Thiophenium Salts as New Oxidant for Redox Polymerization under Mild- and Low-Toxicity Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Thiophenium Salts
2.1.1. Synthesis of 3,7-Ditertiobutyl-5-(trifluoromethyl)dibenzothiophenium Trifluoromethanesulfonate (Thiophenium (I))
2.1.2. Synthesis of 2,8-Difluoro-S-(trifluoromethyl)dibenzothiophenium Hexafluorophosphate (Thiophenium IV)
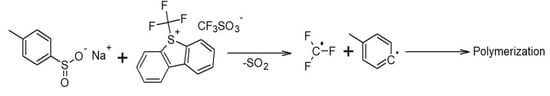
2.2. Thiophenium Salts in Redox Systems
2.2.1. Effect of the Reducing Agent
2.2.2. Effect of the Oxidizing Agent
2.2.3. Effect of the Concentration
2.2.4. Effect of the Monomer
2.2.5. Effect of Light Activation
2.2.6. Study of the System Stability
2.3. Mechanistic Consideration
2.4. Regulatory Labeling of the New Redox Agents
3. Experimental Section
3.1. Chemical Compounds
3.2. Two-Cartridge System Configuration for RFRP
3.3. Efficiency of the RIS Followed by Optical Pyrometry
3.4. Real-Time Fourier Transform Infrared (RT-FTIR) Spectroscopy
3.5. Synthesis of Thiophenium Salts
3.6. Electron Spin Resonance (ESR) Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Misra, G.S.; Bajpai, U.D.N. Redox polymerization. Prog. Polym. Sci. 1982, 8, 61–131. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Redox two-component initiated free radical and cationic polymerizations: Concepts,reactions and applications. Prog. Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Lee, J.H.; Prud’homme, R.K.; Aksay, I.A. Cure depth in photopolymerization: Experiments and theory. J. Mater. Res. 2001, 16, 3536–3544. [Google Scholar] [CrossRef] [Green Version]
- Neckers, D.C.; Jager, W. Photoinitiation for Polymerization: UV & EB at the Millennium, Volume VII, Chemistry & Technology for UV & EB Formulation for Coatings, Inks & Paints; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Pappas, S.P. UV Curing: Science and Technology; Technology Marketing Corp: Shelton, CT, USA, 1985; Volume 2. [Google Scholar]
- Fouassier, J.P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology: Fundamentals and Methods; Elsevier Applied Science: Amsterdam, The Netherlands, 1993; Volume 1. [Google Scholar]
- Fouassier, J.P. Photoinitiation, Photopolymerization, and Photocuring: Fundamentals and Applications; Hanser: Munich, Germany, 1995. [Google Scholar]
- Fouassier, J.P. Photochemistry and UV Curing; Research Signpost: Trivandrum, India, 2006. [Google Scholar]
- Kim, K.; Singstock, N.R.; Childress, K.; Sinha, J.; Salazar, A.; Whitfield, S.; Holder, A.; Stransbury, J.; Musgrave, C. Rational Design of Efficient Amine Reductant Initiators for Amine-Peroxide Redox Polymerization. J. Am. Chem. Soc. 2019, 141, 6279–6291. [Google Scholar] [CrossRef] [PubMed]
- Sarquis, A.M.; Sennet, L.M.; Kittredge, M.C.; Kittredge, K.W.; Sokol, M.S. Investigating the Stability of Benzoyl Peroxide in Over-the-Counter Acne Medications. J. Chem. Educ. 2008, 85, 1655–1657. [Google Scholar] [CrossRef]
- Benigni, R.; Passerini, L. Carcinogenicity of the aromatic amines: From structure–activity relationships to mechanisms of action and risk assessment. Mutat. Res. Mutat. Res. 2002, 511, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Li, G.B.; Zhang, C.; Song, C.; Ma, Y.D. Progress in copper-catalyzed trifluoromethylation. J. Org. Chem. 2018, 14, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Crivello, J. Redox Initiated Cationic Polymerization: Reduction of Triarylsulfonium Salts by Silanes. Silicon 2009, 1, 111–124. [Google Scholar] [CrossRef]
- Wang, D.; Szillat, F.; Fouassier, J.-P.; Lalevée, J. Remarkable Versatility of Silane/Iodonium Salt as Redox Free Radical, Cationic, and Photopolymerization Initiators. Macromolcules 2019, 52, 5638–5645. [Google Scholar] [CrossRef]
- Arar, A.; Wisson, L.; Lalevée, J. New Pure Organic and Peroxide-Free Redox Initiating Systems for Polymerization in Mild Conditions. Polymers 2021, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Sarac, A.S. Redox polymerization. Prog. Polym. Sci. 1999, 24, 1149–1204. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. A New Highly Efficient Amine-Free and Peroxide-Free Redox System for Free Radical Polymerization under Air with Possible Light Activation. Macromolecules 2016, 49, 6296–6309. [Google Scholar] [CrossRef]
- Zhejiang Jiuzhou Pharmaceutical Co. LTD. Halogenated S-(Perfluoroalkyl) Dibenzothiophenium Salt and Its Production Methods. WO2016/107578 A1, 18 December 2018. [Google Scholar]
- Streets, D.; Ceasar, G.P. Inductive and mesomeric effects on the π orbitals of halobenzenes. Mol. Phys. 1973, 26, 1037–1052. [Google Scholar] [CrossRef]
- Saito, M.; Kawaharasaki, S.; Ito, K.; Yamada, S.; Hayamizu, K.; Seki, S. Strategies for fast ion transport in electrochemical capacitor electrolytes from diffusion coefficients, ionic conductivity, viscosity, density and interaction energies based on HSAB theory. RSC Adv. 2017, 7, 14528–14535. [Google Scholar] [CrossRef] [Green Version]
- McCurdy, K.G.; Laidler, K.J. Rates of polymerization of acrylates and methacrylates in emulsion systems. Can. J. Chem. 1964, 42, 825–829. [Google Scholar] [CrossRef]
- Zhang, C. Recent advances in trifluoromethylation of organic compounds using Umemoto’s reagents. Org. Biomol. Chem. 2014, 12, 6580–6589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hu, D.; He, X.; Li, Y.; Chu, Y.; She, Y. Practical and efficient synthesis of aryl trifluoromethyl sulfones from arylsulfonyl chlorides with Umemoto’s reagent II. Tetrahedron Lett. 2020, 61, 151465. [Google Scholar] [CrossRef]
- Wang, Y.; Noble, A.; Sandford, C.; Aggarwal, V. Enantiospecific Trifluoromethyl-Radical-Induced Three-Component Coupling of Boronic Esters with Furans, Angew. Int. Ed. 2017, 56, 1810–1814. [Google Scholar] [CrossRef] [PubMed]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrat, A.; Simon, F.; Mazajczyk, J.; Charriere, B.; Fouquay, S.; Lalevee, J. Thiophenium Salts as New Oxidant for Redox Polymerization under Mild- and Low-Toxicity Conditions. Molecules 2023, 28, 627. https://doi.org/10.3390/molecules28020627
Barrat A, Simon F, Mazajczyk J, Charriere B, Fouquay S, Lalevee J. Thiophenium Salts as New Oxidant for Redox Polymerization under Mild- and Low-Toxicity Conditions. Molecules. 2023; 28(2):627. https://doi.org/10.3390/molecules28020627
Chicago/Turabian StyleBarrat, Alexis, Frédéric Simon, Jérôme Mazajczyk, Bruno Charriere, Stéphane Fouquay, and Jacques Lalevee. 2023. "Thiophenium Salts as New Oxidant for Redox Polymerization under Mild- and Low-Toxicity Conditions" Molecules 28, no. 2: 627. https://doi.org/10.3390/molecules28020627