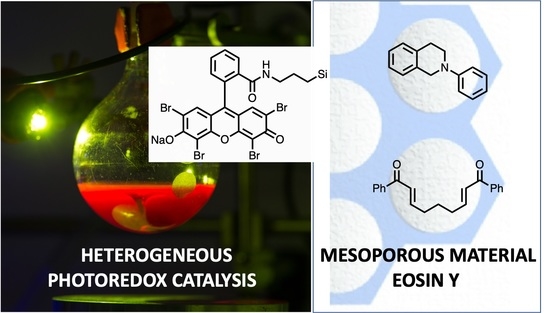
Heterogeneous Photoredox Catalysis Based on Silica Mesoporous Material and Eosin Y: Impact of Material Support on Selectivity of Radical Cyclization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Hybrid Photocatalysts
2.2. Photoredox Aza-Henry Reaction
2.3. Impact of the Support on the Photoreductive Cyclization of Aryl Bis(Enones)
3. Materials and Methods
3.1. Aza Henry Photocatalyzed Reaction
3.2. Photocatalyzed Cycloaddition of 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 16, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, T.; Akita, M. Visible-light radical reaction designed by Ru- and Ir-based photoredox catalysis. Inorg. Chem. Front. 2014, 1, 562–576. [Google Scholar] [CrossRef]
- Nakajima, K.; Miyake, Y.; Nishibayashi, Y. Synthetic Utilization of α-Aminoalkyl Radicals and Related Species in Visible Light Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1946–1956. [Google Scholar] [CrossRef]
- Ravelli, D.; Proti, S.; Fagnoni, M. Carbon–Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016, 49, 1911–1923. [Google Scholar] [CrossRef]
- Goddard, J.-P.; Ollivier, C.; Fensterbank, L. Photoredox Catalysis for the Generation of Carbon Centered Radicals. Acc. Chem. Res. 2016, 49, 1924–1936. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, J.; Chen, X. Cooperative Photoredox Catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038. [Google Scholar] [CrossRef]
- Matsui, J.K.; Lang, S.B.; Heitz, D.R.; Molander, G.A. Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development. ACS Catal. 2017, 7, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Shounak Ray, P.K.S.; Biswas, P. Recent Developments on Visible-Light Photoredox Catalysis by Organic Dyes for Organic Synthesis. In Visible Light-Active Photocatalysis; Ghosh, S., Ed.; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Buzzetti, L.; Crisenza, G.E.M.; Melchiorre, P. Mechanistic Studies in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 3730–3747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, C.R.J.; Yoon, T.P.; MacMillan, D.W.C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- König, B. Chemical Photocatalysis; DeGruyter: Berlin, Germany, 2013. [Google Scholar]
- McAtee, R.C.; McClain, E.J.; Corey, R.J. Stephenson, C.R.J. lluminating Photoredox Catalysis. Trends Chem. 2019, 1, 111–125. [Google Scholar] [CrossRef]
- van Santen, R.A. Modern Heterogeneous Catalysis: An Introduction; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Nørskov, J.K.; Studt, F.; Abild-Pedersen, F.; Bligaard, T. Fundamental Concepts in Heterogeneous Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Mak, C.H.; Han, X.; Du, M.; Kai, J.-J.; Tsang, K.F.; Jia, G.; Cheng, K.-C.; Shen, H.-H.; Hsu, H.-Y. Heterogenization of homogeneous photocatalysts utilizing synthetic and natural support materials. J. Mater. Chem. A. 2021, 9, 4454–4504. [Google Scholar] [CrossRef]
- Gisbertz, S.; Pieber, B. Heterogeneous photocatalysis in organic synthesis. Chem.PhotoChem. 2020, 4, 456–475. [Google Scholar]
- Xie, Z.; Wang, C.; deKrafft, K.E.; Lin, W. Highly Stable and Porous Cross-Linked Polymers for Efficient Photocatalysis. J. Am. Chem. Soc. 2011, 133, 2056–2059. [Google Scholar] [CrossRef]
- Gu, X.; Li, X.; Yang, Q.; Li, P.; Yao, Y. A simple metal-free catalytic sulfoxidation under visible light and air. Green Chem. 2013, 15, 357–361. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Li, Y.; Wu, X.; Xiao, J.; Adams, D.J.; Coopers, A.I. Conjugated Microporous Polymers with Rose Bengal Dye for Highly Efficient Heterogeneous Organo-Photocatalysis. Macromolecules 2013, 46, 8779–8783. [Google Scholar] [CrossRef]
- Jana, A.; Mondal, J.; Borah, P.; Mondal, S.; Bhaumik, A.; Zhao, Y. Ruthenium bipyridyl tethered porous organosilica: A versatile, durable and reusable heterogeneous photocatalyst. Chem. Commun. 2015, 51, 10746–10749. [Google Scholar] [CrossRef]
- Rackl, D.; Kreitmeier, P.; Reiser, O. Synthesis of a polyisobutylene-tagged fac-Ir(ppy)3 complex and its application as recyclable visible-light photocatalyst in a continuous flow process. Green Chem. 2016, 18, 214–219. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Huang, Y.; Zhang, T.; Liu, Y.; Yang, B.; He, C.; Zhou, X.; Zhang, J. Organic sponge photocatalysis. Green Chem. 2017, 19, 2925–2930. [Google Scholar] [CrossRef]
- Shanmugam, S.; Xu, S.; Adnan, N.N.M.; Boyer, C. Heterogeneous Photocatalysis as a Means for Improving Recyclability of Organocatalyst in “Living” Radical Polymerization. Macromolecules 2018, 51, 779–790. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, Y.; Luo, Y.; Yang, B.; Liu, Y.; Zhou, X.; Zhang, J. Organic Cotton Photocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 14759–14766. [Google Scholar] [CrossRef]
- Kumar, G.; Solanki, P.; Nazish, M.; Neogi, S.; Kureshy, R.I.; Khan, N.-U.H. Covalently hooked EOSIN-Y in a Zr(IV) framework as visible-light mediated, heterogeneous photocatalyst for efficient C-H functionalization of tertiary amines. J. Catal. 2019, 371, 298–304. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, W.; Huang, Y.; Li, X.; Liu, Y.; Yang, B.; He, C.; Zhou, X.; Zhang, J. Bifunctional organic sponge photocatalyst for efficient cross-dehydrogenative coupling of tertiary amines to ketones. Chem. Commun. 2017, 53, 12536–12539. [Google Scholar] [CrossRef] [Green Version]
- Kundu, T.; Wang, J.; Cheng, Y.; Du, Y.; Qian, Y.; Liu, G.; Zhao, D. Hydrazone-based covalent organic frameworks for Lewis acid catalysis. Dalton Trans. 2018, 47, 13824–13829. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Bo Peh, S.; Liu, G.; Kundu, T.; Dong, J.; Ying, Y.; Qian, Y.; Zhao, D. Cobalt-containing covalent organic frameworks for visible light-driven hydrogen evolution. Sci. China Chem. 2020, 2, 192–197. [Google Scholar] [CrossRef]
- Soria- Castro, S.M.; Lebeau, B.; Cormier, M.; Neunlist, S.; Daou, J.; Goddard, J.-P. Organic/Inorganic Heterogeneous Silica-Based Photoredox Catalyst for Aza-Henry Reactions. Eur. J. Org. Chem. 2020, 2020, 1572–1578. [Google Scholar] [CrossRef]
- Mouarrawis., V.; Plessius, R.; van der Vlugt, J.; Reek, J.N.H. Confinement Effects in Catalysis Using Well-Defined Materials and Cages. Front. Chem. 2018, 6, 623–643. [Google Scholar] [CrossRef] [Green Version]
- Sastre, G.; Corma, A. The confinement effect in zeolites. J. Mol. Cat. A-Chem. 2009, 305, 3–7. [Google Scholar] [CrossRef]
- Kaphan, D.M.; Levin, M.D.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. A supramolecular microenvironment strategy for transition metal catalysis. Science 2015, 350, 1235–1238. [Google Scholar] [CrossRef] [Green Version]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Acid Catalysis in Basic Solution: A Supramolecular Host Promotes Orthoformate Hydrolysis. Science 2007, 316, 85–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecozzi, S.; Rebek, J., Jr. The 55 % Solution: A Formula for Molecular Recognition in the Liquid State. Chem. Eur. J. 1998, 4, 1016–1022. [Google Scholar] [CrossRef]
- Lesthaeghe, D.; Van Speybroeck, V.; Waroquier, M. Theoretical evaluation of zeolite confinement effects on the reactivity of bulky intermediates. Phys. Chem. Chem. Phys. 2009, 11, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Spicer, R.L.; Stergiou, A.D.; Young, T.A.; Duarte, F.; Symes, M.D.; Lusby, P.J. Host–Guest-Induced Electron Transfer Triggers Radical-Cation Catalysis. J. Am. Chem. Soc. 2020, 142, 2134–2139. [Google Scholar] [CrossRef]
- Dalton, D.M.; Ellis, S.R.; Nichols, E.M.; Mathies, R.A.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular Ga4L612– Cage Photosensitizes 1,3-Rearrangement of Encapsulated Guest via Photoinduced Electron Transfer. J. Am. Chem. Soc. 2015, 137, 10128–10131. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Yang, Y.; Duan, C. A Metal–Organic Tetrahedron as a Redox Vehicle to Encapsulate Organic Dyes for Photocatalytic Proton Reduction. J. Am. Chem. Soc. 2015, 137, 3967–3974. [Google Scholar] [CrossRef]
- Lu, Z.; Lavendomme, R.; Burghaus, O.; Nitschke, J.R. A Zn4L6 Capsule with Enhanced Catalytic C−C Bond Formation Activity upon C60 Binding. Angew. Chem. Int. Ed. 2019, 58, 9073–9077. [Google Scholar] [CrossRef]
- Wu, K.; Li, K.; Chen, S.; Hou, Y.-J.; Lu, Y.-L.; Wang, J.-S.; Wei, M.-J.; Pan, M.; Su, C.-Y. The Redox Coupling Effect in a Photocatalytic RuII-PdII Cage with TTF Guest as Electron Relay Mediator for Visible-Light Hydrogen-Evolving Promotion. Angew. Chem. Int. Ed. 2020, 59, 2639–2643. [Google Scholar] [CrossRef]
- Ramamurthy, V. Controlling photochemical reactions via confinement: Zeolites. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 145–166. [Google Scholar] [CrossRef]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient Visible Light Photocatalysis of [2+2] Enone Cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Espelt, L.R.; Guzei, I.A.; Yoon, T.P. Photocatalytic reductive cyclizations of enones: Divergent reactivity of photogenerated radical and radical anion intermediates. Chem. Sci. 2011, 2, 2115–2119. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, G.K.; Scaiano, J.C. Heterogeneous Dual Photoredox-Lewis Acid Catalysis Using a Single Bifunctional Nanomaterial. ACS Catal. 2018, 8, 2914–2922. [Google Scholar] [CrossRef]
- Neumann, M.; Zeitler, K. A Cooperative Hydrogen-Bond-Promoted Organophotoredox Catalysis Strategy for Highly Diastereoselective, Reductive Enone Cyclization Chem. Eur. J. 2013, 19, 6950–6955. [Google Scholar] [CrossRef] [PubMed]
- Spellmeyer, D.C.; Houk, K.N. A Force-Field Model for Intramolecular Radical Additions. J. Org. Chem. 1987, 52, 959–974. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstriom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J.Am.Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Lettow, J.S.; Han, Y.J.; Schmidt-Winkel, P.; Yang, P.; Zhao, D.; Stucky, G.D.; Ying, J.Y. Hexagonal to Mesocellular Foam Phase Transition in Polymer-Templated Mesoporous Silicas. Langmuir 2000, 16, 8291–8295. [Google Scholar] [CrossRef]
- Li, S.; Wan, Q.; Qin, Z.; Fu, Y.; Gu, Y. Understanding Stöber Silica’s Pore Characteristics Measured by Gas Adsorption. Langmuir 2015, 31, 824–832. [Google Scholar] [CrossRef]
- THORLABS. Available online: https://www.thorlabs.com/. (accessed on 14 December 2022).
- Mojr, V.; Svobodová, E.; Straková, K.; Neveselý, T.; Chudoba, J.; Dvořáková, H.; Cibulka, R. Tailoring flavins for visible light photocatalysis: Organocatalytic [2+2] cycloadditions mediated by a flavin derivative and visible light. Chem. Commun. 2015, 51, 12036–12039. [Google Scholar] [CrossRef] [PubMed]







| Entries | Photocatalyst | Mesostructure | SBET (m2/g) | Pore Diameter (nm) | Mesoporous Volume (cm3/g) | EY wt% |
|---|---|---|---|---|---|---|
| 1 | SBA-15-APTES-EY | hexagonal 2D | 202 | 6.5 | 0.31 | 5.7 |
| 2 | SBA-16-APTES-EY | cubic body-centered | 6 | - | - | 2.0 |
| 3 | SBA-15+TMB-APTES-EY | hexagonal 2D | 223 | 7.5 | 0.40 | 6.4 |
| 4 | MCF-APTES-EY | mesocellular foam | 195 | 20 | 0.50 | 8.7 |
| 5 | Stöber-APTES-EY | - | 12 | - | - | 1.6 |
 | ||||
|---|---|---|---|---|
| Entries | PCat | Solvent | Deviation from Standard Conditions | Yield (%) 1 |
| 1 | Eosin Y | CH3NO2 | - | 96 |
| 2 | SBA-15-APTES-EY | CH3NO2 | - | 97 |
| 3 | SBA-15-APTES-RB | CH3NO2 | - | 85 |
| 4 | SBA_15 | CH3NO2 | - | 0 |
| 5 | SBA-15-APTES | CH3NO2 | - | 0 |
| 6 | SBA-15-APTES-EY | CH3NO2 | No light | 0 |
| 7 | SBA-15-APTES-EY | CH3NO2 | 16 h | 97 |
| 8 | SBA-15-APTES-EY | CH3NO2 | 8 h | 80 |
| 9 | Eosin Y | CH3NO2 | 16 h | 96 |
| 10 | Eosin Y | CH3NO2 | 8 h | 98 |
| 11 | SBA-15-APTES-EY | iPrOH | CH3NO2 (10 equiv.), 16 h | 94 |
| 12 | SBA-15-APTES-EY | Water | CH3NO2 (10 equiv.), 24 h | 20 |
| 13 | SBA-15-APTES-EY | Water | CH3NO2 (10 equiv.), 48 h | 34 |
| 14 | SBA-15-APTES-EY | iPrOH | CH3NO2 (10 equiv.), 16 h, PCat (1 mol%) | 92 |
| 15 | SBA-15-APTES-EY | iPrOH | CH3NO2 (10 equiv.), 16 h, PCat (0.5 mol%) | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, N.; Awassa, J.; Toufaily, J.; Lebeau, B.; Daou, T.J.; Cormier, M.; Goddard, J.-P. Heterogeneous Photoredox Catalysis Based on Silica Mesoporous Material and Eosin Y: Impact of Material Support on Selectivity of Radical Cyclization. Molecules 2023, 28, 549. https://doi.org/10.3390/molecules28020549
Mahmoud N, Awassa J, Toufaily J, Lebeau B, Daou TJ, Cormier M, Goddard J-P. Heterogeneous Photoredox Catalysis Based on Silica Mesoporous Material and Eosin Y: Impact of Material Support on Selectivity of Radical Cyclization. Molecules. 2023; 28(2):549. https://doi.org/10.3390/molecules28020549
Chicago/Turabian StyleMahmoud, Nadine, Jazia Awassa, Joumana Toufaily, Bénédicte Lebeau, T. Jean Daou, Morgan Cormier, and Jean-Philippe Goddard. 2023. "Heterogeneous Photoredox Catalysis Based on Silica Mesoporous Material and Eosin Y: Impact of Material Support on Selectivity of Radical Cyclization" Molecules 28, no. 2: 549. https://doi.org/10.3390/molecules28020549