Dark–Light Tandem Catalytic Oxidation of Formaldehyde over SrBi2Ta2O9 Nanosheets
Abstract
:1. Introduction
2. Results and Discussion
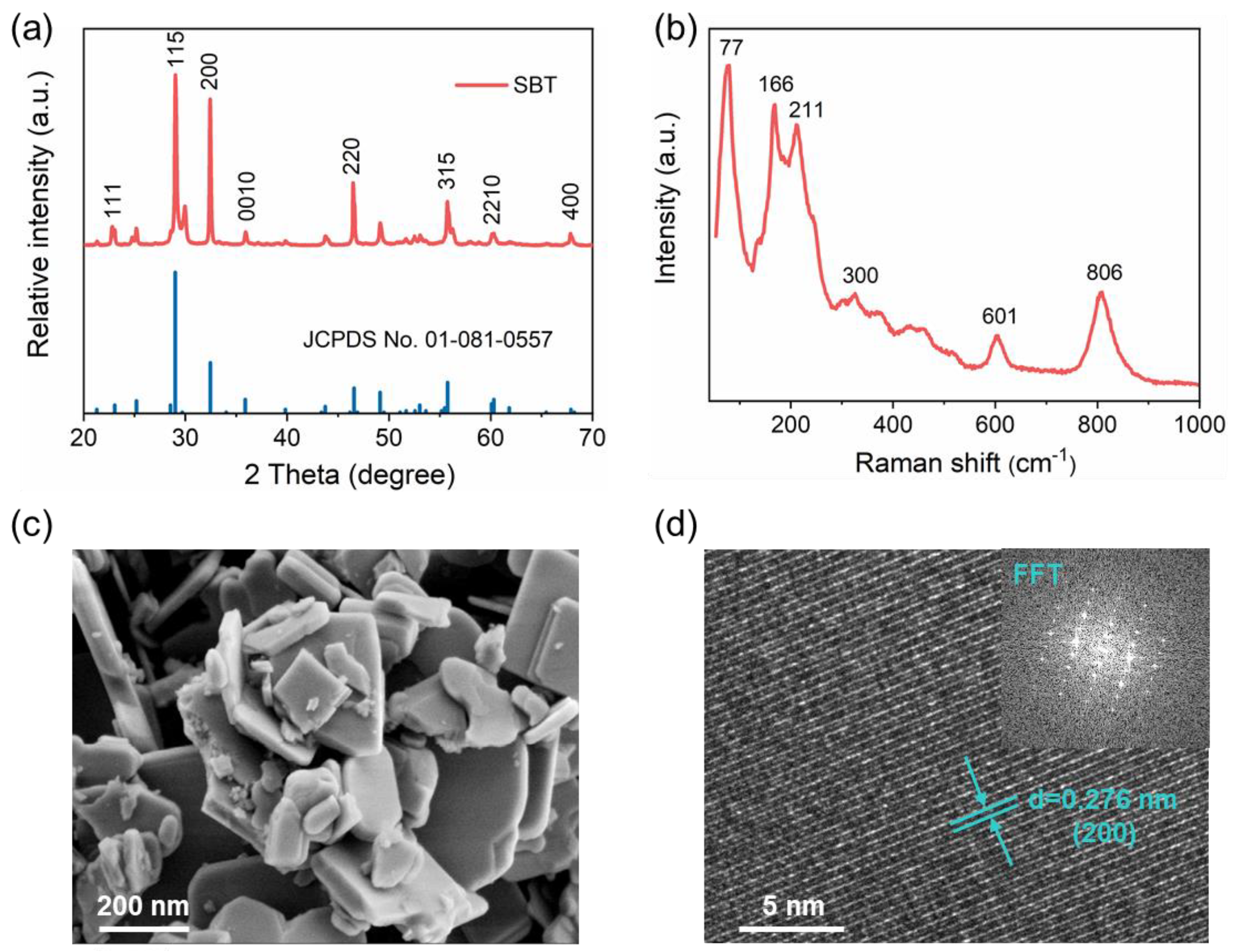
2.1. Catalyst Characterization
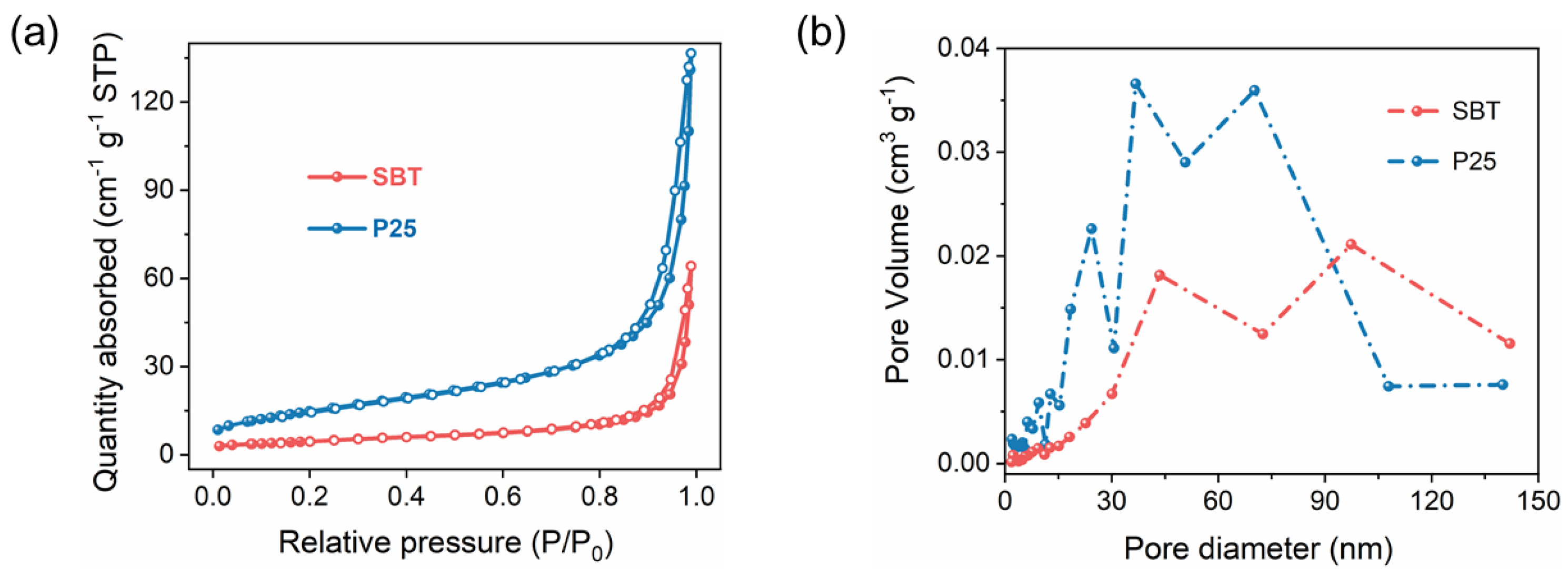
2.2. Brunauer–Emmett–Teller (BET) Analysis
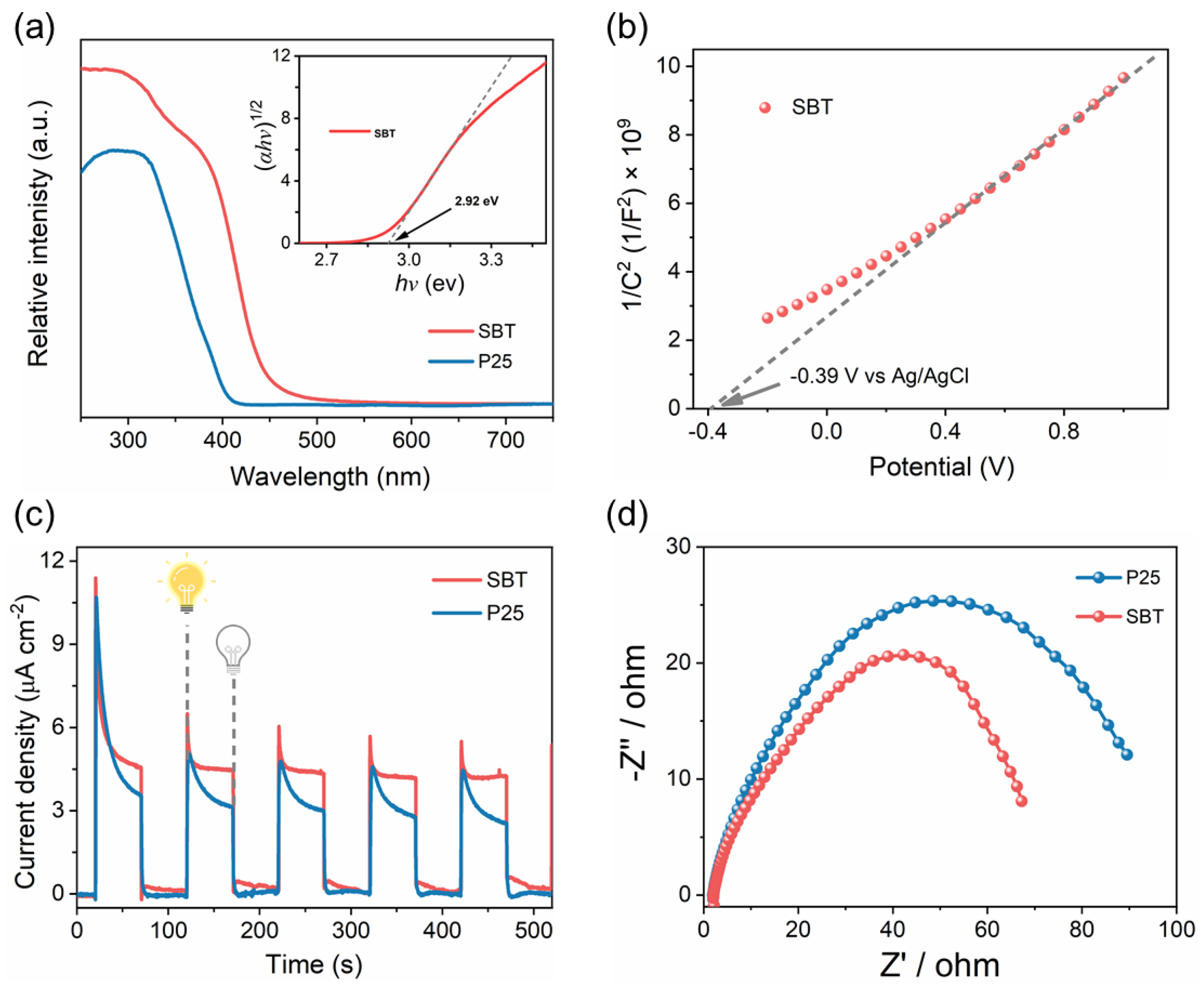
2.3. Optical Properties
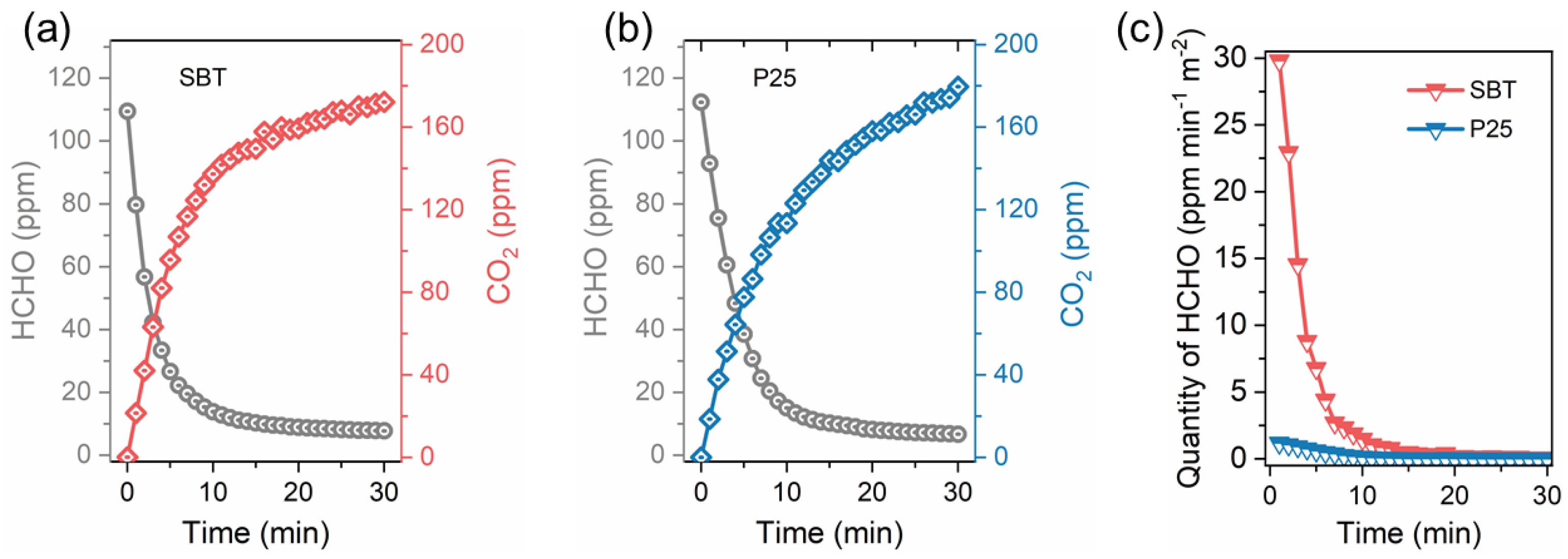
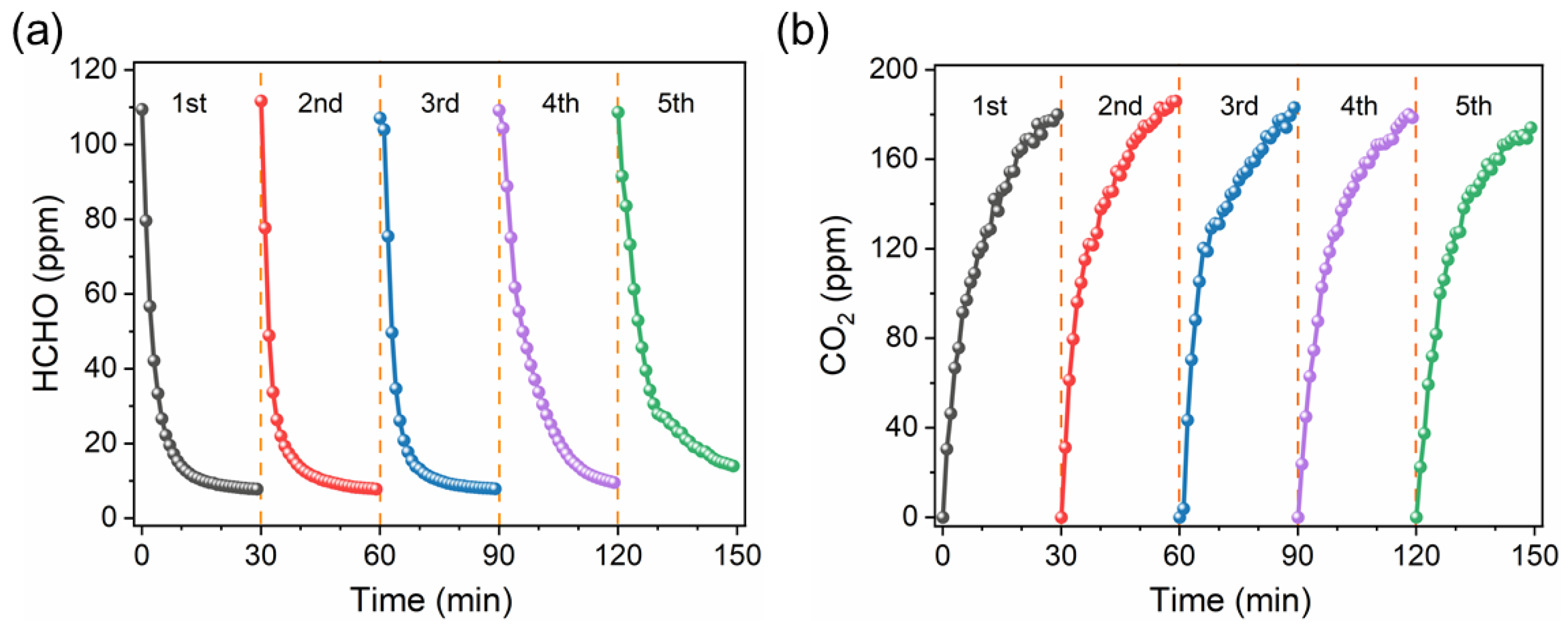
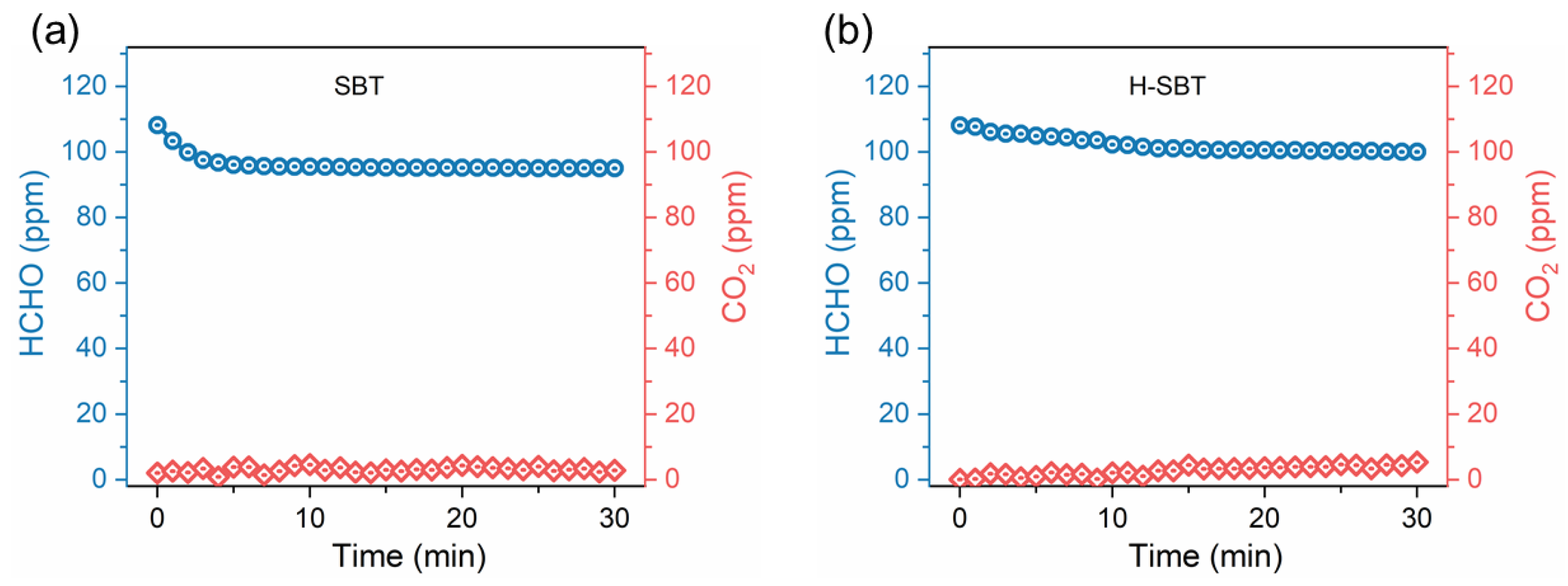
2.4. Photocatalytic Catalytic Performance
2.5. Reaction Mechanism
2.6. In Situ DRIFT Spectra
2.7. EPR Measurements
2.8. Tandem Catalytic Mechanism
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Photocatalytic Tests
3.4. Dark–Light Staged Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, D.; Zhang, G.; Wang, M.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt/MnO2 nanoflowers anchored to boron nitride aerogels for highly efficient enrichment and catalytic oxidation of formaldehyde at room temperature. Angew. Chem. Int. Ed. 2021, 60, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Jiang, X.; Lai, S.N.; Peroulis, D.; Stanciu, L. Nanohybrids of a mxene and transition metal dichalcogenide for selective detection of volatile organic compounds. Nat. Commun. 2020, 11, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Weon, S.; Jeon, W.; Chung, M.W.; Choi, W. Self-wetting triphase photocatalysis for effective and selective removal of hydrophilic volatile organic compounds in air. Nat. Commun. 2021, 12, 6259. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; Choi, E.; Kim, H.; Kim, J.Y.; Park, H.J.; Kim, S.M.; Kim, W.; Choi, W. Active 001 facet exposed TiO2 nanotubes photocatalyst filter for volatile organic compounds removal: From material development to commercial indoor air cleaner application. Environ. Sci. Technol. 2018, 52, 9330–9340. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Y.; Li, M.; Zhao, J.; Zhu, Y.; Wang, C.; Sharma, V.K. Active site-directed tandem catalysis on single platinum nanoparticles for efficient and stable oxidation of formaldehyde at room temperature. Environ. Sci. Technol. 2019, 53, 3610–3619. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xiao, Y.; Zhang, L.; Guo, L.; Zhang, L.; Dong, X. Free-standing composite films of multiple 2D nanosheets: Synergetic photothermocatalysis/photocatalysis for efficient removal of formaldehyde under ambient condition. Chem. Eng. J. 2020, 394, 125014–125025. [Google Scholar] [CrossRef]
- Bellat, J.P.; Bezverkhyy, I.; Weber, G.; Royer, S.; Averlant, R.; Giraudon, J.M.; Lamonier, J.F. Capture of formaldehyde by adsorption on nanoporous materials. J. Hazard. Mater. 2015, 300, 711–717. [Google Scholar] [CrossRef]
- Su, Y.; Li, W.; Li, G.; Ao, Z.; An, T. Density functional theory investigation of the enhanced adsorption mechanism and potential catalytic activity for formaldehyde degradation on Al-decorated C2N monolayer. Chin. J. Catal. 2019, 40, 664–672. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, X.; Cheng, B.; Yu, J.; Jiang, C. Few-layered graphene-like boron nitride: A highly efficient adsorbent for indoor formaldehyde removal. Environ. Sci. Technol. Lett. 2016, 4, 20–25. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Yu, L.; Peng, R.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Ag supported on CeO2 with different morphologies for the catalytic oxidation of HCHO. Chem. Eng. J. 2018, 334, 2480–2487. [Google Scholar] [CrossRef]
- Sun, T.; Li, C.; Bao, Y.; Fan, J.; Liu, E. S-Scheme MnCo2S4/g-C3N4 heterojunction photocatalyst for H2 production. Acta Phys.-Chim. Sin. 2023, 39, 2212009. [Google Scholar] [CrossRef]
- Wang, X.; Hisatomi, T.; Wang, Z.; Song, J.; Qu, J.; Takata, T.; Domen, K. Core-shell-structured LaTaON2 transformed from LaKNaTaO5 plates for enhanced photocatalytic H2 evolution. Angew. Chem. Int. Ed. 2019, 58, 10666–10670. [Google Scholar] [CrossRef]
- Naresh, G.; Mandal, T.K. Excellent sun-light-driven photocatalytic activity by aurivillius layered perovskites, Bi5-xLaxTi3FeO15 (x = 1, 2). ACS Appl. Mater. Interfaces 2014, 6, 21000–21010. [Google Scholar] [CrossRef]
- Naresh, G.; Malik, J.; Meena, V.; Mandal, T.K. pH-mediated collective and selective solar photocatalysis by a series of layered aurivillius perovskites. ACS Omega 2018, 3, 11104–11116. [Google Scholar] [CrossRef]
- Raciulete, M.; Papa, F.; Negrila, C.; Bratan, V.; Munteanu, C.; Pandele-Cusu, J.; Culita, D.C.; Atkinson, I.; Balint, I. Strategy for modifying layered perovskites toward efficient solar light-driven photocatalysts for removal of chlorinated pollutants. Catalysts 2020, 10, 637. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, M.; Le, Y.; Cheng, B.; Yu, J. Three-dimensional carbon foam supported MnO2/Pt for rapid capture and catalytic oxidation of formaldehyde at room temperature. Appl. Catal. B 2020, 267, 118689–118697. [Google Scholar] [CrossRef]
- Liu, W.; Shi, M.; Li, Y.; Wu, Z.; Yang, L.; Zhang, S.; Xiao, X.; Liu, C.; Dai, W.; Chen, C.; et al. Congregated-electrons-strengthened anchoring and mineralization of gaseous formaldehyde on a novel self-supporting Cu2−xSe/Cu2O heterojunction photocatalyst under visible lights: A viable mesh for designing air purifier. Appl. Catal. B 2022, 312, 121427–121440. [Google Scholar]
- Noguchi, T.; Fujishima, A.; Sawunyama, P.; Hashimoto, K. Photocatalytic degradation of gaseous formaldehyde using TiO2 film. Environ. Sci. Technol. 1998, 32, 3831. [Google Scholar] [CrossRef]
- Diao, W.; Cai, H.; Wang, L.; Rao, X.; Zhang, Y. Efficient photocatalytic degradation of gas-phase formaldehyde by Pt/TiO2 nanowires in a continuous flow reactor. ChemCatChem 2020, 12, 5420. [Google Scholar] [CrossRef]
- Kong, L.; Li, X.; Song, P.; Ma, F. Porous graphitic carbon nitride nanosheets for photocatalytic degradation of formaldehyde gas. Chem. Phys. Lett. 2021, 762, 138132. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Sun, M.; Hanif, A.; Wu, H.; Gu, Q.; Ok, Y.S.; Tsang, D.C.W.; Li, J.; Yu, J.; et al. Thermally treated zeolitic imidazolate framework-8 (ZIF-8) for visible light photocatalytic degradation of gaseous formaldehyde. Chem. Sci. 2020, 11, 6670–6681. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Ichibha, T.; Qin, K.S.; Muraoka, K.; Vequizo, J.J.M.; Hibino, K.; Kuriki, R.; Yamashita, S.; Hongo, K.; Uchiyama, T.; et al. Undoped layered perovskite oxynitride Li2LaTa2O6N for photocatalytic CO2 reduction with visible light. Angew. Chem. Int. Ed. 2018, 57, 8154–8158. [Google Scholar] [CrossRef] [Green Version]
- Hailili, R.; Wang, Z.-Q.; Ji, H.; Chen, C.; Gong, X.-Q.; Sheng, H.; Zhao, J. Mechanistic insights into the photocatalytic reduction of nitric oxide to nitrogen on oxygen-deficient quasi-two-dimensional bismuth-based perovskites. Environ. Sci. Nano 2022, 9, 1453–1465. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Q.; Liu, G.; Zhao, Q.; Lv, H.; Yuan, M.; Meng, Q.; Zhou, Y.; Xu, J.; Wang, C. Modulation of photocatalytic activity of SrBi2Ta2O9 nanosheets in NO removal by tuning facets exposure. J. Mater. Sci. Technol. 2022, 122, 91–100. [Google Scholar] [CrossRef]
- Zulhadjri; Wendari, T.P.; Ikhram, M.; Putri, Y.E.; Septiani, U.; Imelda. Enhanced dielectric and ferroelectric responses in La3+/Ti4+ co-substituted SrBi2Ta2O9 aurivillius phase. Ceram. Int. 2022, 48, 10328–10332. [Google Scholar] [CrossRef]
- Yamashita, H.; Yoshio, K.; Murata, W.; Onodera, A. Structural changes and ferroelectricity in Bi-layered SrBi2Ta2O9. J. Appl. Phys. 2002, 41(Part 1, No. 11B), 7076–7079. [Google Scholar] [CrossRef]
- Ching-Prado, E.; Pérez, W.; Reynés-Figueroa, A.; Katiyar, R.S.; Ravichandran, D.; Bhalla, A.S. Raman study of SrBi2Ta2O9 thin films. Ferroelectr. Lett. Sect. 2006, 25, 97–102. [Google Scholar] [CrossRef]
- Su, G.; Liu, L.; Zhang, L.; Liu, X.; Xue, J.; Tang, A. Fabrication of magnetic Fe3O4@SiO2@Bi2O2CO3/rGO composite for enhancing its photocatalytic performance for organic dyes and recyclability. Environ. Sci. Pollut. Res. 2021, 28, 50286–50301. [Google Scholar] [CrossRef]
- Huang, B.; Fu, X.; Wang, K.; Wang, L.; Zhang, H.; Liu, Z.; Liu, B.; Li, J. Chemically bonded BiVO4/Bi19Cl3S27 heterojunction with fast hole extraction dynamics for continuous CO2 photoreduction. Adv. Powder Mater. 2023, 100140, in press. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Wang, N.; Tian, F.; Li, J. Surface reconstruction of BiSI nanorods for superb photocatalytic Cr(VI) reduction under near-infrared light irradiation. Chem. Eng. J. 2022, 435, 135152–135162. [Google Scholar] [CrossRef]
- Li, J.; Pan, W.; Liu, Q.; Chen, Z.; Chen, Z.; Feng, X.; Chen, H. Interfacial engineering of Bi19Br3S27 nanowires promotes metallic photocatalytic CO2 reduction activity under near-infrared light irradiation. J. Am. Chem. Soc. 2021, 143, 6551–6559. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, S.; Wu, Y.; Huang, X.; Tian, F.; Liu, S.; Li, C. Enhancing the room-temperature catalytic degradation of formaldehyde through constructing surface lewis pairs on carbon-based catalyst. Appl. Catal. B 2020, 272, 118992–119000. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuo, Y.; Ezugwu, C.I.; Wang, C.C.; Li, C.; Liu, S. Synergetic molecular oxygen activation and catalytic oxidation of formaldehyde over defective MIL-88B(Fe) nanorods at room temperature. Environ. Sci. Technol. 2021, 55, 8341–8350. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Choy, J.-H. A novel hybrid of Bi-based high-Tc superconductor and molecular complex. Inorg. Chem. 2003, 42, 8134–8136. [Google Scholar] [CrossRef]
- Chen, X.; He, G.; Li, Y.; Chen, M.; Qin, X.; Zhang, C.; He, H. Identification of a facile pathway for dioxymethylene conversion to formate catalyzed by surface hydroxyl on TiO2-based catalyst. ACS Catal. 2020, 10, 9706–9715. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Chen, X.; Wang, F.; Qin, X.; Zhang, C.; He, H. High-performance of Cu-TiO2 for photocatalytic oxidation of formaldehyde under visible light and the mechanism study. Chem. Eng. J. 2020, 390, 124481–124489. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Jiang, C.; Zhou, P.; Zhang, P.; Yu, J. The effect of manganese vacancy in birnessite–type MnO2 on room-temperature oxidation of formaldehyde in air. Appl. Catal. B 2017, 204, 147–155. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Shen, J.; Bahi, A.; Zhang, S.; Wan, L.; Ko, F. The role of oxygen vacancies on Pt/NaInO2 catalyst in improving formaldehyde oxidation at ambient condition. Chem. Eng. J. 2020, 395, 125131. [Google Scholar] [CrossRef]
- Miao, L.; Xie, Y.; Xia, Y.; Zou, N.; Wang, J. Facile photo-driven strategy for the regeneration of a hierarchical C@MnO2 sponge for the removal of indoor toluene. Appl. Surf. Sci. 2019, 481, 404–413. [Google Scholar] [CrossRef]
- Abbas, K.; Babic, N.; Peyrot, F. Detection of superoxide radical in adherent living cells by electron paramagnetic resonance (EPR) spectroscopy using cyclic nitrones. Methods Mol. Biol. 2021, 2202, 149–163. [Google Scholar] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Liu, Q.; Lin, Y.; Li, Y. Dark–Light Tandem Catalytic Oxidation of Formaldehyde over SrBi2Ta2O9 Nanosheets. Molecules 2023, 28, 5691. https://doi.org/10.3390/molecules28155691
Ma W, Liu Q, Lin Y, Li Y. Dark–Light Tandem Catalytic Oxidation of Formaldehyde over SrBi2Ta2O9 Nanosheets. Molecules. 2023; 28(15):5691. https://doi.org/10.3390/molecules28155691
Chicago/Turabian StyleMa, Weimin, Qing Liu, Yuhan Lin, and Yingxuan Li. 2023. "Dark–Light Tandem Catalytic Oxidation of Formaldehyde over SrBi2Ta2O9 Nanosheets" Molecules 28, no. 15: 5691. https://doi.org/10.3390/molecules28155691




