Diversity, Antimicrobial, Antioxidant, and Anticancer Activity of Culturable Fungal Endophyte Communities in Cordia dichotoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation, Diversity, Identification, and Characterization of Endophytic Fungi
2.2. Antimicrobial Activity
2.3. Total Phenolic and Flavonoid Content
2.4. Antioxidant Activity
2.5. Anticancerous Activity
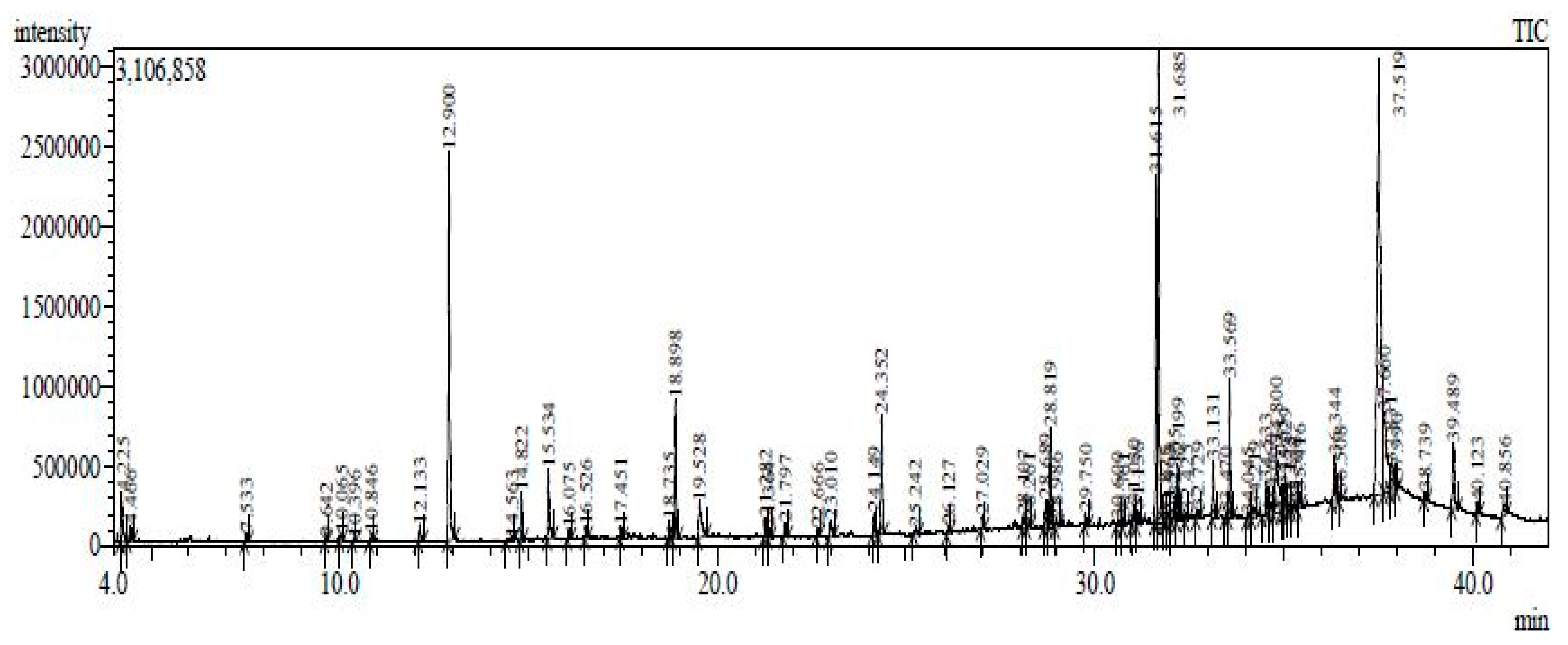
2.6. Chemical Composition of the Endophyic Fungus Cladosporium cladosporioides (MCS2) Analyzed by GC-MS
2.7. Chemical Profiling of Cladosporium cladosporioides (MCS2) Fungal Metabolites by LCMS
3. Materials and Methods
3.1. Collection, Identification, and Authentication of Plant Sample
3.2. Isolation of Fungal Endophytes
3.3. Characterization of Fungal Endophytes—Macroscopic and Microscopic-Based Identification
3.4. Molecular Based Identification
3.5. Bioactivity Assessment Fermentation and Extraction
3.6. Estimation of Total Phenolic and Total Flavonoid Content
3.7. Antimicrobial Activity Agar Disc Diffusion Method
3.8. Tube Dilution Assay
3.9. Antioxidant Activity DPPH Free Radical Scavenging Assay
3.10. Hydrogen Peroxide Scavenging Assay
3.11. Cytotoxic Activity
3.12. Chemical Constituents Using an GC-MS Analysis
3.13. Chemical Profiling of C. cladosporioides (MCS2) Metabolites by LC-MS Analysis
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Rai, N.; Kumari Keshri, P.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Gautam, V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology 2021, 12, 139–159. [Google Scholar] [CrossRef]
- Christian, N.; Sedio, B.E.; Florez, X.; Ramírez, L.A.; Rojas, E.I.; Mejía, L.C.; Herre, E.A. Host affinity of endophytic fungi and the potential for reciprocal interactions involving host secondary chemistry. Am. J. Bot. 2020, 107, 219–228. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Finnie, J.F.; Van Staden, J. The role of endophytes in secondary metabolites accumulation in medicinal plants under abiotic stress. S. Afr. J. Bot. 2020, 134, 126–134. [Google Scholar] [CrossRef]
- El, A.S.; El, M.T.; Rady, A.M.; Zein, N.; Enan, G.; Shindia, A.; Sitohy, B. Exploiting the biosynthetic potency of taxol from fungal endophytes of conifers plants; genome mining and metabolic manipulation. Molecules 2020, 25, 3000. [Google Scholar] [CrossRef]
- Tyagi, R.; Tyagi, S.; Gupta, A. Endophytes: A novel source of biologically active secondary metabolites. Res. J. Pharm. Technol. 2020, 13, 4479–4483. [Google Scholar] [CrossRef]
- Abdel, A.S.; Naggar, M.E.; Allam, A.; Morsy, O.M.; Othman, S.I. Microbial natural products in drug discovery. Processes 2020, 8, 470. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Pharmacological potential of fungal endophytes associated with medicinal plants: A review. J. Fungi 2021, 7, 147. [Google Scholar] [CrossRef]
- Chowdhary, K.; Kaushik, N. Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PLoS ONE 2015, 10, e0141444. [Google Scholar] [CrossRef]
- Gómez, O.C.; Luiz, J.H.H. Endophytic fungi isolated from medicinal plants: Future prospects of bioactive natural products from Tabebuia/Handroanthus endophytes. Appl. Microbiol. Biotechnol. 2018, 102, 9105–9119. [Google Scholar] [CrossRef]
- Nithaniyal, A. Cordia dichotoma G. Forst. Boraginaceae. In Ethnobotany of the Mountain Regions of Southeast Asia; Springer: Berlin/Heidelberg, Germany, 2021; pp. 323–331. [Google Scholar]
- Abdullah, M.; Usmani, S.; Kushwaha, P. A Comprehensive Review on Ethnopharmacology and Phytochemistry of an Underutilized Plant Cordia dichotoma L. Curr. Nutr. Food Sci. 2022, 18, 728–738. [Google Scholar] [CrossRef]
- Raghuvanshi, D.; Sharma, K.; Verma, R.; Kumar, D.; Kumar, H.; Khan, A.; Nepovimova, E. Phytochemistry, and pharmacological efficacy of Cordia dichotoma G. Forst.(Lashuda): A therapeutic medicinal plant of Himachal Pradesh. Biomed. Pharmacother. 2022, 153, 113400. [Google Scholar] [CrossRef]
- Di Bitetti, M.S. The distribution of grooming among female primates: Testing hypotheses with the Shannon-Wiener diversity index. Behaviour 2000, 137, 1517–1540. [Google Scholar] [CrossRef]
- Mollaei, S.; Khanehbarndaz, O.; Gerami, Z.; Ebadi, M. Molecular identification and phytochemical screening of endophytic fungi isolated from Lithospermum officinale L. roots: A new source of shikonin. Phytochemistry 2019, 168, 112116. [Google Scholar] [CrossRef]
- Kaul, S.; Ahmed, M.; Zargar, K.; Sharma, P.; Dhar, M.K. Prospecting endophytic fungal assemblage of Digitalis lanata Ehrh. (foxglove) as a novel source of digoxin: A cardiac glycoside. 3 Biotech 2013, 3, 335–340. [Google Scholar] [CrossRef]
- Kuo, J.; Chang, C.-F.; Chi, W.-C. Isolation of endophytic fungi with antimicrobial activity from medicinal plant Zanthoxylum simulans Hance. Folia Microbiol. 2021, 66, 385–397. [Google Scholar] [CrossRef]
- Raunsai, M.; Wulansari, D.; Fathoni, A.; Agusta, A. Antibacterial and antioxidant activities of endophytic fungi extracts of medicinal plants from Central Sulawesi. J. Appl. Pharm. Sci. 2018, 8, 69–74. [Google Scholar] [CrossRef]
- Phongpaichit, S.; Rungjindamai, N.; Rukachaisirikul, V.; Sakayaroj, J. Antimicrobial activity in cultures of endophytic fungi isolated from Garcinia species. FEMS Microbiol. Immunol. 2006, 48, 367–372. [Google Scholar] [CrossRef]
- Pan, F.; Su, T.-J.; Cai, S.-M.; Wu, W. Fungal endophyte-derived Fritillaria unibracteata var. wabuensis: Diversity, antioxidant capacities in vitro and relations to phenolic, flavonoid or saponin compounds. Sci. Rep. 2017, 7, 42008. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Yang, J.; Xiao, Y.; Cai, Y.; Wan, Y.; Li, C. Isolation and identification of flavonoid-producing endophytic fungi from medicinal plant Conyza blini H. Lév that exhibit higher antioxidant and antibacterial activities. PeerJ 2020, 8, e8978. [Google Scholar] [CrossRef]
- Ayob, F.W.; Mohamad, J.; Simarani, K. Antioxidants and phytochemical analysis of endophytic fungi isolated from a medicinal plant Catharanthus roseus. Borneo J. Sci. Technol. 2019, 1, 62–68. [Google Scholar] [CrossRef]
- Rani, R.; Sharma, D.; Chaturvedi, M.; Yadav, J.P. Total Phenolic Content and In vitro Antioxidant Activity of Endophytic Fungi Isolated from Calotropis procera L. Curr. Bioact. Compd. 2019, 15, 232–241. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management. Innov. Food Sci. Emerg. Technol. 2007, 8, 197–204. [Google Scholar] [CrossRef]
- De, A.T.; Peñalver, P.; Morales, J.C. Synthesis and evaluation of new phenolic-based antioxidants: Structure-activity relationship. Food Chem. 2007, 103, 55–61. [Google Scholar] [CrossRef]
- Lim, S.M.; Agatonovic, S.; Lim, F.T.; Ramasamy, K. High-performance thin layer chromatography-based phytochemical and bioactivity characterisation of anticancer endophytic fungal extracts derived from marine plants. J. Pharm. Biomed. Anal. 2021, 193, 113702. [Google Scholar] [CrossRef]
- Gauchan, D.P.; Kandel, P.; Tuladhar, A.; Acharya, A.; Kadel, U.; Baral, A.; García, M.R. Evaluation of antimicrobial, antioxidant and cytotoxic properties of bioactive compounds produced from endophytic fungi of Himalayan yew (Taxus wallichiana) in Nepal. F1000Research 2020, 9, 379. [Google Scholar] [CrossRef]
- Khiralla, A.; Mohamed, I.; Thomas, J.; Mignard, B.; Spina, R.; Yagi, S.; Laurain, D. A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants. Asian Pac. J. Trop. Med. 2015, 8, 701–704. [Google Scholar] [CrossRef]
- Kumar, S.; Aharwal, R.P.; Jain, R.; Sandhu, S.S. Bioactive molecules of endophytic fungi and their potential in anticancer drug development. Curr. Pharmacol. Rep. 2021, 7, 27–41. [Google Scholar] [CrossRef]
- Li, S.J.; Jiao, F.W.; Li, W.; Zhang, X.; Yan, W.; Jiao, R.H. Cytotoxic xanthone derivatives from the mangrove-derived endophytic fungus Peniophora incarnata Z4. J. Nat. Prod. 2020, 83, 2976–2982. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Li, S.; Zhang, W.; Liu, Z.; Tan, H.; Zhang, W. Trichothecene macrolidesfrom the endophytic fungus Paramyrotheciumroridum and their cytotoxic activity. Fitoterapia 2020, 147, 104768. [Google Scholar] [CrossRef]
- Tapfuma, K.I.; Nchabeleng, E.K.; Adebo, O.A.; Hussan, R.; Williams, R.D.; Ravuluvulu, A.B.; Niemann, N. Antibacterial activity and gas chromatography mass spectrometry (GC–MS)-based metabolite profiles of Celtis africana and its endophytic extracts. Ind. Crops Prod. 2020, 157, 112933. [Google Scholar] [CrossRef]
- Kaur, N.; Arora, D.S.; Kalia, N.; Kaur, M. Bioactive potential of endophytic fungus Chaetomium globosum and GC–MS analysis of its responsible components. Sci. Rep. 2020, 10, 18792. [Google Scholar] [CrossRef]
- Mishra, V.K.; Passari, A.K.; Chandra, P.; Leo, V.V.; Kumar, B.; Uthandi, S.; Singh, B.P. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD-GC-MS analysis. PLoS ONE 2017, 12, e0186234. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, A.; Yan, B.; Niu, S.; Tang, J.; Li, X.; Du, X.; Challis, G.; Che, Y.; Sun, H. LC-MS-guided isolation of penicilfuranone A: A new antifibrotic furancarboxylic acid from the plant endophytic fungus Penicillium sp. sh18. J. Nat. Prod. 2016, 79, 149–155. [Google Scholar] [CrossRef]
- Shaker, K.; Zohair, M.; Hassan, A.; Sweelam, H.; Ashour, W. LC–MS/MS and GC–MS based phytochemical perspectives and antimicrobial effects of endophytic fungus Chaetomium ovatoascomatis isolated from Euphorbia milii. Arch. Microbiol. 2022, 204, 661. [Google Scholar] [CrossRef]
- Tibpromma, S.; Karunarathna, S.; Bhat, J.; Suwannarach, N.; Stephenson, S.; Elgorban, A.; Mortimer, P. Using culture-dependent and molecular techniques to identify endophytic fungi associated with tea leaves (Camellia spp.) in Yunnan Province, China. Diversity 2022, 14, 287. [Google Scholar] [CrossRef]
- Rajabi, M.; Moghadam, M.; Azizi, A.; Soltani, J. Isolation and molecular identification of two rutin-producing endophytic fungi from Caper (Capparis spinosa L.). Biol. J. Microorg. 2022, 11, 169–180. [Google Scholar] [CrossRef]
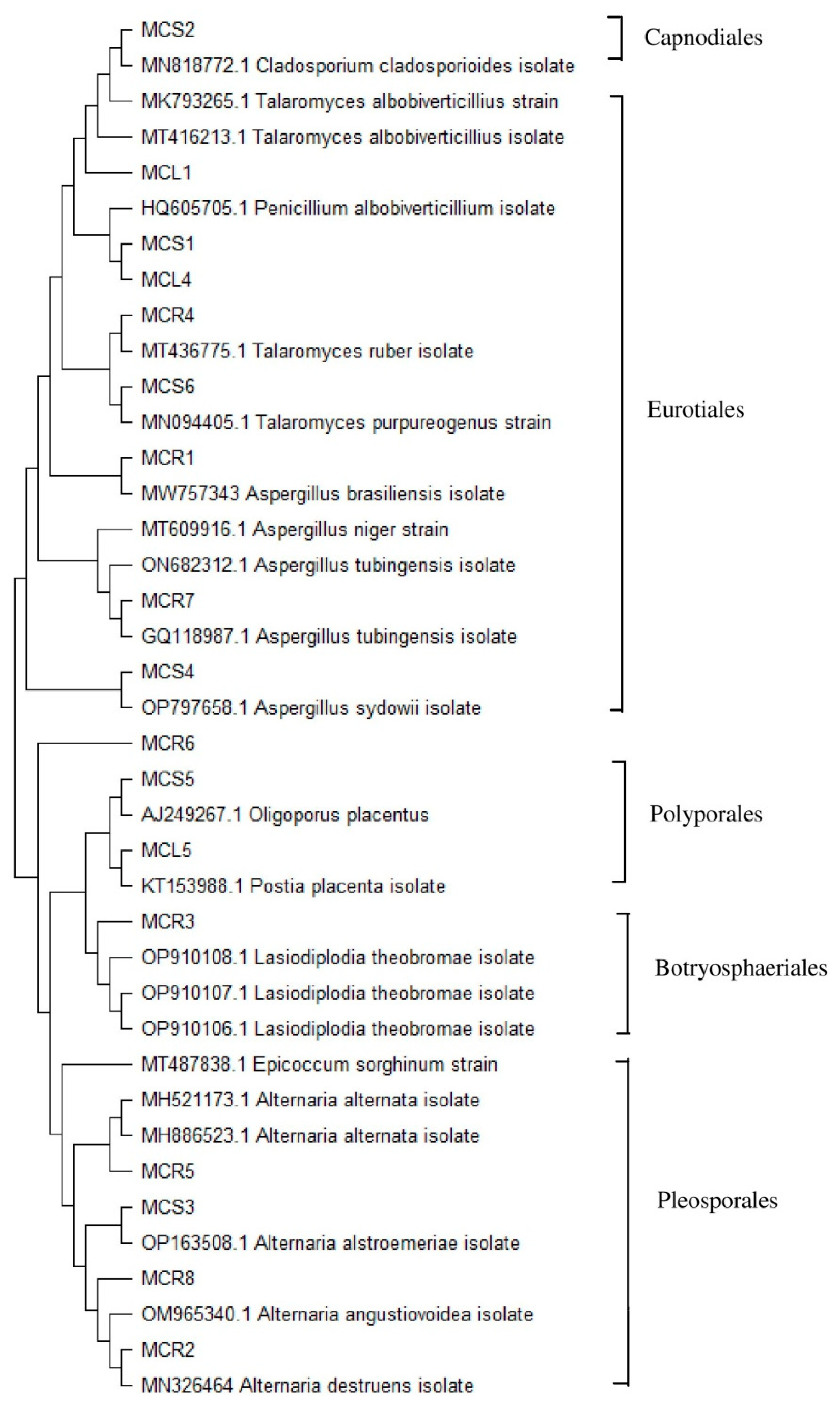

| Tissue | Fungal Codes | GenBank Accession Number | Closest Relatives in NCBI | Reference Sequence Accession Number | Identity (%) |
|---|---|---|---|---|---|
| Root | MCR1 | OP868830 | Aspergillus brasiliensis | MW757343 | 94 |
| MCR2 | OP868835 | Alternaria destruens | MN326464 | 100 | |
| MCR3 | OP868836 | Lasiodiplodiatheobromae | OP910107 | 100 | |
| MCR4 | OP868948 | Talaromycesruber | MT436775 | 100 | |
| MCR5 | OP868964 | Alternaria alternate | MH521173 | 100 | |
| MCR6 | OP869857 | Epicoccumsorghinum | MT487838 | 100 | |
| MCR7 | OP870013 | Aspergillus tubingensis | ON682312 | 99 | |
| MCR8 | OQ028266 | Alternaria angustiovoidea | OM965340 | 99 | |
| Stem | MCS1 | OP870014 | Aspergillus niger | MT609916 | 100 |
| MCS2 | OP870034 | Cladosporium cladosporioides | MN818772 | 100 | |
| MCS3 | OP999368 | Alternaria alstroemeriae | OP163508 | 99 | |
| MCS4 | OP870142 | Aspergillus sydowii | OP797658 | 100 | |
| MCS5 | OP876791 | Oligoporus placenta | AJ249267 | 97 | |
| MCS6 | OP870080 | Talaromycespurpureogenus | MN094405 | 99 |
| Fungal Isolate | Zone of Inhibition in mm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test Organisms | |||||||||
| Bacillus subtilis | Escherichia coli | Staphylococcus aureus | Klebsiella pneumoniae | Salmonella typhi | Pseudomonas aeruginosa | Aspergillus niger | Candida albicans | Candida tropicalis | |
| MCR1 | 15.5 ± 0.4 | 13.5 ± 0.4 | 14 ± 0.8 | 9 ± 0.8 | 13.5 ± 0.4 | 14 ± 0.8 | - | 18 ± 0.4 | 19.8 ± 0.2 |
| MCR2 | 22.1 ± 0.5 | 26.5 ± 0.4 | 19.9 ± 0.3 | 11.9 ± 0.3 | 16 ± 0.4 | 17.5 ± 0.4 | 23.8 ± 0.6 | 28 ± 0.8 | 16 ± 0.8 |
| MCR3 | 30.5 ± 0.4 | 32.1 ± 0.2 | 34.8 ± 0.2 | 22 ± 0.5 | 28.2 ± 0.1 | 19.5 ± 0.4 | 26.8 ± 0.2 | 24 ± 0.2 | 29 ± 0.2 |
| MCR4 | 10.8 ± 0.6 | 16 ± 0.4 | 16.5 ± 0.4 | - | 14.5 ± 0.4 | - | 14 ± 0.4 | - | - |
| MCR5 | - | 13.8 ± 0.6 | 11.5 ± 0.4 | 9 ± 0.4 | - | 6.1 ± 0.2 | - | 11 ± 0.4 | - |
| MCR6 | 23.8 ± 0.2 | 21.9 ± 0.3 | 18.5 ± 0.4 | 15 ± 0.4 | - | 17 ± 0.4 | 17.5 ± 0.4 | - | 19.9 ± 0.3 |
| MCR7 | 13 ± 0.4 | 18.1 ± 0.1 | 20.7 ± 0.2 | - | 15 ± 0.8 | - | - | 14 ± 0.8 | 11.8 ± 0.5 |
| MCR8 | 19.1 ± 0.6 | 22.5 ± 0.4 | 13.5 ± 0.4 | 17.6 ± 0.5 | 14.8 ± 0.6 | 16.1 ± 0.2 | 20.8 ± 0.6 | 17 ± 0.8 | 16.1 ± 0.2 |
| MCS1 | 8.6 ± 0.6 | 10.5 ± 0.4 | 15 ± 0.4 | 12.5 ± 0.4 | 11.5 ± 0.4 | - | - | 8.1 ± 0.2 | 11 ± 0.4 |
| MCS2 | 24 ± 0.8 | 28 ± 0.8 | 24 ± 1.6 | 25.5 ± 0.6 | 27.3 ± 0.8 | 21 ± 0.8 | 26.5 ± 2.0 | 18.4 ± 1.6 | 22 ± 0.8 |
| MCS3 | - | 10 ± 0.4 | - | 14.5 ± 0.4 | - | 11.5 ± 0.4 | - | - | - |
| MCS4 | 10.8 ± 0.6 | 11.5 ± 0.4 | 15.8 ± 0.6 | - | - | 12 ± 0.8 | - | 9 ± 0.4 | - |
| MCS5 | 18.6 ± 0.6 | - | 17.5 ± 0.4 | 12 ± 0.8 | - | - | - | 13 ± 0.4 | 11.1 ± 0.2 |
| MCS6 | 22.5 ± 0.4 | 24.8 ± 0.6 | 26.6 ± 1.24 | 19 ± 0.4 | 20.5 ± 0.6 | 15.1 ± 0.6 | 17 ± 0.8 | 21 ± 0.8 | 24.8 ± 0.6 |
| Root | 11.4 ± 0.9 | 14.7 ± 0.5 | 4.6 ± 1.6 | 3.2 ± 0.8 | - | - | - | 7.9 ± 1.3 | 12.3 ± 0.4 |
| Stem | 9.3 ± 0.7 | 5.8 ± 1.4 | - | - | - | 2.8 ± 0.6 | - | 10.9 ± 0.4 | - |
| Chloramphenicol | 31.5 ± 0.4 | 32.6 ± 0.4 | 35 ± 0.2 | 27.3 ± 0.4 | 28.6 ± 0.4 | 24.8 ± 0.6 | |||
| Fluconazol.e | 29.2 ± 0.2 | 29.5 ± 0.4 | 30 ± 0.4 | ||||||
| DMSO | - | - | - | - | - | - | - | - | - |
| Fungal Isolate | Minimum Inhibitory Concentration (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test Organisms | |||||||||
| Bacillus subtilis | Escherichia coli | Staphylococcus aureus | Klebsiella pneumoniae | Salmonella typhi | Pseudomonas aeruginosa | Aspergillus niger | Candida albicans | Candida tropicalis | |
| MCR1 | - | - | - | - | - | - | - | - | - |
| MCR2 | 100 | - | - | - | - | - | - | 100 | - |
| MCR3 | 25 | 12.5 | 6.25 | 200 | 50 | 100 | - | 50 | 100 |
| MCR4 | - | - | - | - | - | - | - | - | - |
| MCR5 | - | - | - | - | - | - | - | - | - |
| MCR6 | 100 | - | - | - | - | - | - | - | - |
| MCR7 | - | - | 200 | - | 100 | - | - | - | - |
| MCR8 | - | 200 | - | - | - | - | - | 200 | 100 |
| MCS1 | - | - | - | - | - | - | - | - | - |
| MCS2 | 25 | 100 | - | 100 | 50 | - | 100 | - | 200 |
| MCS3 | - | - | - | - | - | - | - | - | - |
| MCS4 | - | - | - | - | - | - | - | - | - |
| MCS5 | - | - | 200 | - | - | - | - | - | - |
| MCS6 | 100 | 200 | - | - | 100 | - | - | 200 | - |
| Root | - | 200 | - | - | - | - | - | - | - |
| Stem | - | - | - | - | - | - | - | - | - |
| Chloramphenicol | 6.25 | 6.25 | 3.125 | 25 | 12.5 | 25 | |||
| Fluconazole | 6.25 | 25 | 25 | ||||||
| Fungal Isolate | Species Identified | Total Phenolic Content (µg GAE/mg Extract) | Total Flavonoid Content (µg Catechin/mg of Extract) | Antioxidant Activity IC50 Value (µg/mL) | |
|---|---|---|---|---|---|
| DPPH Free Radical Scavenging Assay | H2O2 Scavenging Activity | ||||
| MCR1 | Aspergillus brasiliensis | 12.3 ± 1.2 | 17.5 ± 2.0 | 321.21 ± 0.8 | 252.48 ± 2.6 |
| MCR2 | Alternaria destruens | 50.18 ± 1.65 | 38.1 ± 0.8 | 198.09 ± 1.2 | 200.43 ± 0.5 |
| MCR3 | Lasiodiplodiatheobromae | 56.4 ± 3.3 | 35 ± 2.1 | 175.82 ± 0.2 | 165.56 ± 0.2 |
| MCR4 | Talaromycesruber | 22.1 ± 2.9 | 16.9 ± 2.4 | 341.97 ± 0.6 | 322.5 ± 0.9 |
| MCR6 | Epicoccumsorghinum | 92.24 ± 2.9 | 64.1 ± 3.1 | 131.24 ± 0.8 | 156.63 ± 0.4 |
| MCS1 | Aspergillus niger | 24.9 ± 2.1 | 23.4 ± 2.4 | 256.81 ± 0.4 | 269.02 ± 0.8 |
| MCS2 | Cladosporium cladosporioides | 138.4 ± 1.6 | 105.4 ± 2.3 | 95.56 ± 0.4 | 149.51 ± 0.2 |
| MCS4 | Aspergillus sydowii | 18.2 ± 2.8 | 33.8 ± 3.6 | 313.64 ± 0.2 | 371.3 ± 1.2 |
| MCS6 | Talaromycespurpureogenus | 30 ± 2.4 | 18.7 ± 2.0 | 232.09 ± 1.5 | 301.79 ± 3.1 |
| Root | 14 ± 0.6 | 7.8 ± 1.6 | 248.20 ± 0.8 | 302.59 ± 0.4 | |
| Stem | 23.5 ± 1.2 | 18.9 ± 0.8 | 224.92 ± 1.2 | 264.47 ± 1.6 | |
| Positive control (Ascorbic acid) | 18.24 ± 0.4 | 77.49 ± 0.2 | |||
| Fungal Isolate | Species Identified | IC50 (µg/mL) ± SD | ||
|---|---|---|---|---|
| MCF-7 | HCT-116 | PC-3 | ||
| MCR1 | Aspergillus brasiliensis | 25.76 ± 1.23 | >100 | >100 |
| MCR2 | Alternaria destruens | 5.85 ± 0.99 | 3.8 ± 0.46 | >100 |
| MCR3 | Lasiodiplodia theobromae | 1.19 ± 0.69 | 1.045 ± 0.15 | 39.73 ± 1.19 |
| MCR4 | Talaromyces ruber | >100 | >100 | >100 |
| MCR6 | Epicoccum sorghinum | 2.82 ± 0.63 | 19.70 ± 1.22 | >100 |
| MCS1 | Aspergillus niger | 15.01 ± 1.99 | 15.81 ± 1.11 | >100 |
| MCS2 | Cladosporium cladosporioides | 3.96 ± 0.13 | 2.29 ± 0.16 | 0.74 ± 0.008 |
| MCS4 | Aspergillus sydowii | 11.58 ± 1.22 | >100 | >100 |
| MCS6 | Talaromyces purpureogenus | 7.37 ± 0.66 | 1.51 ± 0.16 | >100 |
| Doxorubicin (Positive Control) | 0.11 ± 0.01 | 0.6 ± 0.02 | 1.02 ± 0.05 | |
| Root | 23.4 ± 1.12 | 81.45 ± 1.22 | >100 | |
| Stem | >100 | >100 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Bharti, S.; Goswami, A.; Mallubhotla, S. Diversity, Antimicrobial, Antioxidant, and Anticancer Activity of Culturable Fungal Endophyte Communities in Cordia dichotoma. Molecules 2023, 28, 6926. https://doi.org/10.3390/molecules28196926
Sharma M, Bharti S, Goswami A, Mallubhotla S. Diversity, Antimicrobial, Antioxidant, and Anticancer Activity of Culturable Fungal Endophyte Communities in Cordia dichotoma. Molecules. 2023; 28(19):6926. https://doi.org/10.3390/molecules28196926
Chicago/Turabian StyleSharma, Mahima, Sahil Bharti, Anindya Goswami, and Sharada Mallubhotla. 2023. "Diversity, Antimicrobial, Antioxidant, and Anticancer Activity of Culturable Fungal Endophyte Communities in Cordia dichotoma" Molecules 28, no. 19: 6926. https://doi.org/10.3390/molecules28196926







