MoS2/SnS/CoS Heterostructures on Graphene: Lattice-Confinement Synthesis and Boosted Sodium Storage
Abstract
:1. Introduction
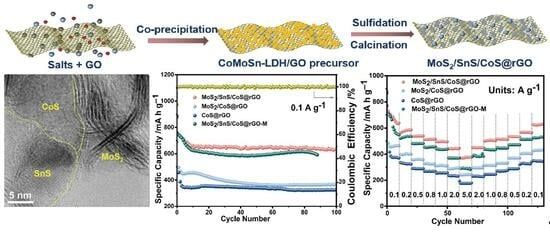
2. Results
3. Materials and Methods
3.1. Preparation of CoMoSn-LDH/GO Precursor
3.2. Preparation of MoS2/SnS/CoS@rGO
3.3. Materials Characterization
3.4. Electrochemical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gervillié-Mouravieff, C.; Boussard-Plédel, C.; Huang, J.; Leau, C.; Blanquer, L.A.; Ben Yahia, M.; Doublet, M.-L.; Boles, S.T.; Zhang, X.H.; Adam, J.L.; et al. Unlocking cell chemistry evolution with operando fibre optic infrared spectroscopy in commercial Na(Li)-ion batteries. Nat. Energy 2022, 7, 1157–1169. [Google Scholar] [CrossRef]
- Su, R.; Zhu, W.; Liang, K.; Wei, P.; Li, J.; Liu, W.; Ren, Y. Mnx+ Substitution to Improve Na3V2(PO4)2F3-Based Electrodes for Sodium-Ion Battery Cathode. Molecules 2023, 28, 1409. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yang, H.; Zhang, Z.; Xu, X.; Xu, Y.; Zhou, J.; Zhou, X.; Pan, Z.; Rao, X.; Gu, Y.; et al. The Progress of Hard Carbon as an Anode Material in Sodium-Ion Batteries. Molecules 2023, 28, 3134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yin, B.; Zhang, Y.; Wu, Q.; Xu, H.; Duan, H.; Shi, M.; He, H. A Multifunctional Coating on Sulfur-Containing Carbon-Based Anode for High-Performance Sodium-Ion Batteries. Molecules 2023, 28, 3335. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, X.-Y.; Chang, B.; Wang, K.-X. Recent progress on germanium-based anodes for lithium ion batteries: Efficient lithiation strategies and mechanisms. Energy Storage Mater. 2020, 30, 146–169. [Google Scholar] [CrossRef]
- Song, N.-J.; Guo, N.; Ma, C.; Zhao, Y.; Li, W.; Li, B. Modulating the Graphitic Domains and Pore Structure of Corncob-Derived Hard Carbons by Pyrolysis to Improve Sodium Storage. Molecules 2023, 28, 3595. [Google Scholar] [CrossRef]
- Sun, X.; Luo, F. Sodium Storage Properties of Carbonaceous Flowers. Molecules 2023, 28, 4753. [Google Scholar] [CrossRef]
- Bommier, C.; Surta, T.W.; Dolgos, M.; Ji, X. New Mechanistic Insights on Na-Ion Storage in Nongraphitizable Carbon. Nano Lett. 2015, 15, 5888. [Google Scholar] [CrossRef]
- Xiao, B.; Soto, F.A.; Gu, M.; Han, K.S.; Song, J.; Wang, H.; Engelhard, M.H.; Murugesan, V.; Mueller, K.T.; Reed, D.; et al. Lithium-Pretreated Hard Carbon as High-Performance Sodium-Ion Battery Anodes. Adv. Energy Mater. 2018, 8, 1801441. [Google Scholar] [CrossRef]
- Yao, X.; Ke, Y.; Ren, W.; Wang, X.; Xiong, F.; Yang, W.; Qin, M.; Li, Q.; Mai, L. Defect-Rich Soft Carbon Porous Nanosheets for Fast and High-Capacity Sodium-Ion Storage. Adv. Energy Mater. 2018, 9, 1803260. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, Y.; Zeng, S.; Yu, Y. Carbon and Carbon Hybrid Materials as Anodes for Sodium-Ion Batteries. Chem. Asian J. 2018, 13, 1248–1265. [Google Scholar] [CrossRef]
- Choi, Y.S.; Byeon, Y.W.; Park, J.H.; Seo, J.H.; Ahn, J.P.; Lee, J.C. Ultrafast Sodiation of Single-Crystalline Sn Anodes. ACS Appl. Mater. Interfaces 2018, 10, 560–568. [Google Scholar] [CrossRef]
- Tan, H.; Chen, D.; Rui, X.; Yu, Y. Peering into Alloy Anodes for Sodium-Ion Batteries: Current Trends, Challenges, and Opportunities. Adv. Funct. Mater. 2019, 29, 1808745. [Google Scholar] [CrossRef]
- Yang, M.; Liu, L.; Yan, H.; Zhang, W.; Su, D.; Wen, J.; Liu, W.; Yuan, Y.; Liu, J.; Wang, X. Porous nitrogen-doped Sn/C film as free-standing anodes for lithium ion batteries. Appl. Surf. Sci. 2021, 551, 149246. [Google Scholar] [CrossRef]
- Chai, W.; Yang, F.; Yin, W.; You, S.; Wang, K.; Ye, W.; Rui, Y.; Tang, B. Bi2S3/C nanorods as efficient anode materials for lithium-ion batteries. Dalton Trans. 2019, 48, 1906–1914. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, S.; Zhai, T.; Yang, M.; Zhao, X.; Xia, H. Yolk-Shell NiS2 Nanoparticle-Embedded Carbon Fibers for Flexible Fiber-Shaped Sodium Battery. Adv. Energy Mater. 2018, 8, 1800054. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Zai, J.; Jin, Y.; Zhan, P.; Huang, Y.; Tie, X.; Qi, R.; Qian, X. Flower-like SnS2 composite with 3D pyrolyzed bacterial cellulose as the anode for lithium-ion batteries with ultralong cycle life and superior rate capability. Dalton Trans. 2019, 48, 833–838. [Google Scholar] [CrossRef]
- Luo, F.; Ma, D.; Li, Y.; Mi, H.; Zhang, P.; Luo, S. Hollow Co3S4/C anchored on nitrogen-doped carbon nanofibers as a free-standing anode for high-performance Li-ion batteries. Electrochim. Acta 2019, 299, 173–181. [Google Scholar] [CrossRef]
- Yang, M.; Chang, X.; Wang, L.; Wang, X.; Gu, M.; Huang, H.; Tang, L.; Zhong, Y.; Xia, H. Interface Modulation of Metal Sulfide Anodes for Long-Cycle-Life Sodium-Ion Batteries. Adv. Mater. 2023, 35, 2208705. [Google Scholar] [CrossRef]
- Lim, Y.V.; Li, X.L.; Yang, H.Y. Recent Tactics and Advances in the Application of Metal Sulfides as High-Performance Anode Materials for Rechargeable Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 31, 2006761. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, X.; Wang, H.; Yu, D.Y.W.; Rogach, A.L. Hierarchical CoS2/N-Doped Carbon@MoS2 Nanosheets with Enhanced Sodium Storage Performance. ACS Appl. Mater. Interfaces 2020, 12, 54644–54652. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wu, N.; Xie, W.; Li, Q.; Guo, D.; Li, J.; Liu, G.; Liu, X.; Mi, H. Realizing Ultrafast and Robust Sodium-Ion Storage of Iron Sulfide Enabled by Heteroatomic Doping and Regulable Interface Engineering. Molecules 2023, 28, 3757. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, C.; Zhang, Q.; Liu, M. Recent progress in the design of metal sulfides as anode materials for sodium ion batteries. Energy Storage Mater. 2019, 22, 66–95. [Google Scholar] [CrossRef]
- Sennua, P.; Christyb, M.; Aravindanc, V.; Lee, Y.-G.; Nahmb, K.S.; Lee, Y.-S. Two Dimensional Mesoporous Cobalt Sulfide Nanosheets as a Superior Anode for a Li-Ion Battery and a Bifunctional Electrocatalyst for the Li-O2 System. Chem. Mater. 2015, 27, 5726–5735. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, R.; Xia, W.; Ma, J.; Qiu, B.; Mahmood, A.; Zhao, R.; Yang, Y.; Xia, D.; Xu, Q. Facile Synthesis of Ultrasmall CoS2 Nanoparticles within Thin N-Doped Porous Carbon Shell for High Performance Lithium-Ion Batteries. Small 2015, 11, 2511–2517. [Google Scholar] [CrossRef]
- Wu, R.; Wang, D.P.; Rui, X.; Liu, B.; Zhou, K.; Law, A.W.; Yan, Q.; Wei, J.; Chen, Z. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv. Mater. 2015, 27, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, J.; Wang, H.; Zhang, J.; Ma, Z.; Yi, Q.; Liu, J.; Wang, S. Synthesis of FeS2/CoS heterostructure microspheres as anodes for high-performance Li-ion batteries. J. Solid. State Electrochem. 2023. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, L.; Huang, Z.; Yi, L.; Wang, X.; Cao, G. Co3S4@polyaniline nanotubes as high-performance anode materials for sodium ion batteries. J. Mater. Chem. A 2016, 4, 5505–5516. [Google Scholar] [CrossRef]
- He, Q.; Xiao, F.; Chen, R.; Yao, T.; Wu, Y.; Wang, H. Covalent sulfur/CoS2-decorated honeycomb-like carbon hierarchies for high-performance sodium storage with ultra-long high-rate cycling stability. J. Alloys Compd. 2023, 960, 170866. [Google Scholar] [CrossRef]
- Mu, M.; He, H.; Gan, Y.; Yu, J.; Mou, J.; Yuan, J.; Li, F.; Wang, X.; Li, X.; Zhang, X.; et al. Three-dimensional porous C/CoS nanocomposites for a long-life and high-rate potassium storage. Mater. Res. Bull. 2023, 165, 112337. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Z.; Wen, B.; Zhang, Z.; Yang, G.; Yan, W. Controlled growth of Co9S8 nanoparticle-embedded carbon nanosheets/carbon nanofibers toward high-performance sodium storage. J. Colloid Interface Sci. 2023, 648, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Kang, H.; Liu, H.; Yang, B.; Li, Z.; Li, Z. Self-assembled CoS2/NiCo2S4/RGO nanohybrids as advanced electrode for hybrid supercapacitor with enhanced energy density and ultra-long durability. J. Energy Storage 2023, 67, 107528. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Zhao, H.; Cui, J.; Zhang, Y.; He, W. A cycle-durable hollow nanoneedle structure with a nanosheet as a conductive substrate CoS1.097/Ni9S8@RGO to enhance supercapacitor performance. Dalton Trans. 2023, 52, 7731–7738. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, Q.; Chen, H.; Li, G.; Zhu, H. Quadra layer core-shell-core-shell structured Co9S8@MnS-NC nanorod composites for enhanced sodium-ion batteries. Electrochim. Acta 2023, 459, 142569. [Google Scholar] [CrossRef]
- Li, Y.; Zan, L.; Chen, J. Improving the Reaction Kinetics by Annealing MoS2/PVP Nanoflowers for Sodium-Ion Storage. Molecules 2023, 28, 2948. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Fan, F.; Zhu, Z.; Zhang, Z.; Gu, Y.; Li, Q. MoS2/CoS heterostructures grown on carbon cloth as free-standing anodes for high-performance sodium-ion batteries. Nanoscale 2023, 15, 6822–6829. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, Y.; Chen, T.; Xia, Q.; Yang, M.; Xia, H.; Yu, Y. Cobalt Sulfide Quantum Dot Embedded N/S-Doped Carbon Nanosheets with Superior Reversibility and Rate Capability for Sodium-Ion Batteries. ACS Nano 2017, 11, 12658–12667. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, T.; Wu, N.; Ding, H.; Yu, G.; Lian, J.; Xu, L.; Qiu, J.; Li, S. Rational Design of the CoS/Co9S8@NC Composite Enabling High-Rate Sodium-Ion Storage. ACS Appl. Energy Mater. 2021, 4, 5574–5582. [Google Scholar] [CrossRef]
- Wan, S.; Cheng, M.; Chen, H.; Zhu, H.; Liu, Q. Nanoconfined bimetallic sulfides (CoSn)S heterostructure in carbon microsphere as a high-performance anode for half/full sodium-ion batteries. J. Colloid Interface Sci. 2022, 609, 403–413. [Google Scholar] [CrossRef]
- Huang, X.; Tao, K.; Han, T.; Li, J.; Zhang, H.; Hu, C.; Niu, J.; Liu, J. Long-Cycling-Life Sodium-Ion Battery Using Binary Metal Sulfide Hybrid Nanocages as Anode. Small 2023, 19, 2302706. [Google Scholar] [CrossRef]
- Duan, Y.-K.; Li, Z.-W.; Zhang, S.-C.; Su, T.; Zhang, Z.-H.; Jiao, A.-J.; Fu, Z.-H. Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review. Molecules 2023, 28, 5037. [Google Scholar] [CrossRef]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al Ordering in Layered Double Hydroxides Revealed by Multinuclear NMR Spectroscopy. Science 2008, 321, 113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cui, G.; Feng, H.; Chen, L.; Wang, H.; Wang, B.; Zhang, X.; Zheng, L.; Hong, S.; Wei, M. Platinum-copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun. 2019, 10, 5812. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. From Layered Double Hydroxides to ZnO-based Mixed Metal Oxides by Thermal Decomposition: Transformation Mechanism and UV-Blocking Properties of the Product. Chem. Mater. 2010, 22, 3933–3942. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Deng, C.; Chen, F.; Su, Y.; Li, S.Y.; Xu, S. Low-content SnO2 nanodots on N-doped graphene: Lattice-confinement preparation and high-performance lithium/sodium storage. Dalton Trans. 2023, 52, 1642–1649. [Google Scholar] [CrossRef]
- Mohili, R.; Hemanth, N.R.; Jin, H.; Lee, K.; Chaudhari, N. Emerging high entropy metal sulphides and phosphides for electrochemical water splitting. J. Mater. Chem. A 2023, 11, 10463–10472. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, M.; Chen, F.; Kang, H.; Xu, S.; Xu, S. IrCo alloy nanoparticles supported on N-doped carbon for hydrogen evolution electrocatalysis in acidic and alkaline electrolytes. Dalton Trans. 2020, 49, 13339–13344. [Google Scholar] [CrossRef]
- Lou, S.J.; Xu, D.Q.; Xu, Z.Y. Mild and versatile nitrate-promoted C-H bond fluorination. Angew. Chem. Int. Ed. Engl. 2014, 53, 10330–10335. [Google Scholar] [CrossRef]
- Sun, N.; Qiu, J.; Xu, B. Understanding of Sodium Storage Mechanism in Hard Carbons: Ongoing Development under Debate. Adv. Energy Mater. 2022, 27, 12. [Google Scholar] [CrossRef]
- Wen, X.; Feng, W.; Li, X.; Yang, J.; Du, R.; Wang, P.; Li, H.; Song, L.; Wang, Y.; Cheng, M.; et al. Diatomite-Templated Synthesis of Single-Atom Cobalt-Doped MoS2/Carbon Composites to Boost Sodium Storage. Adv. Mater. 2023, 2211690. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Liu, Z.; Liu, X.; Duan, J.; Yang, T.; Lan, J.; Tan, Y.; Wang, C.; Chen, M.; et al. Engineering Co—P Alloy Foil to a Well-Designed Integrated Electrode Toward High-Performance Electrochemical Energy Storage. Adv. Mater. 2023, 35, 2209924. [Google Scholar] [CrossRef]
- Ma, Z.; Song, A.; Liu, Z.; Guo, Y.; Yang, X.; Li, Q.; Fan, Y.; Dai, L.; Tian, H.; Qin, X.; et al. Nanoconfined Expansion Behavior of Hollow MnS@Carbon Anode with Extended Lithiation Cyclic Stability. Adv. Energy Mater. 2023, 28, 33. [Google Scholar] [CrossRef]
- Deng, Q.; Liu, X.; Li, Z.; Fan, H.; Zhang, Y.; Yang, H.Y. Cobalt-nickel bimetallic sulfide (NiS2/CoS2) based dual-carbon framework for super sodium ion storage. J. Colloid Interf. Sci. 2023, 633, 480–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, C.; Wang, X.; Chen, S.; Li, D.; Peng, Z.; Yang, D. Boosting Sodium-Ion Storage by Encapsulating NiS (CoS) Hollow Nanoparticles into Carbonaceous Fibers. ACS Appl. Mater. Interfaces 2018, 10, 40531–40539. [Google Scholar] [CrossRef]
- Fang, Y.; Guan, B.Y.; Luan, D.; Lou, X.W.D. Synthesis of CuS@CoS2 Double-Shelled Nanoboxes with Enhanced Sodium Storage Properties. Angew. Chem. Int. Ed. 2019, 58, 7739–7743. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, H.; Qin, B.; Wang, M.; Zhang, Y.; Fan, H. Rational design of heterostructured bimetallic sulfides (CoS2/NC@VS4) with VS4 nanodots decorated on CoS2 dodecahedron for high-performance sodium and potassium ion batteries. J. Colloid Interf. Sci. 2022, 625, 41–49. [Google Scholar] [CrossRef]
- Liu, C.; Lu, Q.; Gorbunov, M.V.; Omar, A.; Martinez, I.G.G.; Zhao, P.; Hantusch, M.; Permana, A.D.C.; He, H.; Gaponik, N.; et al. Ultrasmall CoS nanoparticles embedded in heteroatom-doped carbon for sodium-ion batteries and mechanism explorations via synchrotron X-ray techniques. J. Energy Chem. 2023, 79, 373–381. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.G.; Kong, L.B. Synthesis of polyvalent ion reaction of MoS2/CoS2-RGO anode materials for high-performance sodium-ion batteries and sodium-ion capacitors. J. Colloid Interf. Sci. 2020, 575, 42–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, R.; Xu, Q.; Liu, H.; Tao, M.; Luo, Y.; Bao, S.; Li, C.; Xu, M. Circuit board-like CoS/MXene composite with superior performance for sodium storage. Chem. Eng. J. 2019, 357, 220–225. [Google Scholar] [CrossRef]
- Chen, C.; Hu, X.; Zhang, B.; Miaob, L.; Huang, Y. Architectural design and phase engineering of N/Bcodoped TiO2(B)/anatase nanotube assemblies for high-rate and long-life lithium storage. J. Mater. Chem. A 2015, 3, 22591. [Google Scholar] [CrossRef]
- Bai, D.; Wang, F.; Lv, J.; Zhang, F.; Xu, S. Triple-Confined Well-Dispersed Biactive NiCo2S4/Ni0.96S on Graphene Aerogel for High-Efficiency Lithium Storage. ACS Appl. Mater. Interfaces 2016, 8, 32853–32861. [Google Scholar] [CrossRef]
- Wang, R.; Xue, X.; Lu, W.; Liu, H.; Lai, C.; Xi, K.; Che, Y.; Liu, J.; Guo, S.; Yang, D. Tuning and understanding the phase interface of TiO2 nanoparticles for more efficient lithium ion storage. Nanoscale 2015, 7, 12833–12838. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Huang, F. Graphene inducing graphitization: Towards a hard carbon anode with ultrahigh initial coulombic efficiency for sodium storage. Chem. Eng. J. 2022, 434, 134503. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Dong, Y.; Su, Y.; Zhai, W.; Xu, S. MoS2/SnS/CoS Heterostructures on Graphene: Lattice-Confinement Synthesis and Boosted Sodium Storage. Molecules 2023, 28, 5972. https://doi.org/10.3390/molecules28165972
Zhang R, Dong Y, Su Y, Zhai W, Xu S. MoS2/SnS/CoS Heterostructures on Graphene: Lattice-Confinement Synthesis and Boosted Sodium Storage. Molecules. 2023; 28(16):5972. https://doi.org/10.3390/molecules28165972
Chicago/Turabian StyleZhang, Ruyao, Yan Dong, Yu Su, Wenkai Zhai, and Sailong Xu. 2023. "MoS2/SnS/CoS Heterostructures on Graphene: Lattice-Confinement Synthesis and Boosted Sodium Storage" Molecules 28, no. 16: 5972. https://doi.org/10.3390/molecules28165972