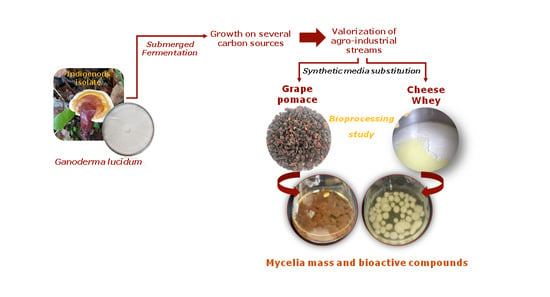
Ganoderma lucidum Mycelia Mass and Bioactive Compounds Production through Grape Pomace and Cheese Whey Valorization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mycelium Growth Rate on Solid State Fermentation (SSF)
2.2. Growth Performance on Conventional Carbon Sources Using Complex Media
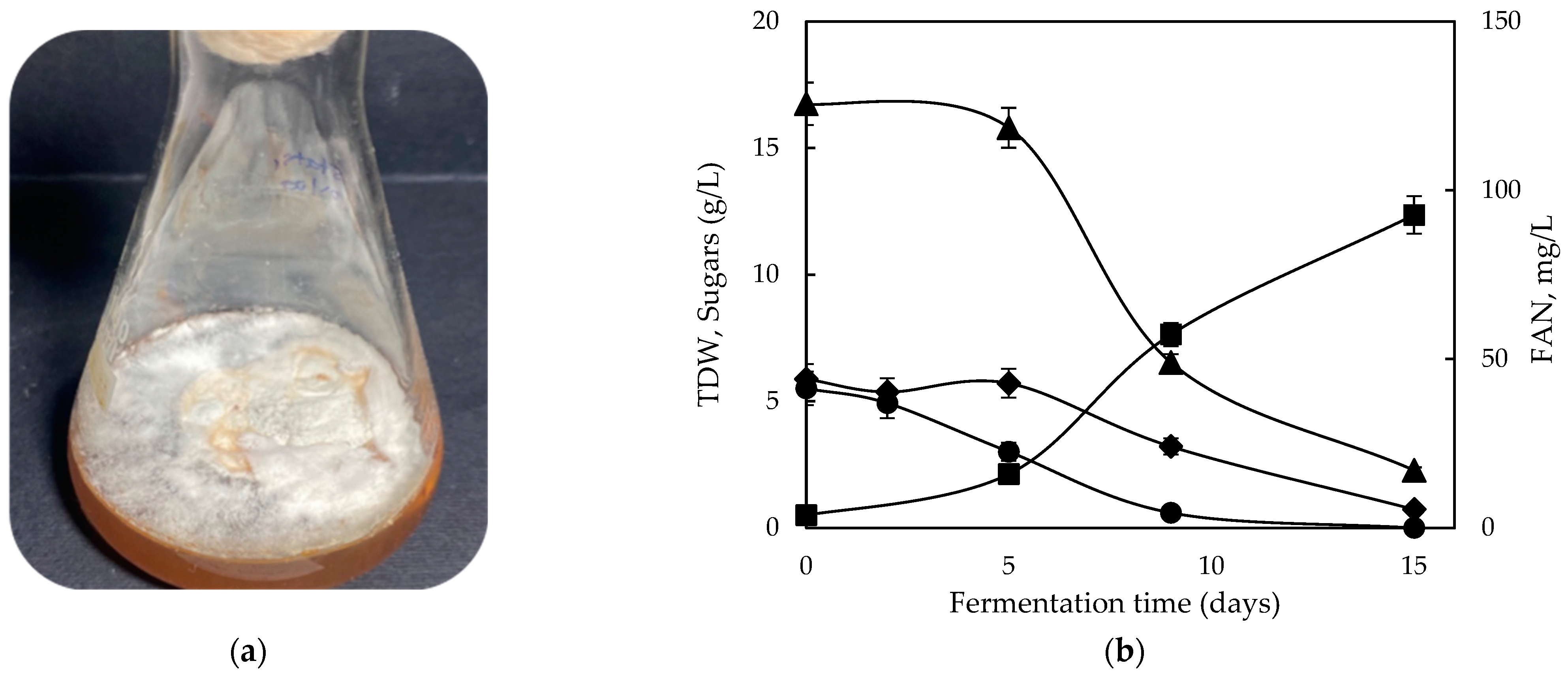
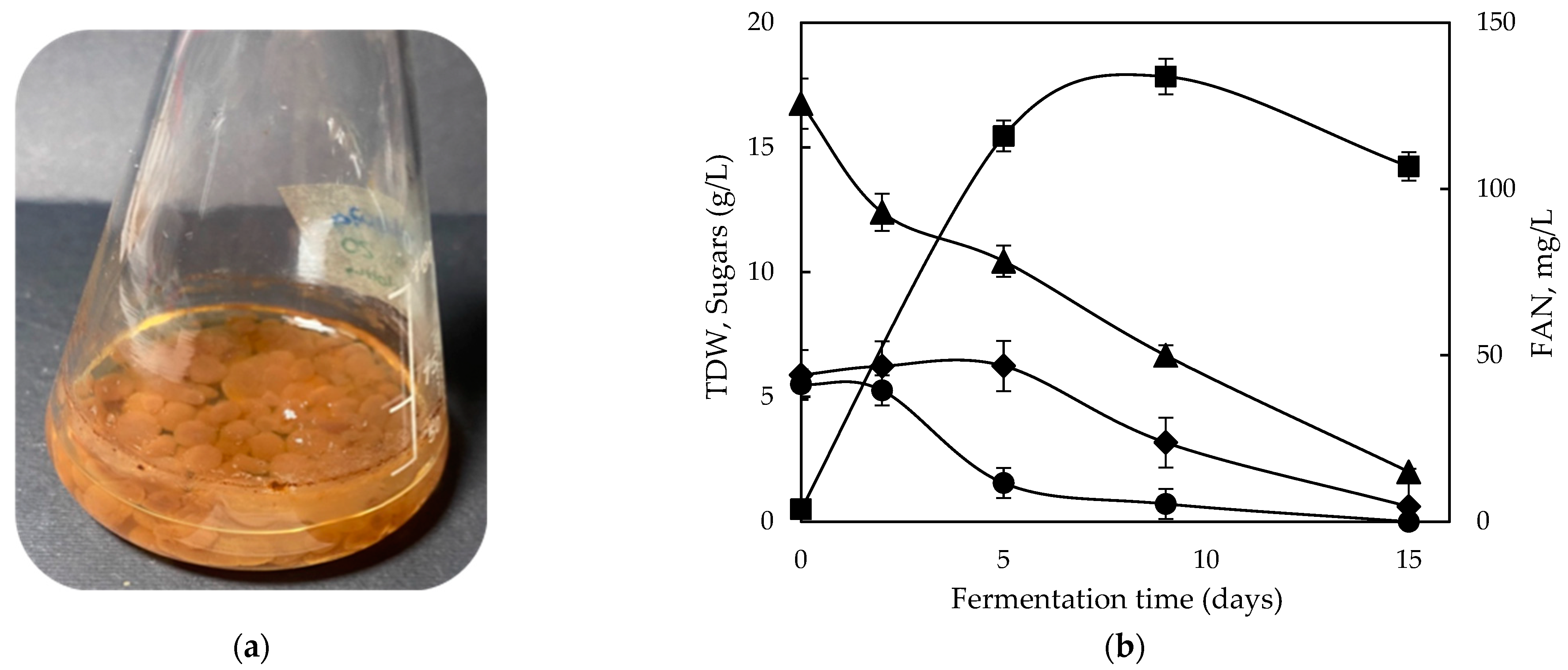
2.3. Growth Behavior on Grape Pomace Extract (GPE) as Nutrient Supplement
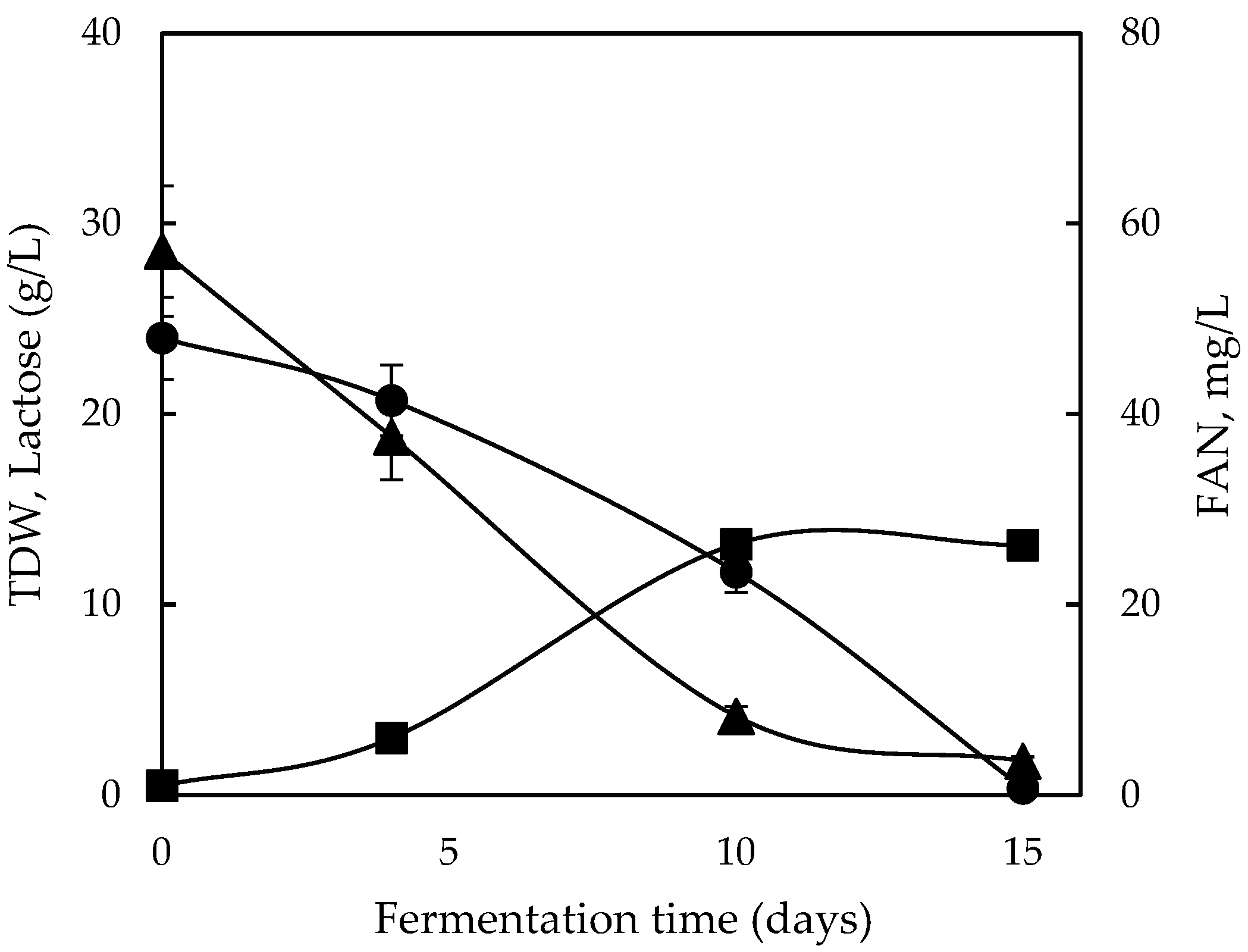
2.4. Growth Behavior on Commercial Lactose and Cheese Whey Permeate (CWP)
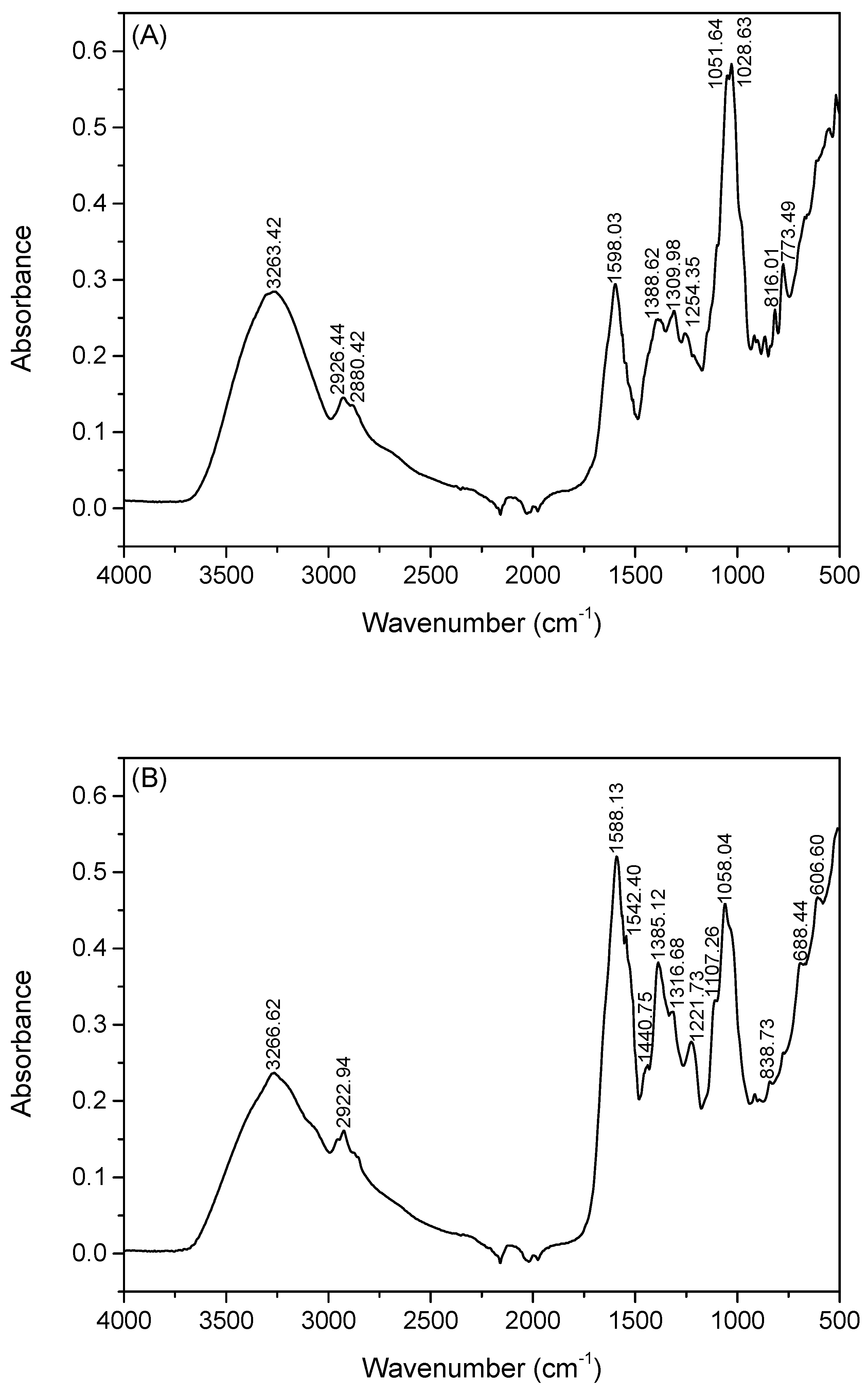
2.5. Production and Characterization of Crude Intracellular (IPS) and Extracellular (EPS) Polysaccharides
3. Materials and Methods
3.1. Fungal Material and Culture Conditions
3.2. Kinetic Growth Rate in Solid State Fermentation (SSF)
3.3. Raw Materials and Submerged Fermentations (SmF)
3.4. Analytical Methods
3.4.1. Total Dry Weight (TDW) Determination
3.4.2. Sugars and Free Amino Nitrogen Quantification
3.4.3. Extraction of Extracellular Polysaccharides (EPS)
3.4.4. Extraction of Fungal Intracellular Polysaccharides (IPS)
3.4.5. Compositional Characterization of the G. lucidum Aqueous Extracts
3.4.6. Fourier-Transform Infrared Spectroscopy (FTIR)
3.4.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A Critical Review on Submerged Production of Mushroom and Their Bioactive Metabolites. 3 Biotech 2020, 10, 337. [Google Scholar] [CrossRef]
- Santos Arteiro, J.M.; Martins, M.R.; Salvador, C.; Candeias, M.F.; Karmali, A.; Caldeira, A.T.; Yang, Q.; Wang, S.W.; Xie, Y.Y.H.; Sun, J.Y.; et al. Biological Activities of a Polysaccharide from the Coculture of Ganoderma Lucidum and Flammulina Velutipes Mycelia in Submerged Fermentation. Microorganisms 2022, 10, 10–18. [Google Scholar]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent Trends in Submerged Cultivation of Mushrooms and Their Application as a Source of Nutraceuticals and Food Additives. Futur. Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative Activities and Chemical Characterization of Polysaccharide Extracts from the Widely Used Mushrooms Ganoderma Applanatum, Ganoderma Lucidum, Lentinus Edodes and Trametes Versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Medicinal Mushrooms: Clinical Perspective and Challenges. Drug Discov. Today 2022, 27, 636–651. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Ka, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma Lucidum Terpenoids and Polysaccharides: A Review. Molecules 2018, 23, 649. [Google Scholar]
- Viceconte, F.R.; Diaz, M.L.; Soresi, D.S.; Lencinas, I.B.; Carrera, A.; Prat, M.I.; Gurovic, M.S.V. Ganoderma Sessile Is a Fast Polysaccharide Producer among Ganoderma Species. Mycologia 2021, 113, 513–524. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyżak, A.; Popiół, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszyńska, B. Bioactivity and Mycochemical Profile of Extracts from Mycelial Cultures of Ganoderma spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef]
- Xu, P.; Ding, Z.Y.; Qian, Z.; Zhao, C.X.; Zhang, K.C. Improved Production of Mycelial Biomass and Ganoderic Acid by Submerged Culture of Ganoderma Lucidum SB97 Using Complex Media. Enzyme Microb. Technol. 2008, 42, 325–331. [Google Scholar] [CrossRef]
- Wagner, R.; Mitchell, D.A.; Sassaki, G.L.; De Almeida Amazonas, M.A.L.; Berovič, M. Current Techniques for the Cultivation of Ganoderma Lucidum for the Production of Biomass, Ganoderic Acid and Polysaccharides. Food Technol. Biotechnol. 2003, 41, 371–382. [Google Scholar]
- Diamantopoulou, P.; Gardeli, C.; Papanikolaou, S. Impact of Olive Mill Wastewaters on the Physiological Behavior of a Wild-Type New Ganoderma Resinaceum Isolate. Environ. Sci. Pollut. Res. 2021, 28, 20570–20585. [Google Scholar] [CrossRef]
- Khoo, S.C.; Ma, N.L.; Peng, W.X.; Ng, K.K.; Goh, M.S.; Chen, H.L.; Tan, S.H.; Lee, C.H.; Luang-In, V.; Sonne, C. Valorisation of Biomass and Diaper Waste into a Sustainable Production of the Medical Mushroom Lingzhi Ganoderma Lucidum. Chemosphere 2022, 286, 131477. [Google Scholar] [CrossRef]
- Soh, E.; Saeidi, N.; Javadian, A.; Hebel, D.E.; Le Ferrand, H. Effect of Common Foods as Supplements for the Mycelium Growth of Ganoderma Lucidum and Pleurotus Ostreatus on Solid Substrates. PLoS ONE 2021, 16, e0260170. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing By-Products in the EU: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Perra, M.; Cuena-Lombraña, A.; Bacchetta, G.; Manca, M.L.; Manconi, M.; Maroun, R.G.; Muntoni, A.; Tuberoso, C.I.; Gil, K.A.; De Gioannis, G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability 2022, 14, 10690. [Google Scholar] [CrossRef]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of Grape Stalks and Pomace for the Production of Bio-Based Succinic Acid by Actinobacillus Succinogenes. Ind. Crop. Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Kuhar, F.; Papinutti, L. Protective Effect of Vanilloids against Chemical Stress on the White-Rot Fungus Ganoderma Lucidum. J. Environ. Manag. 2013, 124, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Fruiting Body, Spores and in Vitro Produced Mycelium of Ganoderma Lucidum from Northeast Portugal: A Comparative Study of the Antioxidant Potential of Phenolic and Polysaccharidic Extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef]
- Fletcher, I.; Freer, A.; Ahmed, A.; Fitzgerald, P. Effect of Temperature and Growth Media on Mycelium Growth of Pleurotus Ostreatus and Ganoderma Lucidum Strains. Cohesive J. Microbiol. Infect. Dis. 2019, 2, 10–15. [Google Scholar] [CrossRef]
- Hassan, N.A.; Supramani, S.; Azzimi Sohedein, M.N.; Ahmad Usuldin, S.R.; Klaus, A.; Ilham, Z.; Chen, W.H.; Wan-Mohtar, W.A.A.Q.I. Efficient Biomass-Exopolysaccharide Production from an Identified Wild-Serbian Ganoderma Lucidum Strain BGF4A1 Mycelium in a Controlled Submerged Fermentation. Biocatal. Agric. Biotechnol. 2019, 21, 101305. [Google Scholar] [CrossRef]
- Rosales-López, C.; Vargas-López, A.; Monge-Artavia, M.; Rojas-Chaves, M. Evaluation of Conditions to Improve Biomass Production by Submerged Culture of Ganoderma Sp. Microorganisms 2022, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, J.P.; Supramani, S.; Ahmad Usuldin, S.R.; Ilham, Z.; Klaus, A.; Khairul Ikram, N.K.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. Efficient Biomass-Endopolysaccharide Production from an Identified Wild-Serbian Ganoderma Applanatum Strain BGS6Ap Mycelium in a Controlled Submerged Fermentation. Biocatal. Agric. Biotechnol. 2021, 37, 102166. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, L.; Guo, X.; Li, Y.; Hou, B.; Fan, Q.; Wang, K.; Luo, Y.; Zhong, J.-J. Sucrose Fed-Batch Strategy Enhanced Biomass, Polysaccharide, and Ganoderic Acids Production in Fermentation of Ganoderma Lucidum 5.26. Bioprocess Biosyst. Eng. 2016, 39, 37–44. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Chatzikonstantinou, A.V.; Xiros, C.; Katapodis, P.; Stamatis, H. Mycoprotein Production by Submerged Fermentation of the Edible Mushroom Pleurotus Ostreatus in a Batch Stirred Tank Bioreactor Using Agro-Industrial Hydrolysate. Foods 2023, 12, 2295. [Google Scholar] [CrossRef]
- Pessoni, R.A.B.; Tersarotto, C.C.; Mateus, C.A.P.; Zerlin, J.K.; Simões, K.; de Cássia, L.; Figueiredo-Ribeiro, R.; Braga, M.R. Fructose Affecting Morphology and Inducing β-Fructofuranosidases in Penicillium Janczewskii. Springerplus 2015, 4, 487. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Huang, Q.; Hu, B.; Liang, L.; Wang, Q. Gllac7 Is Induced by Agricultural and Forestry Residues and Exhibits Allelic Expression Bias in Ganoderma Lucidum. Front. Microbiol. 2022, 13, 890686. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome Sequence of the Model Medicinal Mushroom Ganoderma Lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Papadaki, A.; Alexandri, M.; Poulios, V.; Gonou-Zagou, Z.; Kopsahelis, N. Sepedonium Sp. and Phellinus Sp. Novel Isolates: Growth Pattern and Production of Polysaccharide-Protein Complexes on Conventional and Grape Pomace Substrates. Waste Biomass Valoriz. 2023. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Grgić, J.; Perković, G.; Komlenić, D.K.; Bucić-Kojić, A. A Comparative Study of the Influence of Various Fungal-Based Pretreatments of Grape Pomace on Phenolic Compounds Recovery. Foods 2022, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-C.; Ke, Y.-F.; Kuo, S.-S. Effect of Fatty Acids on the Mycelial Growth and Polysaccharide Formation by Ganoderma Lucidum in Shake Flask Cultures. Enzyme Microb. Technol. 2000, 27, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Papanikolaou, S.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Patterns of Major Metabolites Biosynthesis by Different Mushroom Fungi Grown on Glucose-Based Submerged Cultures. Bioprocess Biosyst. Eng. 2014, 37, 1385–1400. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Zhang, Y.; He, J.; Xie, Y. Enhanced Exopolysaccharide Production in Submerged Fermentation of Ganoderma Lucidum by Tween 80 Supplementation. Bioprocess Biosyst. Eng. 2021, 44, 47–56. [Google Scholar] [CrossRef]
- Tu, G.; Wang, Y.; Ji, Y.; Zou, X. The Effect of Tween 80 on the Polymalic Acid and Pullulan Production by Aureobasidium Pullulans CCTCC M2012223. World J. Microbiol. Biotechnol. 2015, 31, 219–226. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhong, J.J. Exopolysaccharide Biosynthesis and Related Enzyme Activities of the Medicinal Fungus, Ganoderma Lucidum, Grown on Lactose in a Bioreactor. Biotechnol. Lett. 2002, 24, 1023–1026. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.; Hwang, S. Optimizing Bioconversion of Deproteinated Cheese Whey to Mycelia of Ganoderma Lucidum. Process Biochem. 2003, 38, 1685–1693. [Google Scholar] [CrossRef]
- Song, M.; Kim, N.; Lee, S.; Hwang, S. Use of Whey Permeate for Cultivating Ganoderma Lucidum Mycelia. J. Dairy Sci. 2007, 90, 2141–2146. [Google Scholar] [CrossRef]
- Zhu, L.W.; Zhong, J.J.; Tang, Y.J. Significance of Fungal Elicitors on the Production of Ganoderic Acid and Ganoderma Polysaccharides by the Submerged Culture of Medicinal Mushroom Ganoderma Lucidum. Process Biochem. 2008, 43, 1359–1370. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhang, W.; Liu, R.S.; Zhu, L.W.; Zhong, J.J. Scale-up Study on the Fed-Batch Fermentation of Ganoderma Lucidum for the Hyperproduction of Ganoderic Acid and Ganoderma Polysaccharides. Process Biochem. 2011, 46, 404–408. [Google Scholar] [CrossRef]
- Fang, Q.-H.; Zhong, J.-J. Submerged Fermentation of Higher Fungus Ganoderma Lucidum for Production of Valuable Bioactive Metabolites—Ganoderic Acid and Polysaccharide. Biochem. Eng. J. 2002, 10, 61–65. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhong, J.-J. Fed-Batch Fermentation of Ganoderma Lucidum for Hyperproduction of Polysaccharide and Ganoderic Acid. Enzyme Microb. Technol. 2002, 31, 20–28. [Google Scholar] [CrossRef]
- Berovič, M.; Popovic, M. Submerged Cultivation of Ganoderma Lucidum Biomass in Stirred Tank Reactor. Chem. Biochem. Eng. Q. 2018, 32, 465–472. [Google Scholar] [CrossRef]
- Fraga, I.; Coutinho, J.; Bezerra, R.M.; Dias, A.A.; Marques, G.; Nunes, F.M. Influence of Culture Medium Growth Variables on Ganoderma Lucidum Exopolysaccharides Structural Features. Carbohydr. Polym. 2014, 111, 936–946. [Google Scholar] [CrossRef]
- Rajalingam, D.; Loftis, C.; Xu, J.J.; Kumar, T.K.S. Trichloroacetic Acid-Induced Protein Precipitation Involves the Reversible Association of a Stable Partially Structured Intermediate. Protein Sci. 2009, 18, 980–993. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef]
- Xu, J.-W.; Ji, S.-L.; Li, H.-J.; Zhou, J.-S.; Duan, Y.-Q.; Dang, L.-Z.; Mo, M.-H. Increased Polysaccharide Production and Biosynthetic Gene Expressions in a Submerged Culture of Ganoderma Lucidum by the Overexpression of the Homologous α-Phosphoglucomutase Gene. Bioprocess Biosyst. Eng. 2015, 38, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, J.; Liu, Y.; Xu, Z.; Wu, J.-Y.; Ding, Z.; Gu, Z.; Zhang, L.; Shi, G. Effects of Mixed Carbon Sources on Galactose and Mannose Content of Exopolysaccharides and Related Enzyme Activities in Ganoderma Lucidum. RSC Adv. 2016, 6, 39284–39291. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Liu, X.-C.; Dong, F.-Y.; Guo, M.-Z.; Wang, X.-T.; Wang, Z.; Zhang, Y.-M. Influence of Fermentation Conditions on Polysaccharide Production and the Activities of Enzymes Involved in the Polysaccharide Synthesis of Cordyceps Militaris. Appl. Microbiol. Biotechnol. 2016, 100, 3909–3921. [Google Scholar] [CrossRef]
- Srour, B.; Bruechert, S.; Andrade, S.L.A.; Hellwig, P. Secondary Structure Determination by Means of ATR-FTIR Spectroscopy. In Membrane Protein Structure and Function Characterization: Methods and Protocols; Lacapere, J.-J., Ed.; Springer: New York, NY, USA, 2017; pp. 195–203. ISBN 978-1-4939-7151-0. [Google Scholar]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.; Ganguly, J.; Paul, A.K. Partial Characterization of an Extracellular Polysaccharide Produced by the Moderately Halophilic Bacterium Halomonas Xianhensis SUR308. Biofouling 2015, 31, 735–744. [Google Scholar] [CrossRef]
- Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Gonou-Zagou, Z.; Kopsahelis, N. Trametes Versicolor as a Natural Source of Bioactive Compounds for the Production of Whey Protein Films with Functional Properties: A Holistic Approach to Valorize Cheese Whey. Waste Biomass Valoriz. 2022, 13, 3989–3998. [Google Scholar] [CrossRef]
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. Evaluation of Biomass and Chitin Production of Morchella Mushrooms Grown on Starch-Based Substrates. Foods 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, V.; Alimpoumpa, D.; Papadaki, A.; Lappa, I.; Alexopoulos, K.; Kopsahelis, N. Cheese Whey Utilization for Biosurfactant Production: Evaluation of Bioprocessing Strategies Using Novel Lactobacillus Strains. Biomass Convers. Biorefin. 2022, 12, 4621–4635. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.; Koutinas, A.A. Utilisation of By-Products from Sunflower-Based Biodiesel Production Processes for the Production of Fermentation Feedstock. Waste Biomass Valoriz. 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom Polysaccharides and Lipids Synthesized in Liquid Agitated and Static Cultures. Part I: Screening Various Mushroom Species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef]
- Yang, L.; Kang, X.; Dong, W.; Wang, L.; Liu, S.; Zhong, X.; Liu, D. Prebiotic Properties of Ganoderma Lucidum Polysaccharides with Special Enrichment of Bacteroides Ovatus and B. Uniformis in Vitro. J. Funct. Foods 2022, 92, 105069. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]

| Carbon Source | Glucose | Fructose | Mixed Sugars |
|---|---|---|---|
| Static conditions | |||
| TDWmax (g/L) | 11.44 ± 0.63 | 5.87 ± 0.18 | 6.60 ± 0.27 |
| QX (g/L/d) | 0.73 ± 0.02 | 0.27 ± 0.06 | 0.30 ± 0.10 |
| QS (g/L/d) | 0.78 ± 0.04 | 0.44 ± 0.11 | 0.39 ± 0.02 |
| YX/S (g/g) | 0.94 ± 0.04 | 0.61 ± 0.01 | 0.77 ± 0.03 |
| Shaking conditions | |||
| TDWmax (g/L) | 10.44 ± 0.52 | 6.18 ± 0.01 | 9.05 ± 0.59 |
| QX (g/L/d) | 0.67 ± 0.03 | 0.57 ± 0.14 | 0.86 ± 0.06 |
| QS (g/L/d) | 0.78 ± 0.04 | 0.92 ± 0.21 | 0.96 ± 0.23 |
| YX/S (g/g) | 0.85 ± 0.04 | 0.62 ± 0.01 | 0.82 ± 0.03 |
| Carbon Source | Lactose | CWP | CWP Supplemented |
|---|---|---|---|
| Static conditions | |||
| TDWmax (g/L) | 9.20 ± 0.52 | 4.90 ± 0.17 | 7.01 ± 0.07 |
| QX (g/L/d) | 0.46 ± 0.14 | 0.25 ± 0.11 | 0.54 ± 0.08 |
| QS (g/L/d) | 0.61 ± 0.09 | 0.63 ± 0.26 | 0.86 ± 0.28 |
| YX/S (g/g) | 0.76 ± 0.04 | 0.40 ± 0.03 | 0.47 ± 0.02 |
| Shaking conditions | |||
| TDWmax (g/L) | 8.60 ± 0.45 | 3.90 ± 0.44 | 7.52 ± 0.28 |
| QX (g/L/d) | 0.68 ± 0.12 | 0.20 ± 0.12 | 0.70 ± 0.09 |
| QS (g/L/d) | 0.87 ± 0.11 | 0.64 ± 0.32 | 1.20 ± 0.30 |
| YX/S (g/g) | 0.79 ± 0.14 | 0.31 ± 0.02 | 0.58 ± 0.09 |
| Fermentation Time (Days) | EPS Production (g/L) | IPS Production (g/L) | IPS Content (mg/100 mg Biomass) | μg Trolox Equivalents/mg Extract * | % Inhibition ** |
|---|---|---|---|---|---|
| GPE supplemented with yeast extract and peptone | |||||
| 5 | 1.28 ± 0.14 | 5.94 ± 0.88 | 44.15 ± 0.27 | 12.72 ± 0.31 | 63.6 ± 0.5 |
| 15 | 0.50 ± 0.15 | 5.35 ± 0.11 | 39.80 ± 0.13 | 13.41 ± 0.52 | 95.2 ± 0.2 |
| CWP with 0.25% Tween 80 addition | |||||
| 4 | 3.42 ± 0.16 | 0.20 ± 0.02 | 6.97 ± 0.35 | 3.42 ± 0.22 | 59.7 ± 0.2 |
| 10 | 3.28 ± 0.13 | 1.61 ± 0.07 | 11.69 ± 0.41 | 5.86 ± 0.16 | 81.1 ± 0.3 |
| 15 | 3.02 ± 0.11 | 2.88 ± 0.11 | 22.03 ± 0.28 | 4.43 ± 0.19 | 67.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachrimanidou, V.; Papadaki, A.; Papapostolou, H.; Alexandri, M.; Gonou-Zagou, Z.; Kopsahelis, N. Ganoderma lucidum Mycelia Mass and Bioactive Compounds Production through Grape Pomace and Cheese Whey Valorization. Molecules 2023, 28, 6331. https://doi.org/10.3390/molecules28176331
Kachrimanidou V, Papadaki A, Papapostolou H, Alexandri M, Gonou-Zagou Z, Kopsahelis N. Ganoderma lucidum Mycelia Mass and Bioactive Compounds Production through Grape Pomace and Cheese Whey Valorization. Molecules. 2023; 28(17):6331. https://doi.org/10.3390/molecules28176331
Chicago/Turabian StyleKachrimanidou, Vasiliki, Aikaterini Papadaki, Harris Papapostolou, Maria Alexandri, Zacharoula Gonou-Zagou, and Nikolaos Kopsahelis. 2023. "Ganoderma lucidum Mycelia Mass and Bioactive Compounds Production through Grape Pomace and Cheese Whey Valorization" Molecules 28, no. 17: 6331. https://doi.org/10.3390/molecules28176331