Research Progress on Displacement Mechanism of Supercritical CO2 in Low-Permeability Heavy Oil Reservoir and Improvement Mechanism of Displacement Agents
Abstract
:1. Introduction
2. Overview of SC-CO2 and Heavy Oil
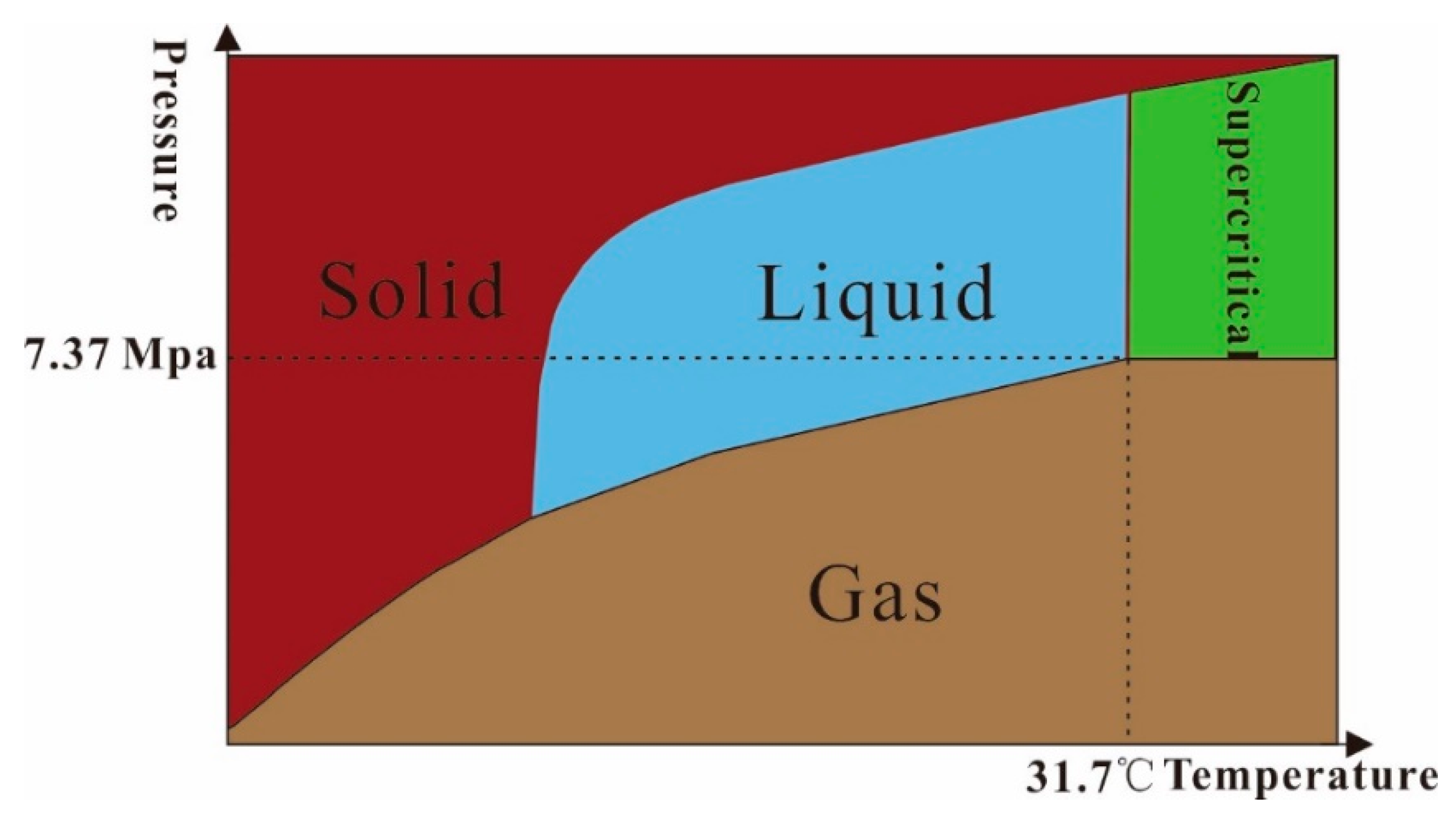
2.1. Basic Properties of SC-CO2
2.2. Composition and Viscous Mechanism of Heavy Oil
2.3. Geological Characteristics of Low-Permeability Reservoirs and EOR Techniques—CO2 Flooding
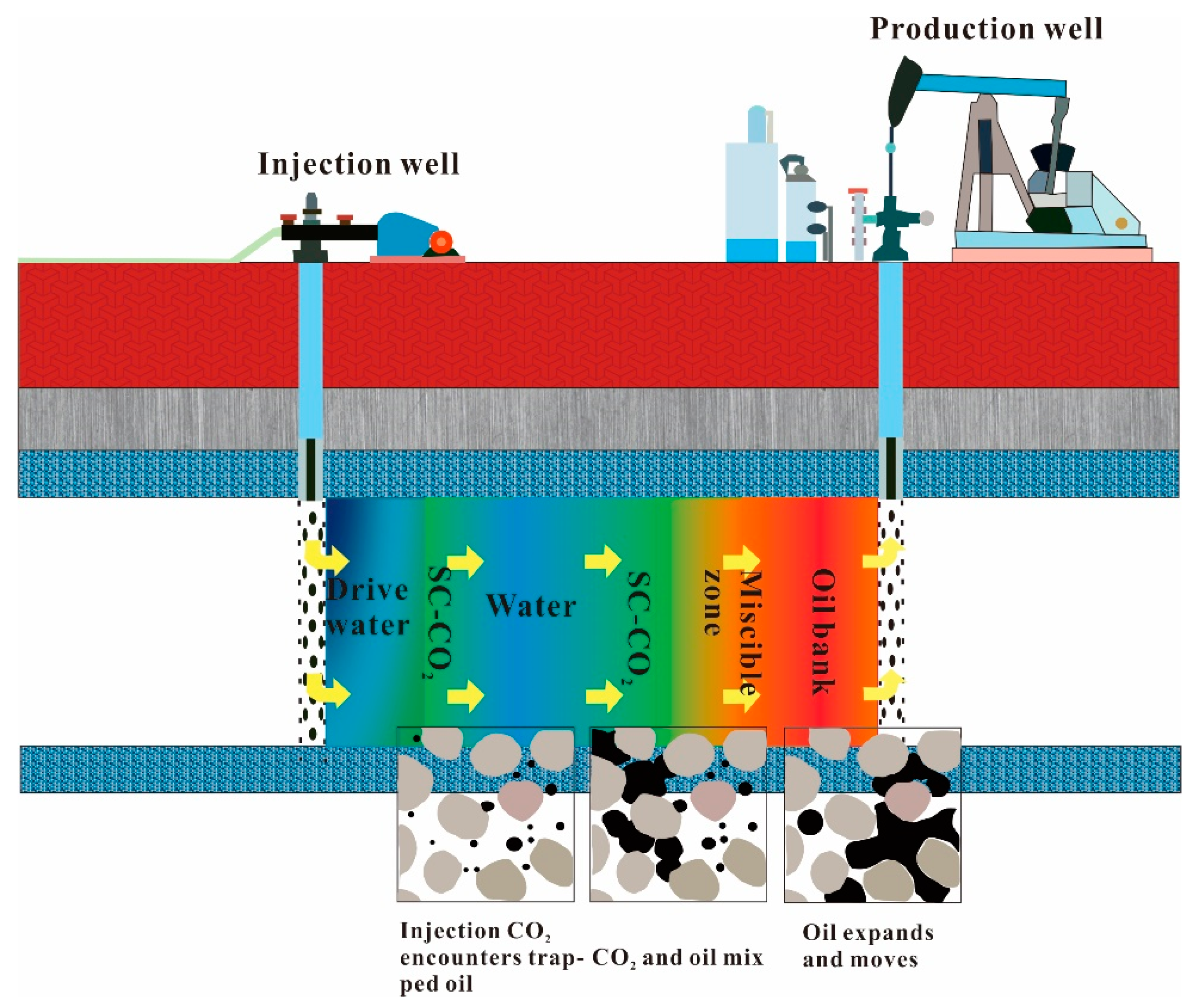
3. Main Mechanism of SC-CO2 Flooding of Heavy Oil
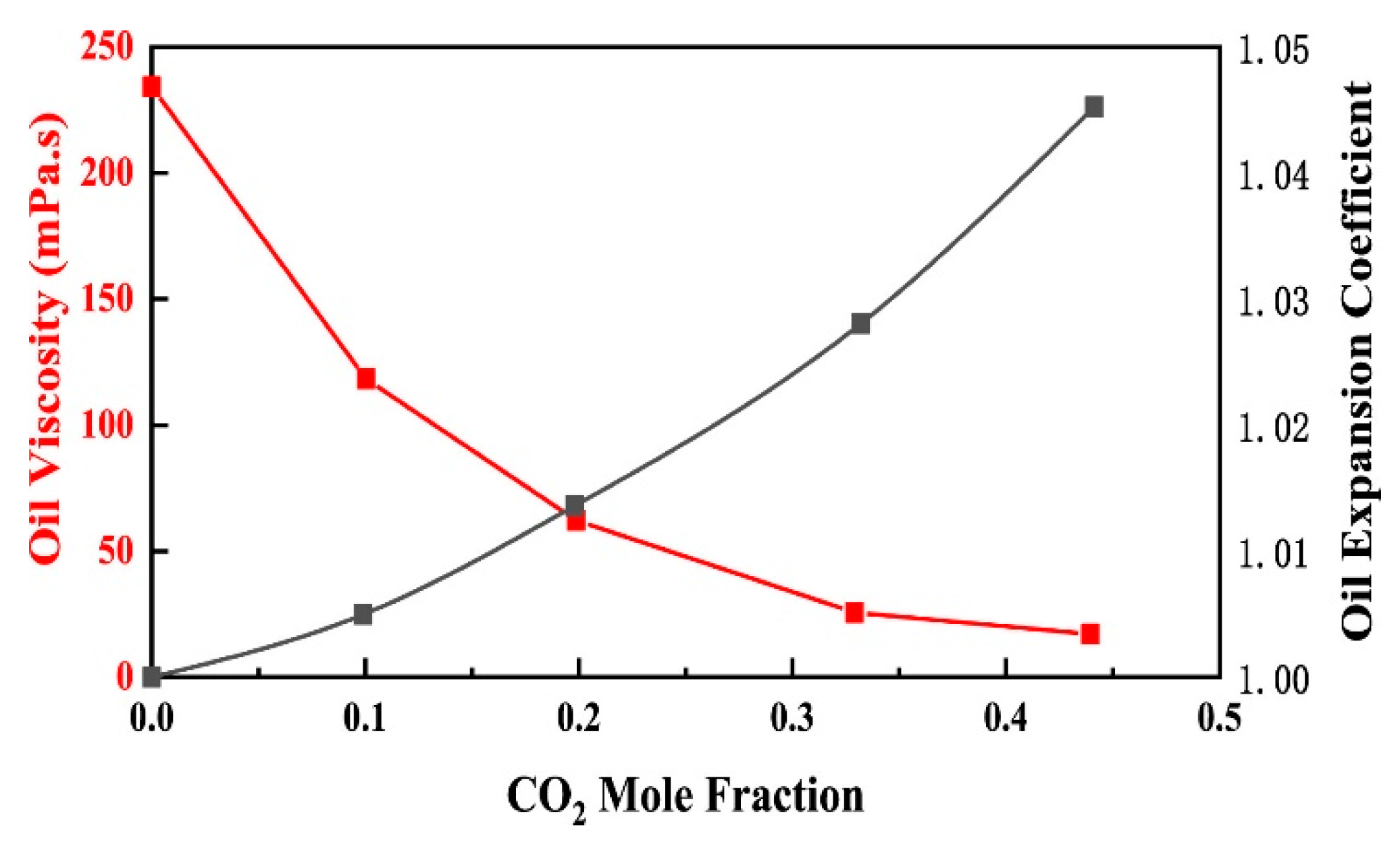
3.1. The Swelling of Heavy Oil by SC-CO2
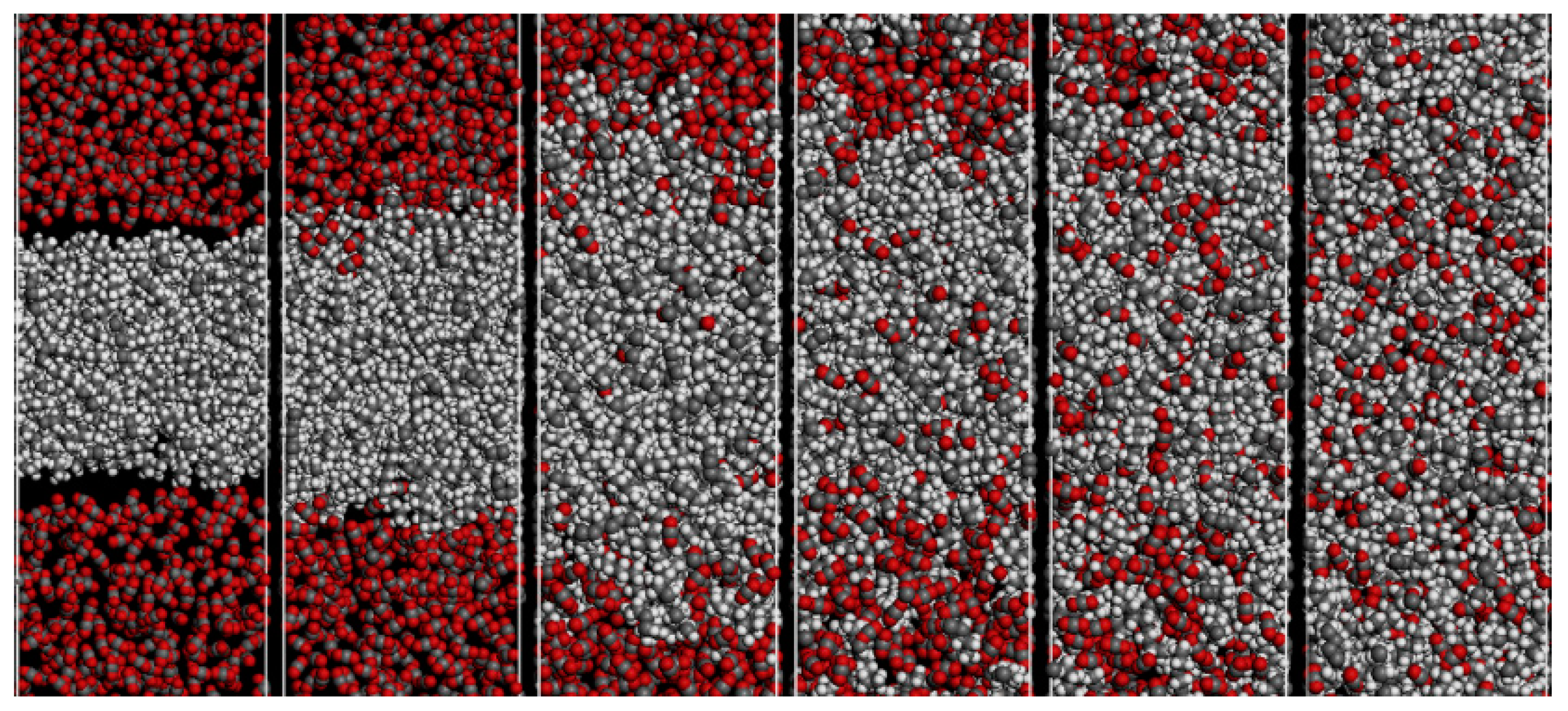
3.2. SC-CO2 Viscosity Reduction Characteristics in Heavy Oil
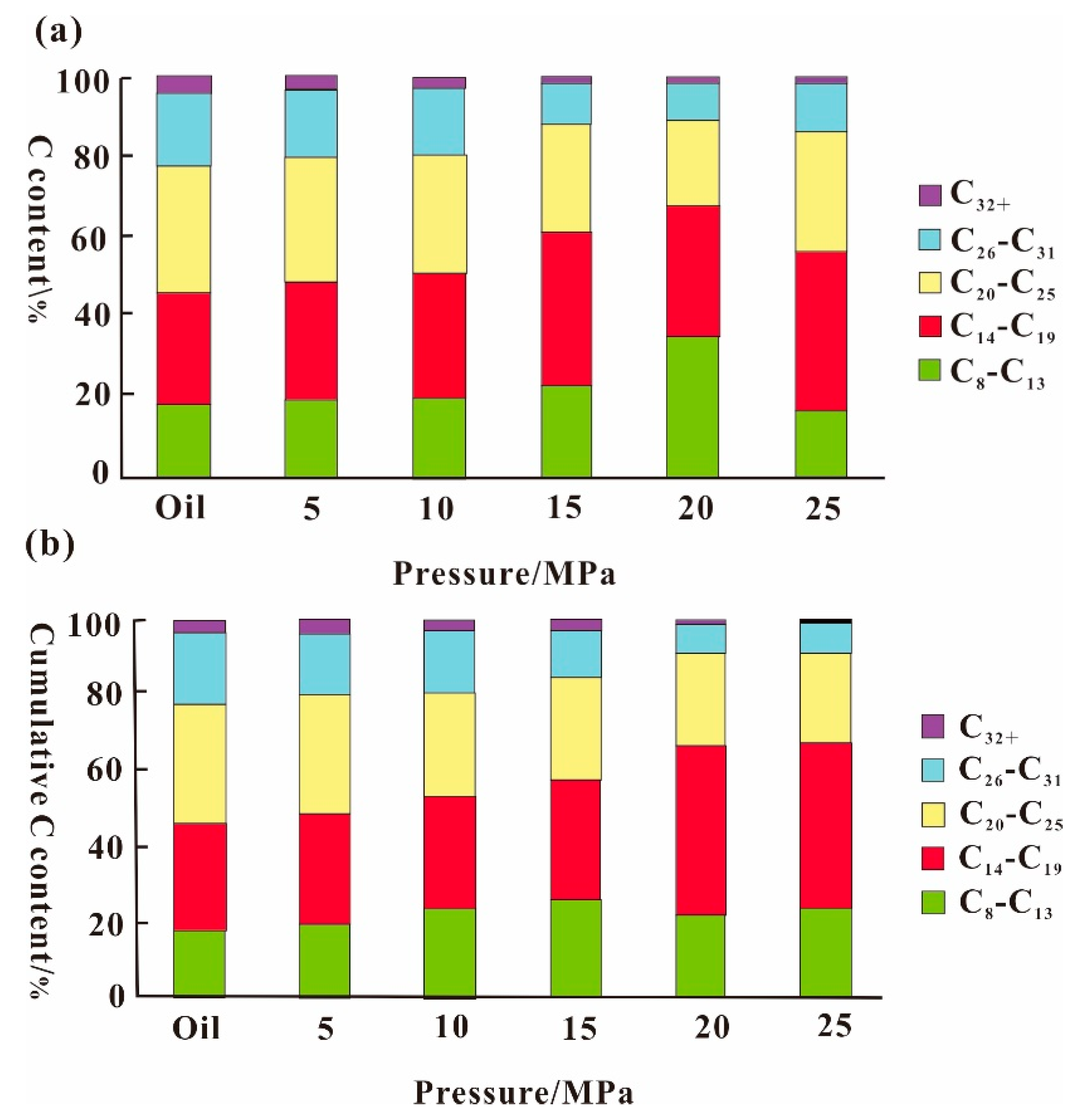
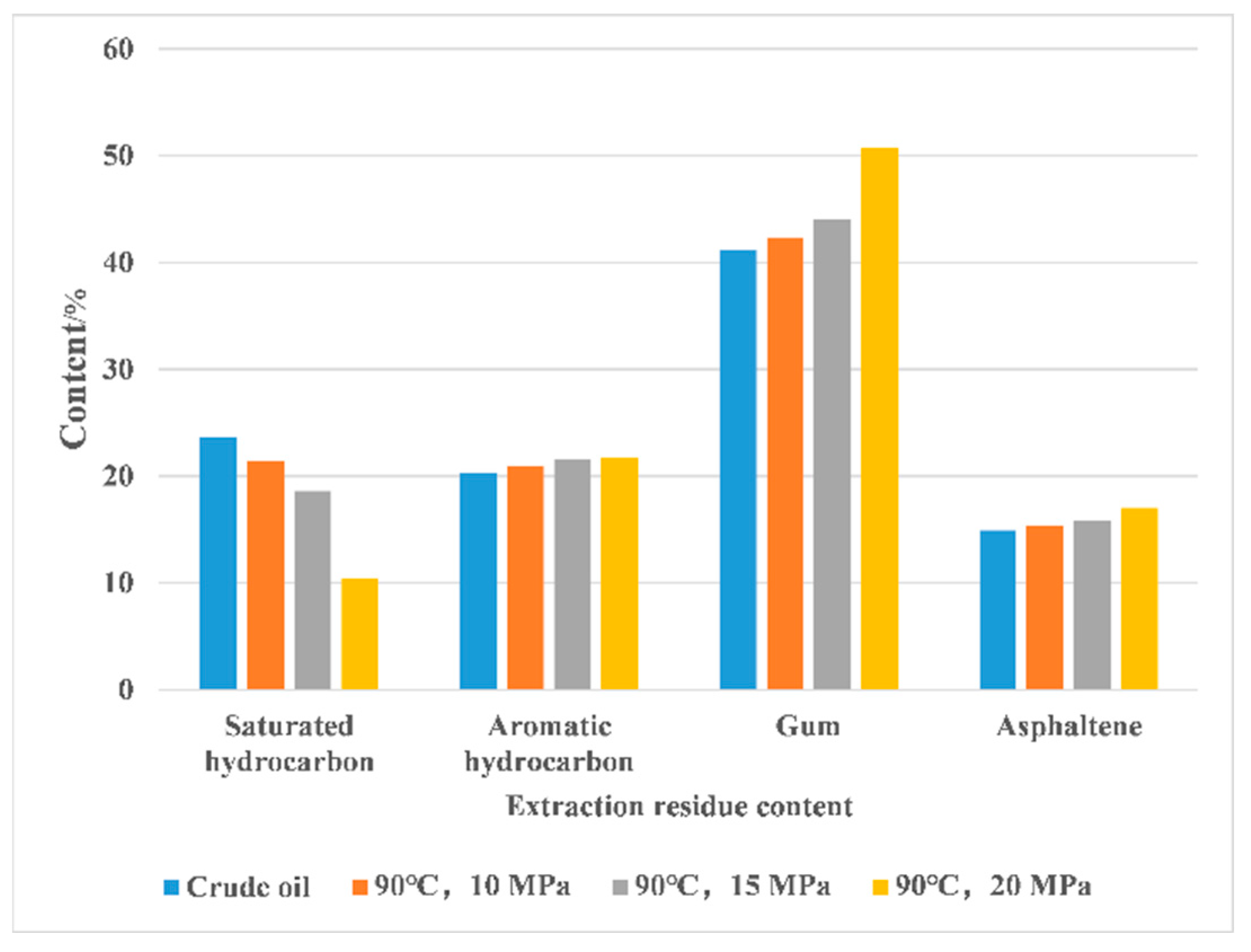
3.3. SC-CO2 Extraction
4. Research Progress on the Mechanism of CO2 Flooding Improved by Typical Oil Flooding Agents
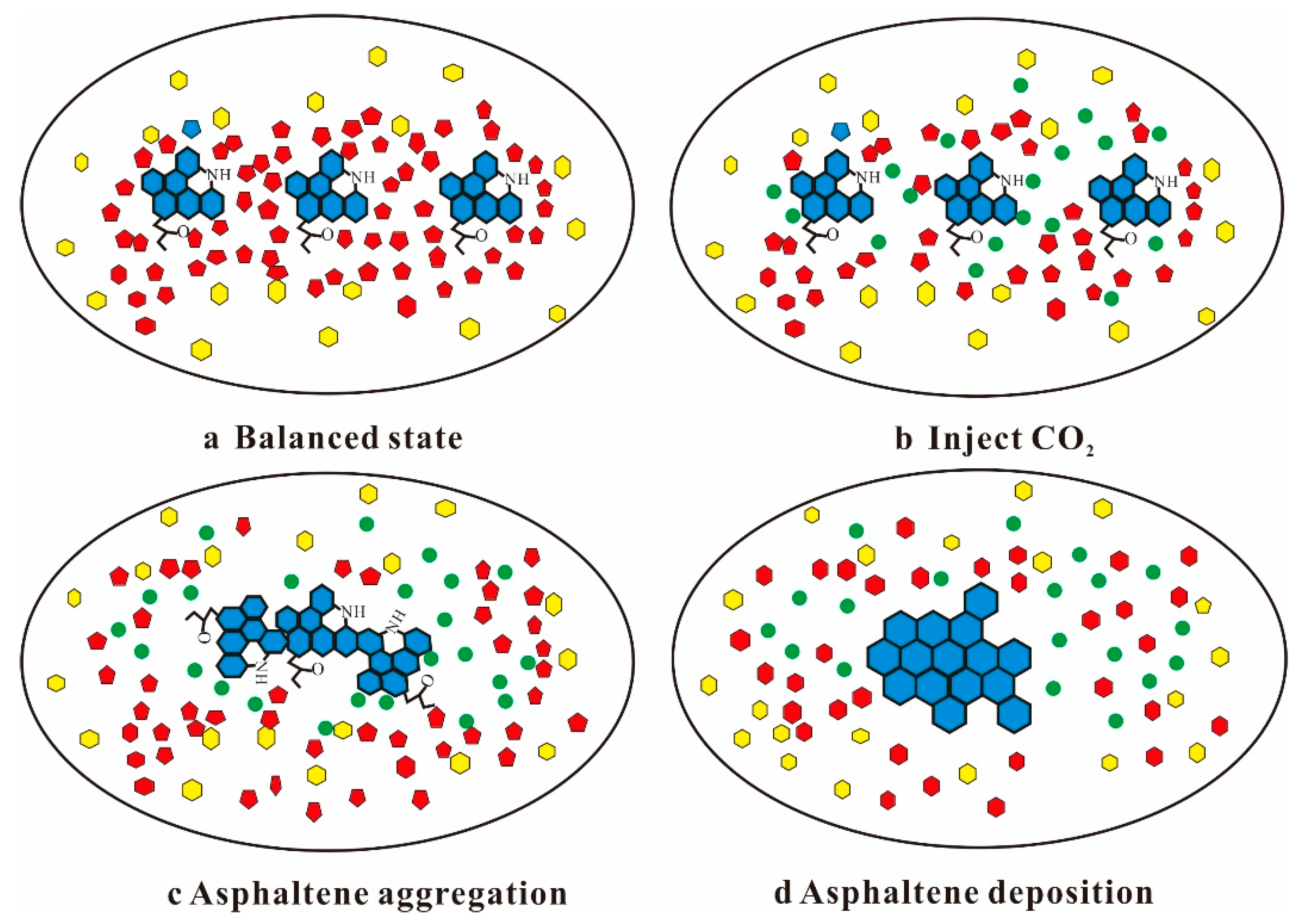
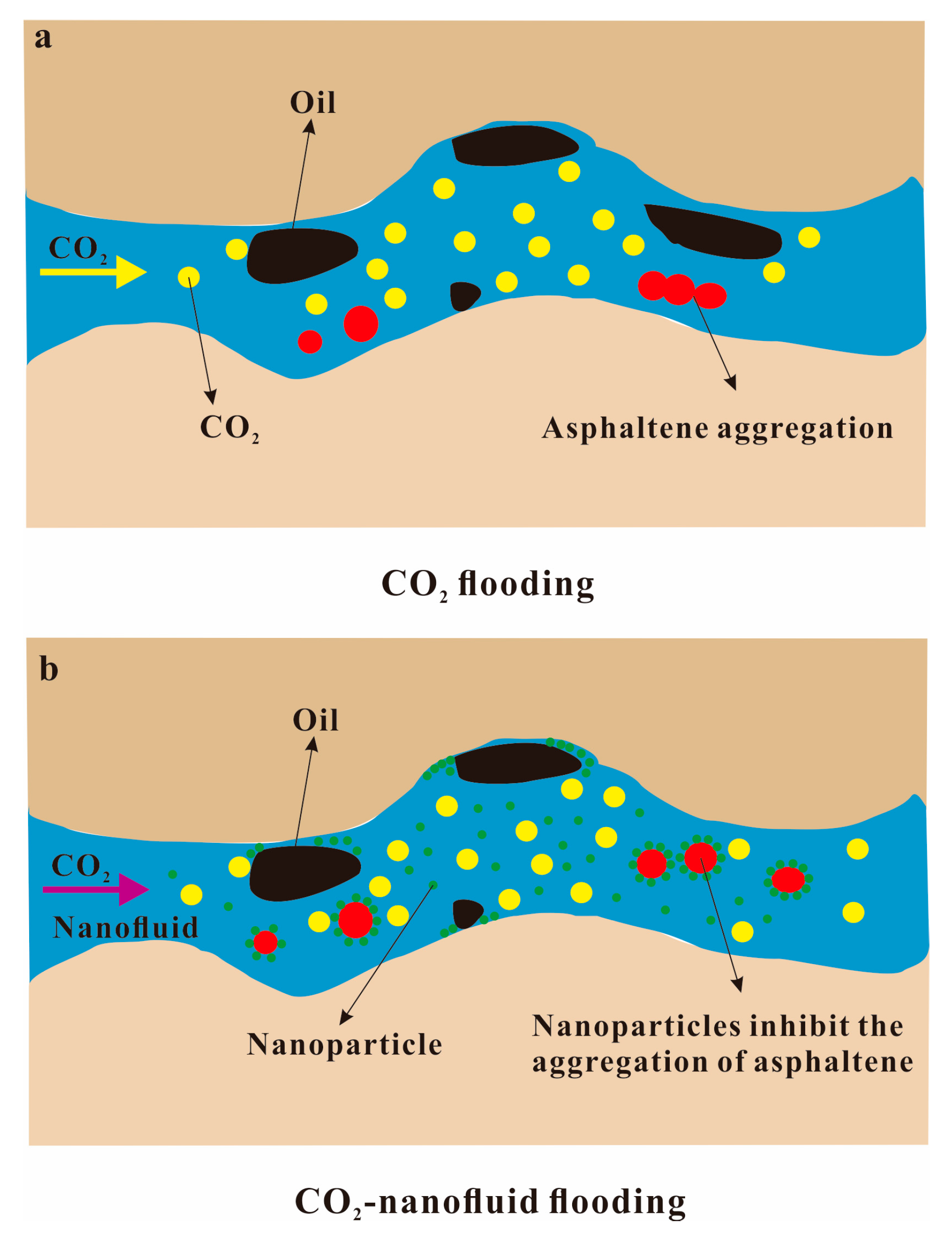
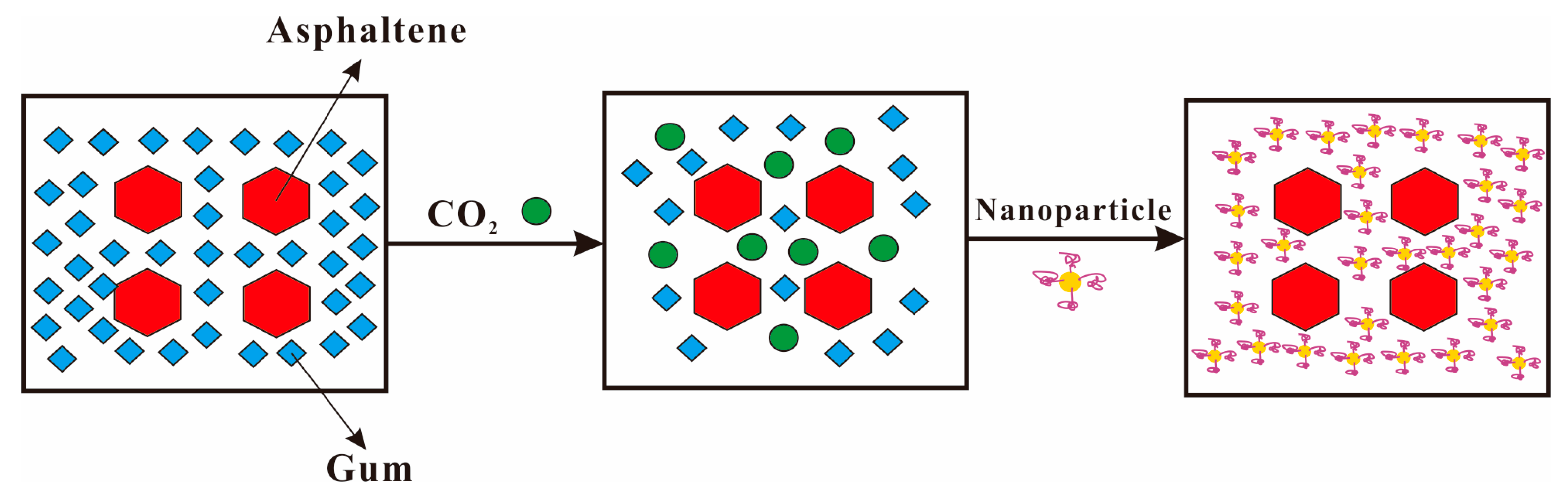
4.1. Nanoparticles Inhibit Asphaltene Deposition during CO2 Flooding
4.1.1. Adsorption of Nanoparticles
4.1.2. Dispersion of Nanoparticles
4.2. Polymer Enhanced CO2 Flooding
4.2.1. Polymer Improves CO2 Channeling
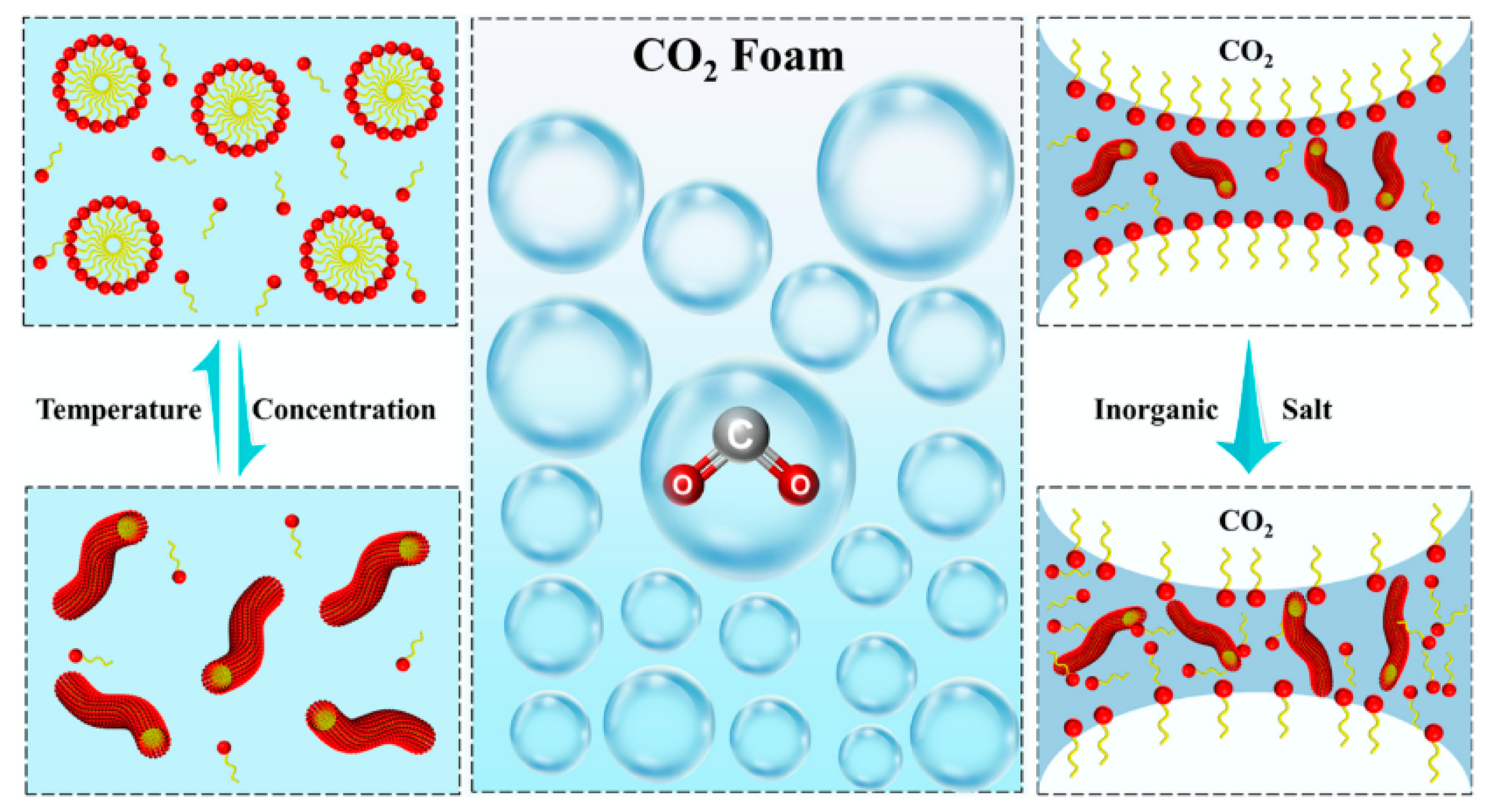
4.2.2. Polymer Enhanced Stability of CO2 Foam
4.3. Surfactants Improve CO2 Flooding
4.3.1. Surfactants on Foaming Properties and Foam Stability of CO2 Foam
4.3.2. Surfactants Reduce the Minimum Miscible Pressure (MMP) of CO2 Flooding
5. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, S.; Liu, Y.; Zhang, J.; Li, P.; Tang, X.; Li, Z.; Dong, Z.; Xu, L.; Zhao, X. Formation conditions and evolution of fractures in multiple tight rocks: Implications for unconventional reservoir exploitation. J. Pet. Sci. Eng. 2021, 200, 108354. [Google Scholar] [CrossRef]
- Dong, K.; Jiang, M.; Li, J.; Zhang, D. Research progresses in formation mechanism of complex fracture network for unconventional reservoir. Arab. J. Geosci. 2020, 13, 750. [Google Scholar] [CrossRef]
- Pei, Y.; Zhou, H.; Li, D.; Zhang, S.; Tian, F.; Zhao, L. A Novel Unconventional Reservoir Fracturing Method-Liquid Self-Propping Fracturing Technology. In Proceedings of the International Petroleum Technology Conference, Dhahran, Kingdom of Saudi Arabia, 13–15 January 2020. [Google Scholar]
- Zhou, X.; Yuan, Q.; Peng, X.; Zeng, F.; Zhang, L. A critical review of the CO2 huff ‘n’ puff process for enhanced heavy oil recovery. Fuel 2018, 215, 813–824. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Y.; Huang, S. A promising chemical-augmented WAG process for enhanced heavy oil recovery. Fuel 2013, 104, 333–341. [Google Scholar] [CrossRef]
- Luo, T.; Yao, Z.; Duan, J.; Li, J.; Zhao, Y. Research on Geologic Characterization and 3D Geological Modeling for Low Permeability Reservoir. J. Liaoning Petrochem. Univ. 2020, 40, 40–44. [Google Scholar]
- Fang, Y.; Yang, E.; Yin, D.; Gan, Y. Study on distribution characteristics of microscopic residual oil in low permeability reservoirs. J. Dispers. Sci. Technol. 2019, 41, 575–584. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, Y.; Pan, W.; Huang, L.; Yan, J.; Zheng, R. Potential evaluation on CO2-EGR in tight and low-permeability reservoirs. Nat. Gas Ind. B 2017, 4, 311–318. [Google Scholar] [CrossRef]
- Salmo, I.C.; Pettersen, Ø.; Skauge, A. Polymer Flooding at Adverse Mobility Ratio -Acceleration of Oil Production by Crossflow into Water Channels. Energy Fuels 2017, 31, 5948–5958. [Google Scholar] [CrossRef]
- Li, L.; Su, Y.; Hao, Y.; Zhan, S.; Lv, Y.; Zhao, Q.; Wang, H. A comparative study of CO2 and N2 huff-n-puff EOR performance in shale oil production. J. Pet. Sci. Eng. 2019, 181, 106174. [Google Scholar] [CrossRef]
- Wan, T.; Wang, X.; Jing, Z.; Gao, Y. Gas injection assisted steam huff-n-puff process for oil recovery from deep heavy oil reservoirs with low-permeability. J. Pet. Sci. Eng. 2020, 185, 106613. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Z.; Zhu, G.; Zhang, H. Research and Application of CO2 Flooding Enhanced Oil Recovery in Low Permeability Oilfield. Open J. Geol. 2017, 7, 1435–1440. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Gao, Y.; Zhang, Y.; Yan, Y.; Zhang, J. Enhanced oil recovery with CO2/N2 slug in low permeability reservoir: Molecular dynamics simulation. Chem. Eng. Sci. 2019, 197, 204–211. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, J.; Li, J.; Zhou, X.; Dong, W.; Cheng, Z. Experimental study on oil recovery mechanism of CO2 associated enhancing oil recovery methods in low permeability reservoirs. J. Pet. Sci. Eng. 2021, 197, 108047. [Google Scholar] [CrossRef]
- Zhao, F.; Hao, H.; Hou, J.; Hou, L.; Song, Z. CO2 mobility control and sweep efficiency improvement using starch gel or ethylenediamine in ultra-low permeability oil layers with different types of heterogeneity. J. Pet. Sci. Eng. 2015, 133, 52–65. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Liao, G.; Zhang, F.; Jiaqiang, E. Density fluctuations and transport properties of supercritical carbon dioxide calculated by molecular dynamics simulation near the Widom line. J. Supercrit. Fluids 2023, 200, 106003. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Shiau, B.; Harwell, J.H. In-situ CO2 generation for EOR by using urea as a gas generation agent. Fuel 2018, 217, 499–507. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Xiong, J.; Liang, L. Experimental on the pore structure characteristics of Longmaxi Formation shale in southern Sichuan Basin, China. Petroleum 2021, 7, 135–141. [Google Scholar] [CrossRef]
- Su, X.; Yue, X. Mechanism study of the relation between the performance of CO2 immiscible flooding and rock permeability. J. Pet. Sci. Eng. 2020, 195, 107891. [Google Scholar] [CrossRef]
- Xiao, P.; Yang, Z.; Wang, X.; Xiao, H.; Wang, X. Experimental investigation on CO2 injection in the Daqing extra/ultra-low permeability reservoir. J. Pet. Sci. Eng. 2017, 149, 765–771. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, B.; Zhu, T.; Wang, P.; Zhang, X.; Wang, T.; Wu, F.; Zhang, L.; Kang, W.; Ketova, Y.A.; et al. Conformance control mechanism of low elastic polymer microspheres in porous medium. J. Pet. Sci. Eng. 2021, 196, 107708. [Google Scholar] [CrossRef]
- Fakher, S.; Imqam, A. An experimental investigation of immiscible carbon dioxide interactions with crude oil: Oil swelling and asphaltene agitation. Fuel 2020, 269, 117380. [Google Scholar] [CrossRef]
- Rangriz Shokri, A.; Babadagli, T. Feasibility assessment of heavy-oil recovery by CO2 injection after cold production with sands: Lab-to-field scale modeling considering non-equilibrium foamy oil behavior. Appl. Energy 2017, 205, 615–625. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, X.; Yang, H.; Liao, G.; Zeng, F. CO2 flooding strategy to enhance heavy oil recovery. Petroleum 2017, 3, 68–78. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, W.; Wang, X.; Guo, S. 2-D Pore-Scale Experimental Investigations of Asphaltene Deposition and Heavy Oil Recovery by CO2 Flooding. Energy Fuels 2018, 32, 3194–3201. [Google Scholar] [CrossRef]
- Rezk, M.G.; Foroozesh, J. Effect of CO2 mass transfer on rate of oil properties changes: Application to CO2-EOR projects. J. Pet. Sci. Eng. 2019, 180, 298–309. [Google Scholar] [CrossRef]
- Wanat, E.; Teletzke, G.; Newhouse, D.; Lawrence, J.; Shehhi, R.A.; Willingham, T.; Jawhari, M.O. Quantification of Oil Recovery Mechanisms during Gas Injection EOR Coreflood Experiments. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017. [Google Scholar]
- Al-Shargabi, M.; Davoodi, S.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Carbon Dioxide Applications for Enhanced Oil Recovery Assisted by Nanoparticles: Recent Developments. ACS Omega 2022, 7, 9984–9994. [Google Scholar] [CrossRef]
- Qian, K.; Yang, S.; Dou, H.; Wang, Q.; Wang, L.; Huang, Y. Experimental Investigation on Microscopic Residual Oil Distribution During CO2 Huff-and-Puff Process in Tight Oil Reservoirs. Energies 2018, 11, 2843. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D. Quantification of Gas Exsolution and Mass Transfer of Foamy Oil in Bulk Phase under Solution Gas Drive Conditions. In Proceedings of the SPE Western Regional Meeting, Garden Grove, CA, USA, 22–26 April 2018. [Google Scholar]
- Bakhshi, P.; Kharrat, R.; Hashemi, A.; Zallaghi, M. Experimental evaluation of carbonated waterflooding: A practical process for enhanced oil recovery and geological CO2 storage. Greenh. Gases Sci. Technol. 2018, 8, 238–256. [Google Scholar] [CrossRef]
- Fakher, S.; Imqam, A. Asphaltene precipitation and deposition during CO2 injection in nano shale pore structure and its impact on oil recovery. Fuel 2019, 237, 1029–1039. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Li, J.; Han, R.; Lu, C. Mechanism and Performance Analysis of Nanoparticle-Polymer Fluid for Enhanced Oil Recovery: A Review. Molecules 2023, 28, 4331. [Google Scholar] [CrossRef]
- Li, N.; Mao, G.; Liu, Y. Effect of the Evaluation and Mechanism Analysis of a Novel Nanohybrid Pour Point Depressant on Facilitating Flow Properties of Crude Oil. Energy Fuels 2018, 32, 10563–10570. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Shojaei, S.; Riazi, M.; Sharifi, M. Review on application of nanoparticles for EOR purposes: A critical review of the opportunities and challenges. Chin. J. Chem. Eng. 2019, 27, 237–246. [Google Scholar] [CrossRef]
- Ke, H.; Yuan, M.; Xia, S. A review of nanomaterials as viscosity reducer for heavy oil. J. Dispers. Sci. Technol. 2020, 43, 1271–1282. [Google Scholar] [CrossRef]
- Montes, D.; Henao, J.; Taborda, E.A.; Gallego, J.; Cortes, F.B.; Franco, C.A. Effect of Textural Properties and Surface Chemical Nature of Silica Nanoparticles from Different Silicon Sources on the Viscosity Reduction of Heavy Crude Oil. ACS Omega 2020, 5, 5085–5097. [Google Scholar] [CrossRef]
- Rezaeiakmal, F.; Parsaei, R.; Shafiabadi, A.; Rezaei, A. Insights into the flow behaviour of the pre-generated polymer enhanced foam in heterogeneous porous media during tertiary oil recovery: Effect of gravitational forces. J. Pet. Sci. Eng. 2022, 213, 110385. [Google Scholar] [CrossRef]
- Yang, H.; Lv, Z.; Wang, L.; Feng, C.; Wang, J.; Xu, Z.; Huang, Y.; Li, Z.; Kang, W. Stability mechanism of controlled acid-resistant hydrophobic polymer nanospheres on CO2 foam. Fuel 2023, 346, 128332. [Google Scholar] [CrossRef]
- Abdelaal, A.; Gajbhiye, R.; AlShehri, D.; Mahmoud, M.; Patil, S.; Zhou, X. Improvement of supercritical carbon dioxide foam performance for EOR in sandstone reservoirs: An experimental and optimization study. Gas Sci. Eng. 2023, 110, 204859. [Google Scholar] [CrossRef]
- Shen, H.; Yang, Z.; Li, X.; Peng, Y.; Lin, M.; Zhang, J.; Dong, Z. CO2-responsive agent for restraining gas channeling during CO2 flooding in low permeability reservoirs. Fuel 2021, 292, 120306. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.; Fan, F.; Zhang, L.; Yao, E. Study on the Influence of CO2 Finger-Channeling Flooding on Oil Displacement Efficiency and Anti-Channeling Method. In Proceedings of the ARMA-CUPB Geothermal International Conference, Beijing, China, 5–8 August 2019. [Google Scholar]
- Zhang, L.; Zhao, F.; Hou, J. Experimental Study of Improving the CO2 Flooding Development Effect in Ultra-Low Permeability Reservoir. Adv. Mater. Res. 2012, 594, 2470–2474. [Google Scholar]
- Li, Q.; Zhao, D.; Yin, J.; Zhou, X.; Li, Y.; Chi, P.; Han, Y.; Ansari, U.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-bearing Sediment in Northern South China Sea by Horizontal Wellbore: Evolution and Mechanism. Nat. Resour. Res. 2023, 321, 1595–1620. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Yang, Y.; Ansari, U.; Han, Y.; Li, X.; Cheng, Y. Preliminary experimental investigation on long-term fracture conductivity for evaluating the feasibility and efficiency of fracturing operation in offshore hydrate-bearing sediments. Ocean Eng. 2023, 281, 114949. [Google Scholar] [CrossRef]
- Delgado-García, Y.I.; Luna, S.; López-Malo, A.; Morales-Camacho, J.I. Effect of supercritical carbon dioxide on physicochemical and techno-functional properties of amaranth flour. Chem. Eng. Process. 2022, 178, 109031. [Google Scholar] [CrossRef]
- Daş, E.; Gürsel, S.A.; Yurtcan, A.B. Simultaneously deposited Pt-alloy nanoparticles over graphene nanoplatelets via supercritical carbon dioxide deposition for PEM fuel cells. J. Alloys Compd. 2021, 874, 159919. [Google Scholar] [CrossRef]
- Senyay-Oncel, D.; Kimiz-Gebologlu, I.; Yesil-Celiktas, O. New developments in supercritical fluids as a green technology: Processing of β-glucanase with sub- and supercritical carbon dioxide. Biochem. Eng. J. 2023, 195, 108934. [Google Scholar] [CrossRef]
- Jung, B.; Im, S.; Westerhoff, P.; Park, K. Supercritical carbon dioxide fluid cleaning of extracellular polymeric substance surrogates on seawater reverse osmosis membranes. Desalination 2023, 559, 116637. [Google Scholar] [CrossRef]
- Cai, X.; Long, J.; Ren, Q.; Dong, M.; Wang, W.; Tian, S.; Liu, Z. Aggregation Mechanism of Asphaltene Molecular Aggregates. Acta Pet. Sin. (Pet. Process.) 2019, 35, 920–928. [Google Scholar]
- Liu, G.; Yang, J.; Song, J.; Xu, X. Inhibition of asphaltene precipitation in blended crude oil using novel oil-soluble maleimide polymers. Energy Sources Part A Recovery Util. Environ. Effects 2019, 41, 2460–2470. [Google Scholar] [CrossRef]
- Gray, M.R.; Tykwinski, R.R.; Stryker, J.M.; Tan, X. Supramolecular Assembly Model for Aggregation of Petroleum Asphaltenes. Energy Fuels 2011, 25, 3125–3134. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Ren, S. Molecular Dynamics Simulation of Self-Aggregation of Asphaltenes at an Oil/Water Interface: Formation and Destruction of the Asphaltene Protective Film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Technically Recoverable Shale Oil and Shale Gas Resources: An Assessment of 137 Shale Formations in 41 Countries Outside the United States; U.S. Energy Information Administration: Washington, DC, USA, 2013.
- Ji, B.; Fang, J. An overview of efficient development practices at low permeability sandstone reservoirs in China. Energy Geosci. 2023, 4, 100179. [Google Scholar] [CrossRef]
- Cong, S.; Li, J.; Liu, W.; Shi, Y.; Li, Y.; Zheng, K.; Luo, X.; Luo, W. EOR mechanism of fracture oil displacement agent for ultra-low permeability reservoir. Energy Rep. 2023, 9, 4893–4904. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Meng, X.; Chen, Y.; Song, W.; Yuan, C. Key parameters and dominant EOR mechanism of CO2 miscible flooding applied in low-permeability oil reservoirs. Geoenergy Sci. Eng. 2023, 225, 211724. [Google Scholar] [CrossRef]
- Song, S.; Ghanizadeh, A.; Younis, A.; Clarkson, C.R. Experimental evaluation of the impact of induced fractures on cyclic solvent injection performance in low-permeability hydrocarbon reservoirs. Fuel 2023, 354, 129327. [Google Scholar] [CrossRef]
- Wang, W.; Guo, X.; Duan, P.; Kang, B.; Zheng, D.; Zafar, A. Investigation of plugging performance and enhanced oil recovery of multi-scale polymer microspheres in low-permeability reservoirs. Nat. Gas Ind. B 2023, 10, 223–232. [Google Scholar] [CrossRef]
- Li, W.; Ji, Z. Finite element analysis of temporary plugging and fracturing mechanism. Fault-Block Oil Gas Field 2016, 23, 514–517. [Google Scholar]
- Zhou, D.; Xiong, X.; He, J.; Dong, B.; He, Y. Multi-Stage Deflective Fracturing Technology for Low Permeability Reservoir. Pet. Drill. Technol. 2020, 48, 85–89. [Google Scholar]
- Li, H.; Li, J.; Li, W.; Hu, J.; Yuan, G.; Liang, Y.; Zhang, X.; Guo, Y. Synthesis of associative polymer for flooding in low permeability reservoir and its reservoir adaptability. Pet. Reserv. Eval. Dev. 2020, 10, 24–32. [Google Scholar]
- Wang, G. Microscopic residual oil utilization mechanism of low viscosity polymer flooding in low permeability reservoir. Fault-Block Oil Gas Field 2018, 25, 776–780. [Google Scholar]
- Peng, X.; Wang, Y.; Diao, Y.; Zhang, L.; Yazid, I.M.; Ren, S. Experimental investigation on the operation parameters of carbon dioxide huff-n-puff process in ultra low permeability oil reservoirs. J. Pet. Sci. Eng. 2019, 174, 903–912. [Google Scholar] [CrossRef]
- Wang, G. Carbon dioxide capture, enhanced-oil recovery and storage technology and engineering practice in Jilin Oilfield, NE China. Pet. Explor. Dev. 2023, 50, 245–254. [Google Scholar] [CrossRef]
- Sun, X.; Du, M.; Han, B.; Sun, Y.; Zhao, M.; Guan, B.; Dai, C. Review on Carbon Dioxide Fracturing Technology. Oilfield Chem. 2017, 34, 374–380. [Google Scholar]
- Zhang, L.; Wang, S.; Zhang, L.; Ren, S.; Guo, Q. Assessment of CO2 EOR and its geo-storage potential in mature oil reservoirs, Shengli Oilfield, China. Pet. Explor. Dev. 2009, 36, 737–742. [Google Scholar]
- Pedersen, K.S.; Christensen, P.L.; Shaikh, J.A. Phase Behavior of Petroleum Reservoir Fluids; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Cai, X.; Zhou, Z.; Du, F. Increase production mechanism and application of CO2 huff and puff technology. Oil Drill. Prod. Technol. 2002, 24, 45–46. [Google Scholar]
- Zhang, T.; Ming, T.; Yuan, L.; Zhu, G.; Zhang, C.; Liu, Y.; Li, Y.; Wang, W.; Yang, X. Experimental study on stress-dependent multiphase flow in ultra-low permeability sandstone during CO2 flooding based on LF-NMR. Energy 2023, 278, 127874. [Google Scholar] [CrossRef]
- Sambo, C.; Liu, N.; Shaibu, R.; Ahmed, A.A.; Hashish, R.G. A Technical Review of CO2 for Enhanced Oil Recovery in Unconventional Oil Reservoirs. Geoenergy Sci. Eng. 2023, 221, 111185. [Google Scholar] [CrossRef]
- Sharafi, M.S.; Ghasemi, M.; Ahmadi, M.; Kazemi, A. An experimental approach for measuring carbon dioxide diffusion coefficient in water and oil under supercritical conditions. Chin. J. Chem. Eng. 2021, 34, 160–170. [Google Scholar] [CrossRef]
- Whorton, L.P.; Brownscombe, E.R.; Dyes, A.B. Method for Producing Oil by Means of Carbon Dioxide. U.S. Patent No. 2,623,596; U.S. Patent and Trademark Office, Washington, DC, USA, 30 December 1950. [Google Scholar]
- Yang, Z.; Li, M.; Peng, B.; Lin, M.; Dong, Z. Dispersion Property of CO2 in Oil. 1. Volume Expansion of CO2+ Alkane at near Critical and Supercritical Condition of CO2. J. Chem. Eng. Data 2012, 57, 1305–1311. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Z.; Liu, K.; Burke, N. Molecular Simulation of CO2 Solubility and Its Effect on Octane Swelling. Energy Fuels 2013, 27, 2741–2747. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Sun, B.; Shen, Y.; Zhang, J.; Chen, X.; Wang, M. Molecular dynamics simulation on volume swelling of CO2–alkane system. Fuel 2015, 143, 194–201. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Peng, B.; Lin, M.; Dong, Z. Volume expansion of CO2+oil at near critical and supercritical conditions of CO2. Fuel 2013, 112, 283–288. [Google Scholar] [CrossRef]
- Han, H.; Yuan, S.; Li, S.; Liu, X.; Chen, X. Dissolving capacity and volume expansion of carbon dioxide in chain n-alkanes. Pet. Explor. Dev. 2015, 42, 97–103. [Google Scholar] [CrossRef]
- Li, C.; Pu, H.; Zhao, J. Molecular Simulation Study on the Volume Swelling and the Viscosity Reduction of n-Alkane/CO2 Systems. Ind. Eng. Chem. Res. 2019, 58, 8871–8877. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wang, J.; Pan, Z.; Liu, J. Molecular Simulation Study on the Density Behavior of n-Alkane/CO2 Systems. ACS Omega 2021, 6, 29618–29628. [Google Scholar] [CrossRef] [PubMed]
- Sima, S.; Milanesio, J.M.; Ramello, J.I.; Cismondi, M.; Secuianu, C.; Feroiu, V.; Geană, D. The effect of the naphthenic ring on the VLE of (carbon dioxide + alkane) mixtures. J. Chem. Thermodyn. 2016, 93, 374–385. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, P.; Ge, X.; Du, J.; Wang, Z. Influence factors on CO2 solubility in cycloalkanes and cycloalkane volume expansion: Temperature, pressure and molecular structure. J. Mol. Liq. 2021, 332, 115859. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.; Bei, K.; Chou, I.M.; Lin, C.; Pan, Z. A new approach for the measurement of the volume expansion of a CO2+ n-dodecane mixture in a fused silica capillary cell by Raman spectroscopy. Fuel 2017, 203, 113–119. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Xia, S.; Ma, P. The solubility of CO2 in (hexane + cyclohexane) and (cyclopentane + ethylbenzene) and (toluene + undecane) systems at high pressures. J. Chem. Thermodyn. 2021, 154, 106324. [Google Scholar] [CrossRef]
- Souza, J.L.; Fabri, F.; Buffon, R.; Schuchardt, U. Reduced anatase on silica as a support for a Ziegler catalyst. Appl. Catal. A Gen. 2007, 323, 234–241. [Google Scholar] [CrossRef]
- He, F.; Zhang, L. Organo-modified ZnAl layered double hydroxide as new catalyst support for the ethylene polymerization. J. Colloid Interface Sci. 2007, 315, 439–444. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Li, M. Research on Supercritical CO2-Heavy Oil Viscosity Reduction Characteristics and Calculation Model. Sci. Technol. Eng. 2013, 13, 294–298. [Google Scholar]
- Seyyedsar, S.M.; Sohrabi, M. Intermittent CO2 and viscosity-reducing gas (VRG) injection for enhanced heavy oil recovery. Fuel Process. Technol. 2017, 164, 1–12. [Google Scholar] [CrossRef]
- Seyyedsar, S.M.; Farzaneh, S.A.; Sohrabi, M. Experimental investigation of tertiary CO2 injection for enhanced heavy oil recovery. J. Nat. Gas Sci. Eng. 2016, 34, 1205–1214. [Google Scholar] [CrossRef]
- Sun, G.; Li, C.; Wei, G.; Yang, F. Characterization of the viscosity reducing efficiency of CO2 on heavy oil by a newly developed pressurized stirring-viscometric apparatus. J. Pet. Sci. Eng. 2017, 156, 299–306. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Q.; Li, Z. Experimental investigation of carbon dioxide flooding in heavy oil reservoirs for enhanced oil recovery. Energy Rep. 2022, 8, 10754–10761. [Google Scholar] [CrossRef]
- Miller, J.S.; Jones, R.A. A Laboratory Study To Determine Physical Characteristics Of Heavy Oil After CO2 Saturation. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 5–8 April 1981. [Google Scholar]
- Li, Z.; Li, X.; Yuan, M.; Huang, D.; Zhang, G. Study on laboratory experiments of CO2 drive in Shang 13–22 unit. Pet. Geol. Recovery Effic. 2000, 3, 005. [Google Scholar]
- Zhao, G.; Liu, R.; Zhang, W.; Fu, M. Experimental Research on Affection of CO2 to the Physical Property of High Solidification Point Oil. J. Jianghan Pet. Univ. Staff. 2003, 4, 35–37. [Google Scholar]
- Xin, S.; Luo, T.; Zhang, Y.; Zhang, L.; He, Y.; Zhang, H. Laboratory test evaluation of CO2 injection miscible flooding in BN reservoir, North China. Pet. Geol. Eng. 2004, 18, 30–32. [Google Scholar]
- Zhao, M. Laboratory and Numerical Simulation Study on CO2 Flooding in Ultra Low Permeability Reservoir; Northeast Petroleum University: Daqing, China, 2008; Volume 11, pp. 1469–1472. [Google Scholar]
- Liu, S. Rsearch on Laboratory experiment of CO2 drive in ultra-low permeability reservoir. Sci. Technol. Ed. 2011, 33, 133–136. [Google Scholar]
- Xiang, P. Research and application of carbon dioxide oil displacement technology. Petrochem. Ind. Technol. 2021, 28, 33–34. [Google Scholar]
- Jaffé, R.; Diaz, D.; Hajje, N.; Chen, L.; Eckardt, C.; Furton, K.G. Hydrocarbon speciation in ancient sediments studied by stepwise high-temperature supercritical carbon dioxide extraction. Org. Geochem. 1997, 26, 59–65. [Google Scholar] [CrossRef]
- Akinlua, A.; Torto, N.; Ajayi, T.R. Supercritical fluid extraction of aliphatic hydrocarbons from Niger Delta sedimentary rock. J. Supercrit. Fluids 2008, 45, 57–63. [Google Scholar] [CrossRef]
- Kesavan, S.K.; Ghosh, A.; Polasky, M.E.; Parameswaran, V.; Lee, S. Supercritical Extraction of Stuart Oil Shale. Fuel Sci. Technol. Int. 1988, 6, 505–523. [Google Scholar] [CrossRef]
- Deo, M.D.; Hwang, J.; Hanson, F.V. Supercritical fluid extraction of a crude oil, bitumen-derived liquid and bitumen by carbon dioxide and propane. Fuel 1992, 71, 1519–1526. [Google Scholar] [CrossRef]
- Guiliano, M.; Boukir, A.; Doumenq, P.; Mille, G.; Crampon, C.; Badens, E.; Charbit, G. Supercritical Fluid Extraction of Bal 150 Crude Oil Asphaltenes. Energy Fuels 2000, 14, 89–94. [Google Scholar] [CrossRef]
- Koel, M.; Ljovin, S.; Bondar, Y. Supercritical carbon dioxide extraction of Estonian oil shale. Oil Shale 2000, 17, 225–235. [Google Scholar] [CrossRef]
- Jaffé, R.; Diaz, D.; Furton, K.G.; Lafargue, E. High temperature supercritical carbon dioxide extractions of geological samples: Effects and contributions from the sample matrix. Appl. Geochem. 2000, 15, 79–89. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, S.; Chen, H.; Wang, L.; Qian, K.; Meng, Z.; Lei, H.; Wang, Z. Effect and influencing factors of CO2 huff and puff in a tight oil reservoir-Taking the Lucaogou formation in the Xinjiang Jimsar sag as an example. Pet. Sci. Bull. 2018, 3, 434–445. [Google Scholar]
- Li, M.; Shan, W.; Liu, X.; Shang, G. Laboratory study on miscible oil displacement mechanism of supercritical carbon dioxide. Acta Pet. Sinica 2006, 27, 80. [Google Scholar]
- Wang, Q.; Zhang, N.; Wu, Q.; Zhang, P.; Zhao, J. Research progress on the solubility of supercritical CO2. Refin. Chem. Ind. 2011, 22, 1–5. [Google Scholar]
- Peng, B. Study On the Mechanism of Carbon Dioxide Auxiliary Steam Thermal Recovery; China University of Petroleum: Beijing, China, 2012. [Google Scholar]
- Fan, P.; Zhu, W.; Lin, j.; Gao, T.; Wang, Y. Factors and Regularity of Supercritical cOExtraction of Heavy Oil lmpact. Sci. Technol. Eng. 2017, 17, 31–36. [Google Scholar]
- Allawzi, M.; Al-Otoom, A.; Allaboun, H.; Ajlouni, A.; Al Nseirat, F. CO2 supercritical fluid extraction of Jordanian oil shale utilizing different co-solvents. Fuel Process. Technol. 2011, 92, 2016–2023. [Google Scholar] [CrossRef]
- Priyanto, S.; Mansoori, G.A.; Suwono, A. Measurement of property relationships of nano-structure micelles and coacervates of asphaltene in a pure solvent. Chem. Eng. Sci. 2001, 56, 6933–6939. [Google Scholar] [CrossRef]
- Ni, H.; Hsu, C.S.; Lee, P.; Wright, J.; Chen, R.; Xu, C.; Shi, Q. Supercritical carbon dioxide extraction of petroleum on kieselguhr. Fuel 2015, 141, 74–81. [Google Scholar] [CrossRef]
- Cao, X.; Peng, B.; Ma, S.; Ni, H.; Zhang, L.; Zhang, W.; Li, M.; Hsu, C.S.; Shi, Q. Molecular Selectivity in Supercritical CO2 Extraction of a Crude Oil. Energy Fuels 2017, 31, 4996–5002. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Qian, M.; Jiang, Q.; Cao, T.; Liu, P.; Bao, Y.; Tao, G. Movable oil extraction from shale with supercritical carbon dioxide. Pet. Geol. Exp. 2020, 42, 646–652. [Google Scholar]
- Li, H. Molecular Dynamics Simulation for Supercritical Carbon Dioxide Extraction of Heavy Oil Components in Deep Reservoir. Sci. Technol. Eng. 2021, 21, 12543–12550. [Google Scholar]
- Ding, C. Research and Analysis of the Influencement of Carbon Dioxide Flooding on Asphaltene Precipitation; China University of Petroleum: Beijing, China, 2013. [Google Scholar]
- Chen, L.; Yv, H.; Tang, R.; Wang, W.; Yao, Z. Effect of Asphalt Deposition on CO2 Flooding in Light Oil Reservoir. Oilfield Chem. 2017, 34, 87–91. [Google Scholar]
- Kazemzadeh, Y.; Sourani, S.; Doryani, H.; Reyhani, M.; Shabani, A.; Fallah, H. Recovery of Asphaltenic Oil During Nano Fluid Injection. Pet. Sci. Technol. 2014, 33, 139–146. [Google Scholar] [CrossRef]
- Mohammadi, M.; Akbari, M.; Fakhroueian, Z.; Bahramian, A.; Azin, R.; Arya, S. Inhibition of Asphaltene Precipitation by TiO2, SiO2, and ZrO2 Nanofluids. Energy Fuels 2011, 25, 3150–3156. [Google Scholar] [CrossRef]
- Cao, C.; Song, Z.; Su, S.; Tang, Z.; Xie, Z.; Chang, X.; Shen, P. Water-based nanofluid-alternating-CO2 injection for enhancing heavy oil recovery: Considering oil-nanofluid emulsification. J. Pet. Sci. Eng. 2021, 205, 108934. [Google Scholar] [CrossRef]
- Hassanpour, S.; Malayeri, M.R.; Riazi, M.; Mousavi, S.M.; Negahban, Z. On the impact of Co3O4 nanoparticles on interaction of heavy oil and brine mixtures. J. Pet. Sci. Eng. 2018, 171, 680–686. [Google Scholar] [CrossRef]
- Mohammadi, M.; Dadvar, M.; Dabir, B. Application of response surface methodology for optimization of the stability of asphaltene particles in crude oil by TiO2/SiO2 nanofluids under static and dynamic conditions. J. Dispers. Sci. Technol. 2017, 39, 431–442. [Google Scholar] [CrossRef]
- Panahi, S.; Sardarian, A.R.; Esmaeilzadeh, F.; Mowla, D. Synthesize and characterization of chitosan and silica supported on Fe3O4 nanoparticles for the adsorption and removal of asphaltene molecules from crude oil. Mater. Res. Express 2018, 5, 095022. [Google Scholar] [CrossRef]
- Li, A.; Zhang, W.; Shu, F.; Li, L.; Li, M.; Lei, B. Properties of asphaltene in BA crude oil and mechanism of deposition inhibitor. Chem. Ind. Eng. Prog. 2019, 38, 110–115. [Google Scholar]
- Wang, X. Unlocking the Mechanism of Asphaltene Dispersion in Petroleum Fluids; China University of Petroleum: Beijing, China, 2014. [Google Scholar]
- Setoodeh, N.; Darvishi, P.; Lashanizadegan, A. A comparative study to evaluate the performance of coated Fe3O4nanoparticles for adsorption of asphaltene from crude oil in bench scale. J. Dispers. Sci. Technol. 2017, 39, 711–720. [Google Scholar] [CrossRef]
- Xu, L.; Li, Q.; Myers, M.; Cao, X. Investigation of the enhanced oil recovery mechanism of CO2 synergistically with nanofluid in tight glutenite. Energy 2023, 273, 127275. [Google Scholar] [CrossRef]
- Ju, B.; Fan, T.; Li, Z. Improving water injectivity and enhancing oil recovery by wettability control using nanopowders. J. Pet. Sci. Eng. 2012, 86, 206–216. [Google Scholar] [CrossRef]
- Dezfuli, M.G.; Jafari, A.; Gharibshahi, R. Optimum volume fraction of nanoparticles for enhancing oil recovery by nanosilica/supercritical CO2 flooding in porous medium. J. Pet. Sci. Eng. 2020, 185, 106599. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam–oil interaction in porous media: Implications for foam assisted enhanced oil recovery. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012; Volume 183, pp. 1–13. [Google Scholar]
- Zhang, S. Study on identification method for gas channeling of CO2 flooding in low permeability reservoirs. Pet. Geol. Recovery Effic. 2020, 27, 101–106. [Google Scholar]
- Cui, C.; Zhou, Z.; He, Z. Enhance oil recovery in low permeability reservoirs: Optimization and evaluation of ultra-high molecular weight HPAM/phenolic weak gel system. J. Pet. Sci. Eng. 2020, 195, 107908. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Wang, J.; Xu, F. Flow regularity and new knowledge of enhancing recovery mechanism of weak gel. Pet. Geol. Recovery Effic. 2006, 13, 85–87. [Google Scholar]
- Ma, Y.; Zhang, H.; Yuan, S. Hydration Structure of Partially Hydrolyzed Preformed Particle Gel. Chem. J. Chin. Univ. 2015, 36, 386–394. [Google Scholar]
- Peng, H. Research on Methods of Anti-channeling of CO2-Flooding in Low-permeability Reservoirs; China University of Petroleum: Beijing, China, 2018. [Google Scholar]
- Al-Ali, A.H.; Schechter, D.S.; Lane, R.H. Application of Polymer Gels as Conformance Control Agents for Carbon Dioxide EOR WAG Floods. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. [Google Scholar]
- Sun, X.; Bai, B.; Alhuraishawy, A.K.; Zhu, D. Understanding the Plugging Performance of HPAM-Cr (III) Polymer Gel for CO2 Conformance Control. SPE J. 2021, 26, 3109–3118. [Google Scholar] [CrossRef]
- Zhou, B.; Kang, W.; Yang, H.; Zhu, T.; Zhang, H.; Li, X.; Sarsenbekuly, B.; Sarsenbek, T. Preparation and properties of an acid-resistant preformed particle gel for conformance control. J. Pet. Sci. Eng. 2021, 197, 107964. [Google Scholar] [CrossRef]
- Pu, W.; Du, D.; Fan, H.; Chen, B.; Yuan, C.; Varfolomeev, M.A. CO2-responsive preformed gel particles with interpenetrating networks for controlling CO2 breakthrough in tight reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126065. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, W.; Zhang, T.; Ge, J.; Jiang, P.; Zhang, G. CO2 in water foam stabilized with CO2-dissolved surfactant at high pressure and high temperature. J. Pet. Sci. Eng. 2019, 178, 930–936. [Google Scholar] [CrossRef]
- Du, D.; Beni, A.N.; Farajzadeh, R.; Zitha, P.L.J. Effect of Water Solubility on Carbon Dioxide Foam Flow in Porous Media: An X-ray Computed Tomography Study. J. Ind. Eng. Chem. 2008, 47, 6298–6306. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Bruining, H.; Zitha, P.L.J. Comparative Study of CO2 and N2 Foams in Porous Media at Low and High Pressure-Temperatures. Ind. Eng. Chem. Res. 2009, 48, 4542–4552. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Y.; Zhang, L.; Ren, S.; Lu, J.; Wang, X.; Fan, N. The stability study of CO2 foams at high pressure and high temperature. J. Pet. Sci. Eng. 2017, 154, 234–243. [Google Scholar] [CrossRef]
- Lv, M.; Wang, S. Stability of carbon dioxide foam and effect of polymer on its foam properties. CIESC J. 2014, 65, 2219–2224. [Google Scholar]
- Adkins, S.S.; Chen, X.; Chan, I.; Torino, E.; Nguyen, Q.P.; Sanders, A.W.; Johnston, K.P. Morphology and stability of CO2-in-water foams with nonionic hydrocarbon surfactants. Langmuir 2010, 26, 5335–5348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zheng, J. Effect of Polymer on Foam Stability of CO2 Flooding Anti-Channeling System. Shandong Chem. Ind. 2022, 51, 9–11. [Google Scholar]
- Zhang, X.; Zhang, G.; Ge, J.; Jiang, J. Research on the stability of polymer enhanced CO2 foam. Fault-Block Oil Gas Field 2020, 27, 799–802. [Google Scholar]
- Davarpanah, A.; Shirmohammadi, R.; Mirshekari, B. Experimental evaluation of polymer-enhanced foam transportation on the foam stabilization in the porous media. Int. J. Environ. Sci. Technol. 2019, 16, 8107–8116. [Google Scholar] [CrossRef]
- Pu, W.; Wei, P.; Suna, L.; Wang, S. Stability, CO2 sensitivity, oil tolerance and displacement efficiency of polymer enhanced foam. RSC Adv. 2017, 7, 6251–6258. [Google Scholar] [CrossRef]
- Qi, G.; Zhou, L.; Long, M.; Wu, S.; Zhou, X. Experimental Study of the CO2 Miscible Modulator Oil-displacement Mechanism. Sci. Technol. Eng. 2016, 16, 180–183. [Google Scholar]
- Lv, W.; Gong, H.; Li, Y.; Li, Z.; Dong, M. The potential and mechanism of nonionic polyether surfactants dissolved in CO2 to improve the miscibility of CO2-hydrocarbon systems. Fuel 2022, 326, 125012. [Google Scholar] [CrossRef]
- Sun, L. Experimental Study on Reducing CO2 Miscibility Pressure by Modifiers; China University of Petroleum: Beijing, China, 2016. [Google Scholar]
- Liu, Y. Technology Research of Improving the Oil Displacement Efficiency for CO2 Flooding; Northeast Petroleum University: Daqing, China, 2017. [Google Scholar]
- Yang, S.; Lian, L.; Yang, Y.; Li, S.; Tang, J.; Ji, Z.; Zhang, Y. Molecular Optimization Design and Evaluation of Miscible Processing Aids Applied to CO2 Flooding. Xinjiang Pet. Geol. 2015, 36, 555–559. [Google Scholar]
- Wang, F.; Luo, H.; Ren, Y.; Fan, W.; Liang, M.; Zhang, C. Progress of Miscibility Pressure Reduction of Carbon Dioxide Flooding. J. Petrochem. Univ. 2015, 28, 93–97. [Google Scholar]
- Wu, C.; Shen, Z.; Li, Y.; Sha, O. Methods of Lowering the Minimum Miscibility Pressure of CO2 Flooding. Chem. World 2016, 57, 451–456. [Google Scholar]
- Lv, W.; Dong, M.; Sarma, H.; Li, Y.; Li, Z.; Sun, J.; Gong, H. Effects of CO2-philic nonionic polyether surfactants on miscibility behaviors of CO2-hydrocarbon systems: Experimental and simulation approach. Chem. Eng. J. 2023, 464, 142701. [Google Scholar] [CrossRef]
- Wang, X. Performance evaluation and oil displacement experiment study of CO2 foam system. Pet. Geol. Recovery Effic. 2020, 27, 69–74. [Google Scholar]
- Jones, S.A.; Laskaris, G.; Vincent-Bonnieu, S.; Farajzadeh, R.; Rossen, W.R. Effect of surfactant concentration on foam: From coreflood experiments to implicit-texture foam-model parameters. J. Ind. Eng. Chem. 2016, 37, 268–276. [Google Scholar] [CrossRef]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Colloid Interface Sci. 2016, 228, 55–70. [Google Scholar] [CrossRef]
- Kanokkarn, P.; Shiina, T.; Santikunaporn, M.; Chavadej, S. Equilibrium and dynamic surface tension in relation to diffusivity and foaming properties: Effects of surfactant type and structure. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 135–142. [Google Scholar] [CrossRef]
- Tamura, T.; Ohyama, M.; Ohyama, M. Dynamic Surface Tension and Foaming Properties of Aqueous Polyoxyethylene n-Dodecyl Ether Solutions. J. Colloid Interface Sci. 1995, 173, 493–499. [Google Scholar] [CrossRef]
- AlQuaimi, B.I.; Rossen, W.R. Study of foam generation and propagation in fully characterized physical-model fracture. J. Pet. Sci. Eng. 2019, 175, 1169–1181. [Google Scholar] [CrossRef]
- Fu, T.; Ma, Y. Bubble formation and breakup dynamics in microfluidic devices: A review. Chem. Eng. Sci. 2015, 135, 343–372. [Google Scholar] [CrossRef]
- Wei, P.; Pu, W.; Sun, L.; Pu, Y.; Li, D.; Chen, Y. Role of water-soluble polymer on foam-injection process for enhancing oil recovery. J. Ind. Eng. Chem. 2018, 65, 280–289. [Google Scholar] [CrossRef]
- Adkins, S.S.; Chen, X.; Nguyen, Q.P.; Sanders, A.W.; Johnston, K.P. Effect of branching on the interfacial properties of nonionic hydrocarbon surfactants at the air-water and carbon dioxide-water interfaces. J. Colloid Interface Sci. 2010, 346, 455–463. [Google Scholar] [CrossRef]
- Xing, D.; Wei, B.; McLendon, W.; Enick, R.; McNulty, S.; Trickett, K.; Mohamed, A.; Cummings, S.; Eastoe, J.; Rogers, S.; et al. CO2-Soluble, Nonionic, Water-Soluble Surfactants That Stabilize CO2-in-Brine Foams. SPE J. 2012, 17, 1172–1185. [Google Scholar] [CrossRef]
- Ren, G.; Sanders, A.W.; Nguyen, Q.P. New method for the determination of surfactant solubility and partitioning between CO2 and brine. J. Supercrit. Fluids 2014, 91, 77–83. [Google Scholar] [CrossRef]
- Niu, Q.; Dong, Z.; Lv, Q.; Zhang, F.; Shen, H.; Yang, Z.; Lin, M.; Zhang, J.; Xiao, K. Role of interfacial and bulk properties of long-chain viscoelastic surfactant in stabilization mechanism of CO2 foam for CCUS. J. CO2 Util. 2022, 66, 102297. [Google Scholar] [CrossRef]
- Wu, S.; He, J.; Li, Z.; Lin, W.; Wang, Q.; Wu, F.; Zhao, B.; Zhang, J. Studies on the chemical agent system for reducing in the minimal miscibility pressure of CO2 flooding. China Sci. 2015, 10, 2161–2164. [Google Scholar]
- Almobarak, M.; Wu, Z.; Zhou, D.; Fan, K.; Liu, Y.; Xie, Q. A review of chemical-assisted minimum miscibility pressure reduction in CO2 injection for enhanced oil recovery. Petroleum 2021, 7, 245–253. [Google Scholar] [CrossRef]
- Rommerskirchen, R.; Nijssen, P.; Bilgili, H.; Sottmann, T. Reducing the Miscibility Pressure in Gas Injection Oil Recovery Processes. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016. [Google Scholar]
- Wang, F.; Luo, H.; Ren, Y.; Fan, W.; Liang, M.; Nan, Z. Influences of fatty alcohol polyoxypropylene ether on the minimum miscibility pressure of carbon dioxide flooding. Pet. Geol. Oilfield Dev. Daqing 2016, 35, 118–122. [Google Scholar]
- Guo, P.; Hu, Y.; Qin, J.; Li, S.; Jiao, S.; Chen, F.; He, J. Use of oil-soluble surfactant to reduce minimum miscibility pressure. Pet. Sci. Technol. 2017, 35, 345–350. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Fan, W.; Nan, G.; Li, Z. Effects of the Non-ionic Surfactant (CiPOj) on the Interfacial Tension Behavior between CO2 and Crude Oil. Energy Fuels 2018, 32, 6708–6712. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Li, Z.; Wu, X. Experimental Study on Reducing CO2-Oil Minimum Miscibility Pressure with Hydrocarbon Agents. Energies 2019, 12, 1975. [Google Scholar] [CrossRef]
- Choubineh, A.; Helalizadeh, A.; Wood, D.A. The impacts of gas impurities on the minimum miscibility pressure of injected CO2-rich gas–crude oil systems and enhanced oil recovery potential. Pet. Sci. 2018, 16, 117–126. [Google Scholar] [CrossRef]
- Zhang, C.; Xi, L.; Wu, P.; Li, Z. A novel system for reducing CO2-crude oil minimum miscibility pressure with CO2-soluble surfactants. Fuel 2020, 281, 118690. [Google Scholar] [CrossRef]
- Kuang, N.; Yang, S.; Yuan, Z.; Wang, M.; Zhang, Z.; Zhang, X.; Wang, M.; Zhang, Y.; Li, S.; Wu, J.; et al. Study on Oil and Gas Amphiphilic Surfactants Promoting the Miscibility of CO2 and Crude Oil. Engineering 2021, 6, 27170–27182. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Fu, B. Study on reducing the minimum miscible pressure (MMP) of CO2 flooding by miscible solvent method. Liaoning Chem. Ind. 2017, 46, 3. [Google Scholar]







| Surfactant | Gas | MMP Reduction/% | Method | Reference |
|---|---|---|---|---|
| Acetyl glucose dodecyl ester | CO2 | 27.47 | IFT method | [155] |
| Multiple | CO2 | 17.86 | IFT method | [171] |
| Oil-soluble | CO2 | 17.86 | VIT | [172] |
| Surfactants | CO2 | 16.0–22.0 | Observation cell | [173] |
| QDM, QHB | CO2 | 15.0 | IFT method | [151] |
| Lauryl alcohol polyoxypropylene ether | CO2 | 25.6 | - | [174] |
| Surfactant CAE | CO2 | 20.0 | Slim-tube | [175] |
| Propoxylated | CO2 | 27.7 | VIT | [176] |
| Oil-soluble | CO2 | 8.28 | IFT method | [177] |
| C1, C2, C3 | CO2 | 13.2 | Slim-tube | [178] |
| NP-9 | CO2 | 5.72 | IFT method | [179] |
| 2EH-PO5-EO9 | CO2 | 8.03 | IFT method | [179] |
| Amphiphilic | CO2 | 20.0 | Slim-tube | [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, W.; Tian, J.; Meng, Y.; Zhang, L. Research Progress on Displacement Mechanism of Supercritical CO2 in Low-Permeability Heavy Oil Reservoir and Improvement Mechanism of Displacement Agents. Molecules 2023, 28, 6154. https://doi.org/10.3390/molecules28166154
Sun Y, Zhang W, Tian J, Meng Y, Zhang L. Research Progress on Displacement Mechanism of Supercritical CO2 in Low-Permeability Heavy Oil Reservoir and Improvement Mechanism of Displacement Agents. Molecules. 2023; 28(16):6154. https://doi.org/10.3390/molecules28166154
Chicago/Turabian StyleSun, Yuanxiu, Weijie Zhang, Jinlong Tian, Yanzhao Meng, and Liping Zhang. 2023. "Research Progress on Displacement Mechanism of Supercritical CO2 in Low-Permeability Heavy Oil Reservoir and Improvement Mechanism of Displacement Agents" Molecules 28, no. 16: 6154. https://doi.org/10.3390/molecules28166154




