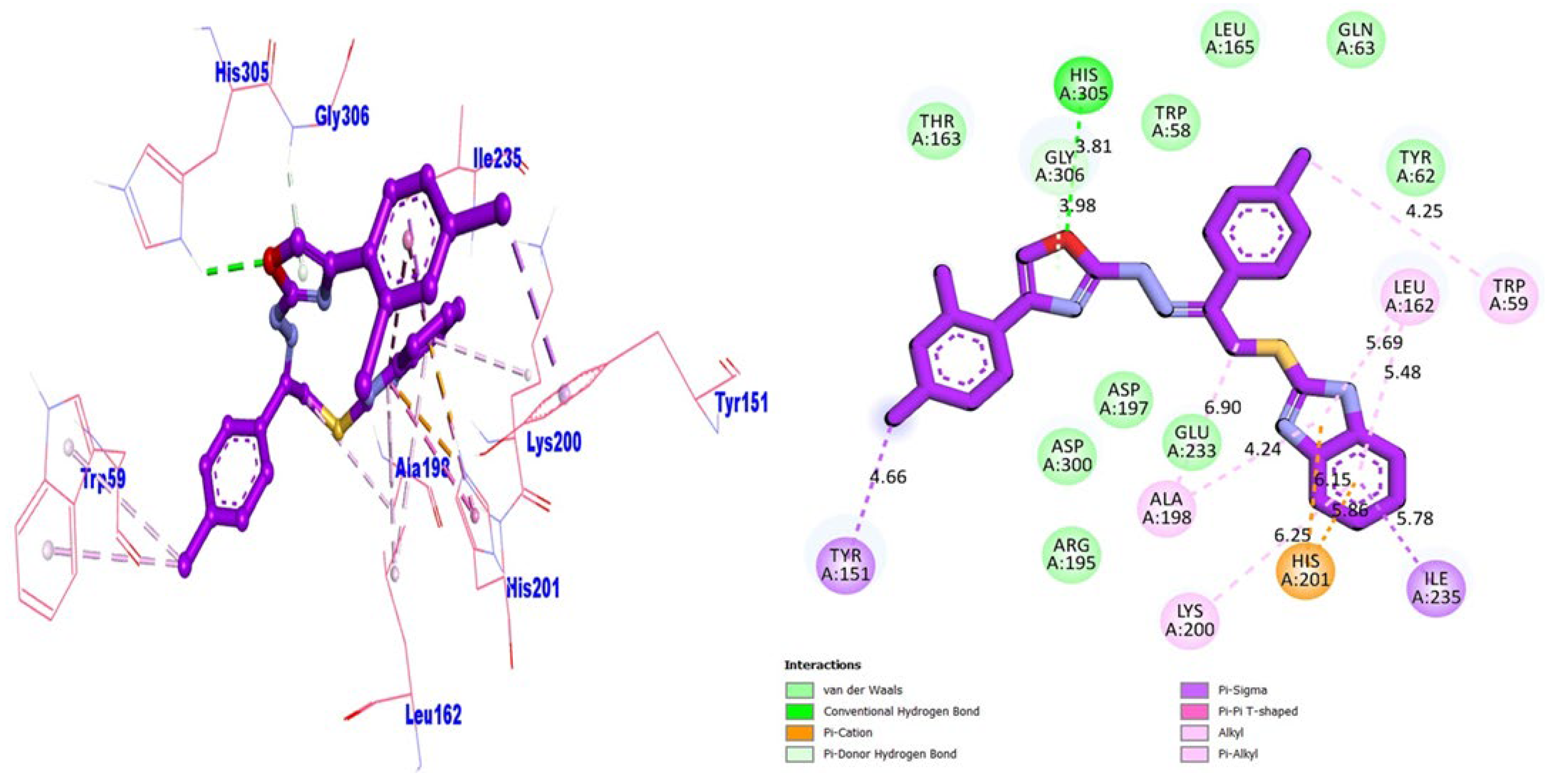
| Analogue-14 | AChE | TYR-151 | π-sigma | −11.17 |
| HIS-305 | HB | |||
| ALA-198 | π-alkyl | |||
| LYS-200 | π-alkyl | |||
| ILE-235 | π-sigma | |||
| LEU-162 | π-alkyl | |||
| TRP-59 | π-alkyl | |||
| HIS-201 | π-cation | |||
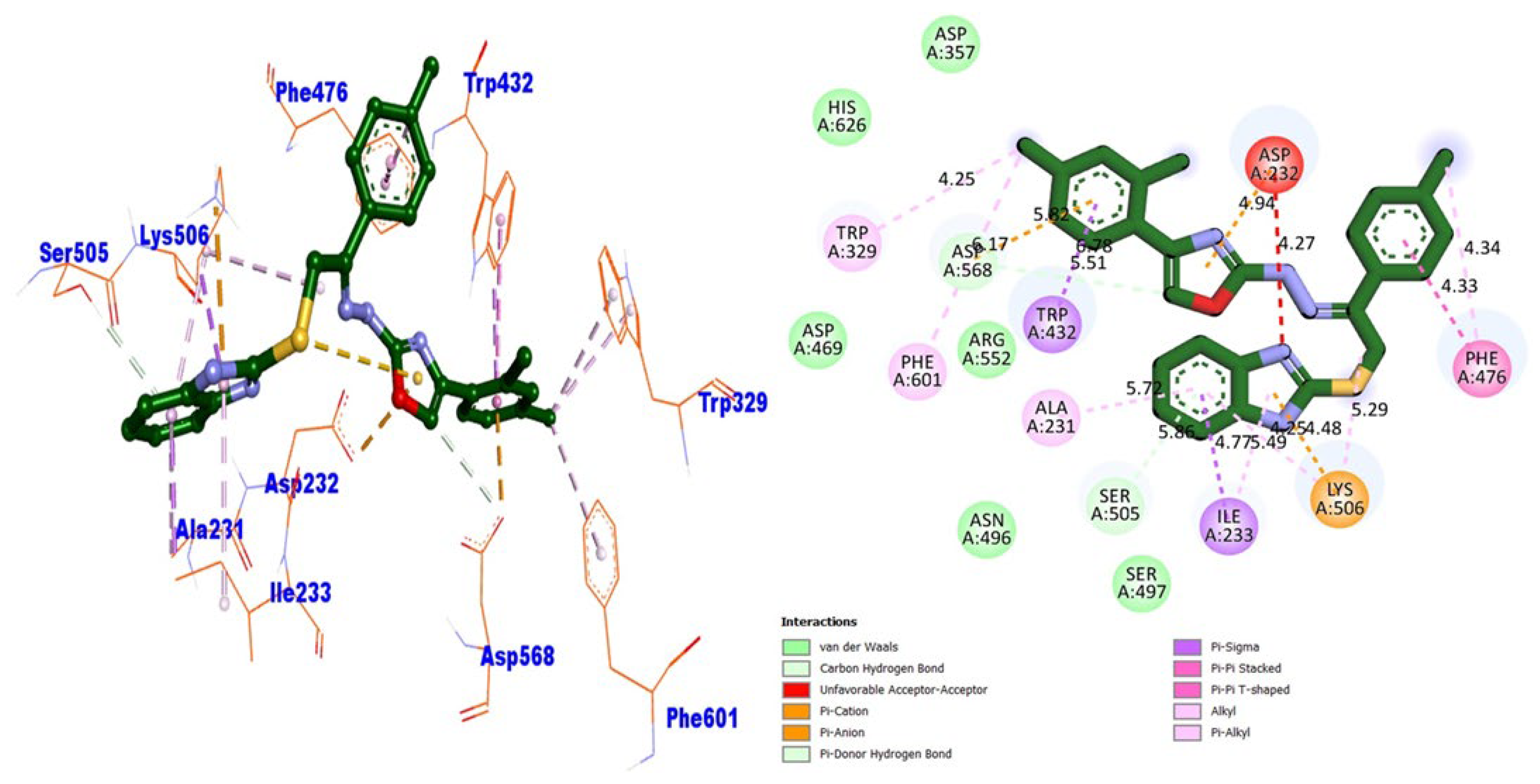
| BuChE | TRP-329 | π-alkyl | −10.87 | |
| PHE-601 | π-alkyl | |||
| ASP-568 | π-cation | |||
| TRP-432 | π-sigma | |||
| ASP-568 | Carbon HB | |||
| ASP-232 | π-cation | |||
| ALA-231 | π-alkyl | |||
| SER-505 | Carbon HB | |||
| ILE-233 | π-sigma and π-π T shaped | |||
| LYS-506 | π-anion and π-π T-shaped | |||
| LYS-506 | π-alkyl | |||
| PHE-476 | π-π T-shaped and π-alkyl | |||
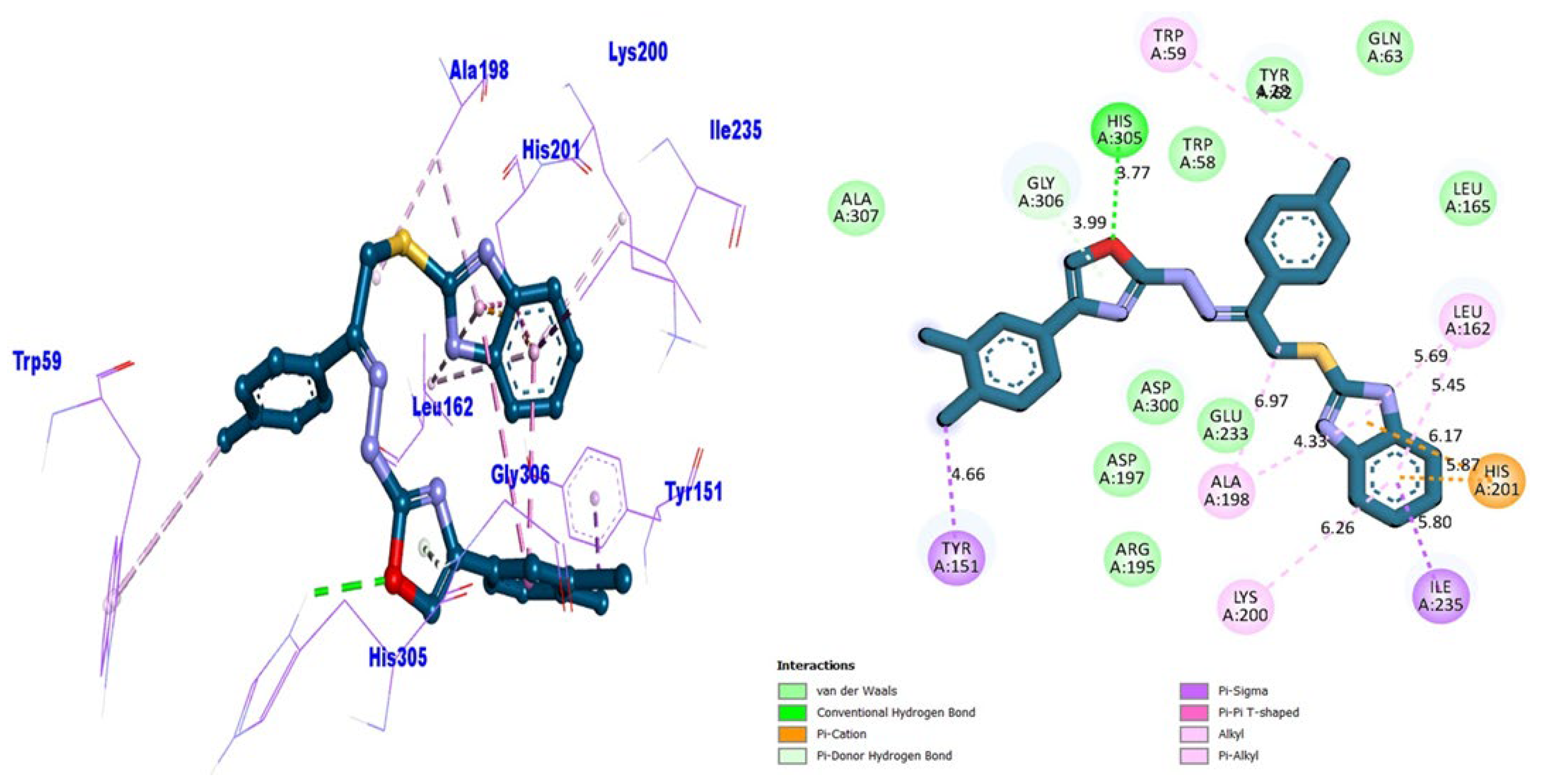
| Analogue-6 | AChE | TYR-151 | π-sigma | −10.12 |
| GLY-306 | Carbon HB | |||
| HIS-305 | HB | |||
| ALA-198 | π-alkyl | |||
| LYS-200 | π-alkyl | |||
| ILE-235 | π-sigma | |||
| HIS-201 | π-cation | |||
| LEU-162 | π-alkyl | |||
| TRP-59 | π-alkyl | |||
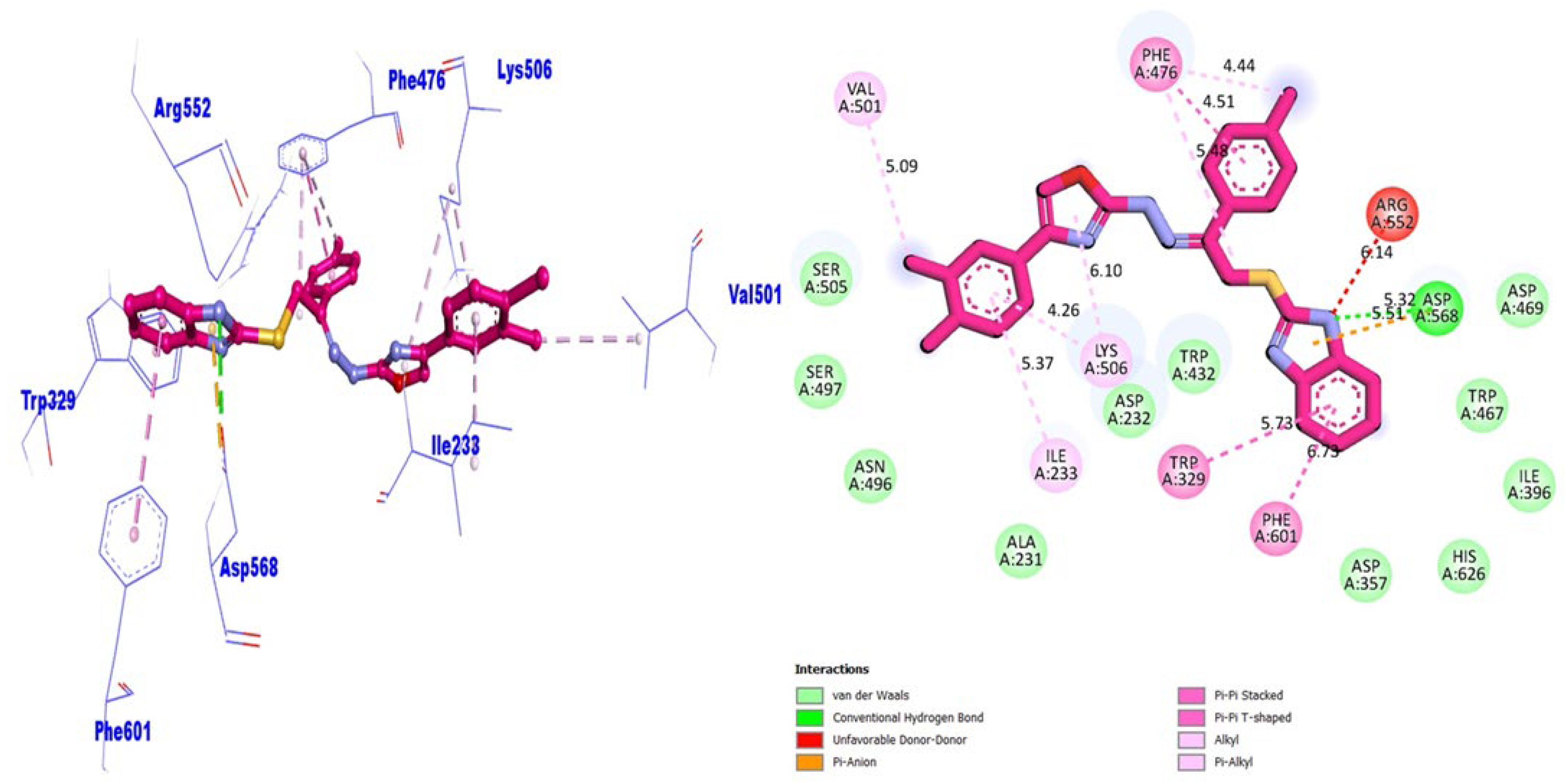
| BuChE | VAL-501 | π-alkyl | −9.76 | |
| ILE-233 | π-alkyl | |||
| LYS-506 | π-alkyl | |||
| TRP-329 | π-π T-shaped | |||
| PHE-601 | π-π T-shaped | |||
| ASP-568 | π-anion | |||
| ASP-568 | HB | |||
| PHE-476 | π-π stacked and π-alkyl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, R.; Rahim, F.; Ullah, H.; Khan, S.; Sarfraz, M.; Iqbal, R.; Suleman, F.; Al-Sadoon, M.K. Design, Synthesis, In Vitro Biological Evaluation and In Silico Molecular Docking Study of Benzimidazole-Based Oxazole Analogues: A Promising Acetylcholinesterase and Butyrylcholinesterase Inhibitors. Molecules 2023, 28, 7015. https://doi.org/10.3390/molecules28207015
Hussain R, Rahim F, Ullah H, Khan S, Sarfraz M, Iqbal R, Suleman F, Al-Sadoon MK. Design, Synthesis, In Vitro Biological Evaluation and In Silico Molecular Docking Study of Benzimidazole-Based Oxazole Analogues: A Promising Acetylcholinesterase and Butyrylcholinesterase Inhibitors. Molecules. 2023; 28(20):7015. https://doi.org/10.3390/molecules28207015
Chicago/Turabian StyleHussain, Rafaqat, Fazal Rahim, Hayat Ullah, Shoaib Khan, Maliha Sarfraz, Rashid Iqbal, Faiza Suleman, and Mohammad Khalid Al-Sadoon. 2023. "Design, Synthesis, In Vitro Biological Evaluation and In Silico Molecular Docking Study of Benzimidazole-Based Oxazole Analogues: A Promising Acetylcholinesterase and Butyrylcholinesterase Inhibitors" Molecules 28, no. 20: 7015. https://doi.org/10.3390/molecules28207015





