Comparison of Gas–Particle Partitioning of Glyoxal and Methylglyoxal in the Summertime Atmosphere at the Foot and Top of Mount Hua
Abstract
:1. Introduction
2. Results and Discussion
2.1. Temporal Variations in Gly and mGly Concentrations
2.1.1. Temporal Variations in Gas-Phase Gly and mGly at the Top and Foot of Mount Hua
2.1.2. Temporal Variations in Particle-Phase Gly and mGly at the Top and Foot of Mount Hua
2.2. Gas–Particle Partitioning of Gly and mGly at the Top and Foot of Mount Hua
2.2.1. Measured and Theoretical Partitioning Coefficients
2.2.2. Gas–Particle Partitioning Ratios and Henry’s Law
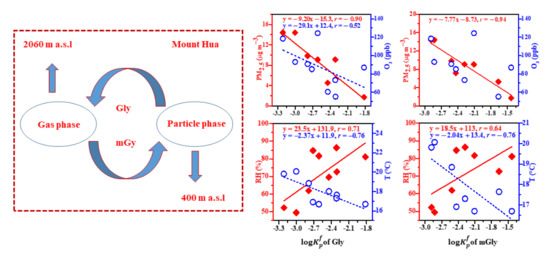
2.3. The Factors Influencing the Gas–Particle Partitioning Process
3. Materials and Methods
3.1. Field Sampling
3.2. Sample Extraction and Analysis
3.3. Estimation of the Partitioning Coefficients
3.4. Quality Assurance and Quality Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, H.Q.; Chen, Z.M.; Li, H.; Qian, X.; Qin, X.; Shi, W.X. Gas-Particle Partitioning of Carbonyl Compounds in the Ambient Atmosphere. Environ. Sci. Technol. 2018, 52, 10997–11006. [Google Scholar] [CrossRef]
- Cui, J.N.; Sun, M.; Wang, L.; Guo, J.Y.; Xie, G.Y.; Zhang, J.B.; Zhang, R.Q. Gas-particle partitioning of carbonyls and its influencing factors in the urban atmosphere of Zhengzhou, China. Sci. Total Environ. 2021, 751, 142027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Lee, A.K.; Abbatt, J.P. Investigation of aqueous-phase photooxidation of glyoxal and methylglyoxal by aerosol chemical ionization mass spectrometry: Observation of hydroxyhydroperoxide formation. J. Phys. Chem. A 2012, 116, 6253–6263. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.N.; Wiedinmyer, C.; Guenther, A.B.; Orlando, J.J.; Karl, T.; Toohey, D.W.; Greenberg, J.P.; Kajii, Y. Isoprene oxidation products are a significant atmospheric aerosol component. Atmos. Chem. Phys. Discuss. 2005, 5, 11143–11156. [Google Scholar]
- Knote, C.; Hodzic, A.; Jimenez, J.L.; Volkamer, R.; Orlando, J.J.; Baidar, S.; Brioude, J.; Fast, J.; Gentner, D.R.; Goldstein, A.H.; et al. Simulation of semi-explicit mechanisms of SOA formation from glyoxal in aerosol in a 3-D model. Atmos. Chem. Phys. 2014, 14, 6213–6239. [Google Scholar] [CrossRef] [Green Version]
- De Haan, D.O.; Hawkins, L.N.; Welsh, H.G.; Pednekar, R.; Casar, J.R.; Pennington, E.A.; de Loera, A.; Jimenez, N.G.; Symons, M.A.; Zauscher, M. Brown carbon production in ammonium-or amine-containing aerosol particles by reactive uptake of methylglyoxal and photolytic cloud cycling. Environ. Sci. Technol. 2017, 51, 7458–7466. [Google Scholar] [CrossRef]
- Lee, H.J.; Aiona, P.K.; Laskin, A.; Laskin, J.; Nizkorodov, S.A. Effect of Solar Radiation on the Optical Properties and Molecular Composition of Laboratory Proxies of Atmospheric Brown Carbon. Environ. Sci. Technol. 2014, 48, 10217–10226. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Ramanathan, V.; Kotamarthi, V.R. Brown carbon: A significant atmospheric absorber of solar radiation? Atmos. Chem. Phys. 2013, 13, 8607–8621. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Lee, A.K.Y.; Huang, L.; Li, X.; Yang, F.; Abbatt, J.P.D. Photochemical processing of aqueous atmospheric brown carbon. Atmos. Chem. Phys. 2015, 15, 6087–6100. [Google Scholar] [CrossRef] [Green Version]
- Pennington, E.A.; Seltzer, K.M.; Murphy, B.N.; Qin, M.; Seinfeld, J.H.; Pye, H.O.T. Modeling secondary organic aerosol formation from volatile chemical products. Atmos. Chem. Phys. 2021, 21, 18247–18261. [Google Scholar] [CrossRef]
- Lerot, C.; Müller, J.F.; Theys, N.; De Smedt, I.; Stavrakou, T.; Van Roozendael, M. Satellite evidence for glyoxal depletion in elevated fire plumes. Geophys. Res. Lett. 2023, 50, e2022GL102195. [Google Scholar] [CrossRef]
- Jang, M.; Czoschke, N.M.; Lee, S.; Kamens, R.M. Heterogeneous atmospheric aerosol production by acid-catalyzed particle-phase reactions. Science 2002, 298, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.L.; Szprengiel, J.; Sareen, N.; Jen, C.N.; Giordano, M.R.; McNeill, V.F. Light-absorbing secondary organic material formed by glyoxal in aqueous aerosol mimics. Atmos. Chem. Phys. 2009, 9, 2289–2300. [Google Scholar] [CrossRef] [Green Version]
- Carlton, A.G.; Turpin, B.J.; Altieri, K.E.; Seitzinger, S.; Reff, A.; Lim, H.-J.; Ervens, B. Atmospheric oxalic acid and SOA production from glyoxal: Results of aqueous photooxidation experiments. Atmos. Environ. 2007, 41, 7588–7602. [Google Scholar] [CrossRef]
- Hu, J.L.; Wang, P.; Ying, Q.; Zhang, H.L.; Chen, J.J.; Ge, X.L.; Li, X.H.; Jiang, J.K.; Wang, S.X.; Zhang, J.; et al. Modeling biogenic and anthropogenic secondary organic aerosol in China. Atmos. Chem. Phys. 2017, 17, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Mao, J.Q.; Min, K.E.; Washenfelder, R.A.; Brown, S.S.; Kaiser, J.; Keutsch, F.N.; Volkamer, R.; Wolfe, G.M.; Hanisco, T.F.; et al. Observational constraints on glyoxal production from isoprene oxidation and its contribution to organic aerosol over the Southeast United States. J. Geophys. Res. 2016, 121, 9849–9861. [Google Scholar] [CrossRef] [Green Version]
- Galloway, M.M.; Loza, C.L.; Chhabra, P.S.; Chan, A.W.H.; Yee, L.D.; Seinfeld, J.H.; Keutsch, F.N. Analysis of photochemical and dark glyoxal uptake: Implications for SOA formation. Geophys. Res. Lett. 2011, 38, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.C.; Chen, Z.M.; Qin, X.; Dong, P. Reversible and irreversible gas–particle partitioning of dicarbonyl compounds observed in the real atmosphere. Atmos. Chem. Phys. 2022, 22, 6971–6987. [Google Scholar] [CrossRef]
- Xie, M.J.; Hannigan, M.P.; Barsanti, K.C. Gas/particle partitioning of 2-methyltetrols and levoglucosan at an urban site in Denver. Environ. Sci. Technol. 2014, 48, 2835–2842. [Google Scholar] [CrossRef]
- Wang, G.H.; Kawamura, K.; Cheng, C.L.; Li, J.J.; Cao, J.J.; Zhang, R.J.; Zhang, T.; Liu, S.X.; Zhao, Z.Z. Molecular distribution and stable carbon isotopic composition of dicarboxylic acids, ketocarboxylic acids, and alpha-dicarbonyls in size-resolved atmospheric particles from Xi’an City, China. Environ. Sci. Technol. 2012, 46, 4783–4791. [Google Scholar] [CrossRef]
- Lv, S.J.; Wang, F.L.; Wu, C.; Chen, Y.B.; Liu, S.J.; Zhang, S.; Li, D.P.; Du, W.; Zhang, F.; Wang, H.; et al. Gas-to-Aerosol Phase Partitioning of Atmospheric Water-Soluble Organic Compounds at a Rural Site in China: An Enhancing Effect of NH3 on SOA Formation. Environ. Sci. Technol. 2022, 56, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, K.; Iwasaki, M.; Takeuchi, M.; Okochi, H.; Kato, S.; Ohira, S.-I.; Toda, K. Diurnal Variations in Partitioning of Atmospheric Glyoxal and Methylglyoxal between Gas and Particles at the Ground Level and in the Free Troposphere. ACS Earth Space Chem. 2018, 2, 915–924. [Google Scholar] [CrossRef]
- Cheng, C.L.; Wang, G.H.; Meng, J.J.; Wang, Q.Y.; Cao, J.; Li, J.J.; Wang, J.Y. Size-resolved airborne particulate oxalic and related secondary organic aerosol species in the urban atmosphere of Chengdu, China. Atmos. Res. 2015, 161–162, 134–142. [Google Scholar] [CrossRef]
- Shen, M.X.; Qi, W.N.; Guo, X.; Dai, W.T.; Wang, Q.Y.; Liu, Y.L.; Fan, Z.Y.; Cao, Y.; Chen, Y.K.; Lu, L.; et al. Influence of vertical transport on chemical evolution of dicarboxylic acids and related secondary organic aerosol from surface emission to the top of Mount Hua, Northwest China. Sci. Total Environ. 2022, 858 Pt 2, 159892. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Xue, L.K.; Dong, C.; Wang, T.; Mellouki, A.; Zhang, Q.Z.; Wang, W.X. Gaseous carbonyls in China’s atmosphere: Tempo-spatial distributions, sources, photochemical formation, and impact on air quality. Atmos. Environ. 2019, 214, 116863. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Dan, L.D.; Ge, M.Y.; Dubois, C.; Ke, W.X.; Perrier, S.; Hui, C.; Li, W.H.; Sheng, J.; Qun, L.Y.; et al. Field Detection of Highly Oxygenated Organic Molecules in Shanghai by Chemical Ionization-Orbitrap. Environ. Sci. Technol. 2022, 56, 7608–7617. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.H.; Yang, L.X.; Chen, J.M.; Kawamura, K.; Sato, M.; Tilgner, A.; van Pinxteren, D.; Chen, Y.; Xue, L.K.; Wang, X.F.; et al. Molecular distributions of dicarboxylic acids, oxocarboxylic acids and α-dicarbonyls in PM2.5 collected at the top of Mt. Tai, North China, during the wheat burning season of 2014. Atmos. Chem. Phys. 2018, 18, 10741–10758. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.J.; Wang, G.H.; Hou, Z.F.; Liu, X.D.; Wei, B.J.; Wu, C.; Cao, C.; Wang, J.Y.; Li, J.J.; Cao, J.J.; et al. Molecular distribution and stable carbon isotopic compositions of dicarboxylic acids and related SOA from biogenic sources in the summertime atmosphere of Mt. Tai in the North China Plain. Atmos. Chem. Phys. 2018, 18, 15069–15086. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.J.; Li, X.; Dong, H.B.; Wu, Z.J.; Chen, S.Y.; Fang, X.; Gao, J.; Guo, S.; Hu, M.; Li, D.Q.; et al. Measurement of gaseous and particulate formaldehyde in the Yangtze River Delta, China. Atmos. Environ. 2020, 224, 117114. [Google Scholar] [CrossRef]
- Healy, R.M.; Wenger, J.C.; Metzger, A.; Duplissy, J.; Kalberer, M.; Dommen, J. Gas/particle partitioning of carbonyls in the photooxidation of isoprene and 1,3,5-trimethylbenzene. Atmos. Chem. Phys. 2008, 8, 3215–3230. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, R.; Shimada, S.; Sekiguchi, K.; Wang, Q.; Sakamoto, K. Measurements of changes in the atmospheric partitioning of bifunctional carbonyls near a road in a suburban area. Atmos. Environ. 2013, 81, 554–560. [Google Scholar] [CrossRef]
- Suciu, L.G.; Masiello, C.A.; Griffin, R.J. Anhydrosugars as tracers in the Earth system. Biogeochemistry 2019, 146, 209–256. [Google Scholar] [CrossRef]
- Xie, M.J.; Hannigan, M.P.; Barsanti, K.C. Gas/particle partitioning of n-alkanes, PAHs and oxygenated PAHs in urban Denver. Atmos. Environ. 2014, 95, 355–362. [Google Scholar] [CrossRef]
- Tang, I.N.; Munkelwitz, H.R. Water activities, densities, and refractive indices of aqueous sulfates and sodium nitrate droplets of atmospheric importance. J. Geophys. Res. 1994, 99, 18801–18808. [Google Scholar] [CrossRef]
- Ervens, B.; Volkamer, R. Glyoxal processing by aerosol multiphase chemistry: Towards a kinetic modeling framework of secondary organic aerosol formation in aqueous particles. Atmos. Chem. Phys. 2010, 10, 8219–8244. [Google Scholar] [CrossRef] [Green Version]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Curry, L.A.; Tsui, W.G.; McNeill, V.F. Technical note: Updated parameterization of the reactive uptake of glyoxal and methylglyoxal by atmospheric aerosols and cloud droplets. Atmos. Chem. Phys. 2018, 18, 9823–9830. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.L.; Kreisberg, N.M.; Worton, D.R.; Isaacman, G.; Weber, R.J.; Liu, S.; Day, D.A.; Russell, L.M.; Markovic, M.Z.; VandenBoer, T.C.; et al. Insights into secondary organic aerosol formation mechanisms from measured gas/particle partitioning of specific organic tracer compounds. Environ. Sci. Technol. 2013, 47, 3781–3787. [Google Scholar] [CrossRef]
- Shi, Q.J.; Zhang, W.N.; Ji, Y.M.; Wang, J.X.; Qin, D.D.; Chen, J.; Gao, Y.P.; Li, G.Y.; An, T.C. Enhanced uptake of glyoxal at the acidic nanoparticle interface: Implications for secondary organic aerosol formation. Environ. Sci. Nano 2020, 7, 1126–1135. [Google Scholar] [CrossRef]
- Kampf, C.J.; Waxman, E.M.; Slowik, J.G.; Dommen, J.; Pfaffenberger, L.; Praplan, A.P.; Prévôt, A.S.H.; Baltensperger, U.; Hoffmann, T.; Volkamer, R. Effective Henry’s Law Partitioning and the Salting Constant of Glyoxal in Aerosols Containing Sulfate. Environ. Sci. Technol. 2013, 47, 4236–4244. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Kamens, R.M. Atmospheric secondary aerosol formation by heterogeneous reactions of aldehydes in the presence of a sulfuric acid aerosol catalyst. Environ. Sci. Technol. 2001, 35, 4758–4766. [Google Scholar] [CrossRef] [PubMed]
- Sorooshian, A.; Varutbangkul, V.; Brechtel, F.J.; Ervens, B.; Feingold, G.; Bahreini, R.; Murphy, S.M.; Holloway, J.S.; Atlas, E.L.; Buzorius, G.; et al. Oxalic acid in clear and cloudy atmospheres: Analysis of data from International Consortium for Atmospheric Research on Transport and Transformation 2004. J. Geophys. Res. 2006, 111, D23S45. [Google Scholar] [CrossRef]
- Shen, C.Y.; Zhang, W.; Choczynski, J.; Davies, J.F.; Zhang, H.F. Phase State and Relative Humidity Regulate the Heterogeneous Oxidation Kinetics and Pathways of Organic-Inorganic Mixed Aerosols. Environ. Sci. Technol. 2022, 56, 15398–15407. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.A.; Hansen, A.S.; Møller, K.H.; Axson, J.L.; Kjaergaard, H.G.; Vaida, V. Ultraviolet Spectroscopy of the Gas Phase Hydration of Methylglyoxal. ACS Earth Space Chem. 2017, 1, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Starr, D.E.; Campbell, C.T. Low-Temperature Adsorption Microcalorimetry: Pb on MgO(100) †. J. Phys. Chem. B 2001, 105, 3776–3782. [Google Scholar] [CrossRef]
- Peckhaus, A.; Grass, S.; Treuel, L.; Zellner, R. Deliquescence and efflorescence behavior of ternary inorganic/organic/water aerosol particles. J. Chem. Phys. 2012, 116, 6199–6210. [Google Scholar] [CrossRef]
- Svenningsson, B.; Rissler, J.; Swietlicki, E.; Mircea, M.; Bilde, M.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Zhou, J.; Mønster, J.; et al. Hygroscopic growth and critical supersaturations for mixed aerosol particles of inorganic and organic compounds of atmospheric relevance. Atmos. Chem. Phys. 2006, 6, 1937–1952. [Google Scholar] [CrossRef] [Green Version]
- Bertram, A.K.; Martin, S.T.; Hanna, S.J.; Smith, M.L.; Bodsworth, A.; Chen, Q.; Kuwata, M.; Liu, A.; You, Y.; Zorn, S.R. Predicting the relative humidities of liquid-liquid phase separation, efflorescence, and deliquescence of mixed particles of ammonium sulfate, organic material, and water using the organic-to-sulfate mass ratio of the particle and the oxygen-to-carbon elemental ratio of the organic component. Atmos. Chem. Phys. 2011, 11, 10995–11006. [Google Scholar]
- Veghte, D.P.; Altaf, M.B.; Freedman, M.A. Size dependence of the structure of organic aerosol. J. Am. Chem. Soc. 2013, 135, 16046–16049. [Google Scholar] [CrossRef]
- Monforte, P.; Ragusa, M.A. Temperature Trend Analysis and Investigation on a Case of Variability Climate. Mathematics 2022, 10, 2202. [Google Scholar] [CrossRef]
- Williams, B.J.; Goldstein, A.H.; Kreisberg, N.M.; Hering, S.V. In situ measurements of gas/particle-phase transitions for atmospheric semivolatile organic compounds. Proc. Natl. Acad. Sci. USA 2010, 107, 6676–6681. [Google Scholar] [CrossRef] [PubMed]




| Season | Gas Phase (ppbv) | Particle Phase (ng m−3) | References | ||
|---|---|---|---|---|---|
| Gly | mGly | Gly | mGly | ||
| Spring | 0.02 ± 0.02 | 0.12 ± 0.08 | 15.24 ± 17.50 | 6.07 ± 2.79 | [18] |
| Summer | 0.13 ± 0.07 | 0.87 ± 0.54 | 10.18 ± 6.63 | 9.50 ± 5.62 | |
| Autumn | 0.07 ± 0.03 | 0.15 ± 0.09 | 9.33 ± 4.24 | 9.15 ± 3.62 | |
| Winter | 0.06 ± 0.05 | 0.11 ± 0.09 | 28.77 ± 25.33 | 14.61 ± 10.15 | |
| Autumn | 0.10 | 0.31 | 12.01 | 8.27 | [1] |
| Spring | 0.17 ± 0.10 | 0.32 ± 0.14 | 61.46 ± 16.26 | 64.33 ± 6.78 | [2] |
| Summer | 0.30 ± 0.13 | 0.90 ± 0.35 | 21.33 ± 11.81 | 17.10 ± 9.28 | |
| Autumn | 0.18 ± 0.05 | 0.59 ± 0.15 | 30.88 ± 16.72 | 25.42 ± 13.53 | |
| Winter | 0.11 ± 0.04 | 0.37 ± 0.15 | 61.46 ± 32.30 | 64.33 ± 33.18 | |
| Season | eff.KH (M atm−1) | References | |||
|---|---|---|---|---|---|
| Gly | mGly | Gly | mGly | ||
| Spring | 1.43 × 10−2 | 1.06 × 10−3 | 2.53 × 109 | 1.33 × 108 | [18] |
| Summer | 8.11 × 10−4 | 1.49 × 10−4 | 1.96 × 108 | 4.92 × 107 | |
| Autumn | 2.14 × 10−3 | 9.55 × 10−4 | 5.08 × 108 | 8.52 × 107 | |
| Winter | 1.30 × 10−2 | 2.60 × 10−3 | 1.04 × 109 | 2.63 × 108 | |
| Autumn | 1.44 × 10−3 | 4.19 × 10−4 | 1.66 × 109 | 5.16 × 108 | [1] |
| Spring | 1.33 × 10−3 | 3.48 × 10−4 | 2.20 × 109 | 6.88 × 108 | [2] |
| Summer | 6.31 × 10−4 | 1.40 × 10−4 | 2.85 × 109 | 4.81 × 108 | |
| Autumn | 1.05 × 10−3 | 2.07 × 10−4 | 2.53 × 1010 | 4.95 × 109 | |
| Winter | 1.71 × 10−3 | 4.27 × 10−4 | 1.07 × 109 | 2.44 × 108 | |
| Position | Compound | Average | ||
|---|---|---|---|---|
| Top | Gly | 4.02 × 10−3 | 5.32 × 10−10 | 7.55 × 106 |
| mGly | 1.01 × 10−2 | 1.12 × 10−9 | 9.08 × 106 | |
| Foot | Gly | 3.59 × 10−3 | 3.83 × 10−10 | 9.40 × 106 |
| mGly | 3.43 × 10−3 | 8.08 × 10−10 | 4.24 × 106 | |
| Total | Gly | 3.79 × 10−3 | 4.57 × 10−10 | 8.30 × 106 |
| mGly | 6.79 × 10−3 | 9.63 × 10−10 | 7.05 × 106 |
| Top | Foot | |||
|---|---|---|---|---|
| Gly | mGly | Gly | mGly | |
| P/G ratios | ||||
| Daytime | 0.016 ± 0.0062 | 0.0460 ± 0.0460 | 0.0468 ± 0.0333 | 0.0857 ± 0.0430 |
| Nighttime | 0.0266 ± 0.0128 | 0.0525 ± 0.0199 | 0.0961 ± 0.0747 | 0.0622 ± 0.0194 |
| Average | 0.0188 ± 0.0119 | 0.0439 ± 0.0350 | 0.0665 ± 0.0559 | 0.0740 ± 0.0333 |
| N/D ratio | 0.799 ± 0.578 | 1.333 ± 1.519 | 1.583 ± 0.951 | 8.022 ± 7.968 |
| Effective Henry’s law constant (mol/kgH2O/atm) | ||||
| Daytime | 4.45 × 108 | 9.81 × 108 | 2.21 × 108 | 9.67 × 108 |
| Nighttime | 2.88 × 109 | 6.82 × 109 | 2.91 × 108 | 3.10 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, W.; Zhang, Y.; Shen, M.; Li, L.; Dai, W.; Chen, Y.; Liu, Y.; Guo, X.; Cao, Y.; Wang, X.; et al. Comparison of Gas–Particle Partitioning of Glyoxal and Methylglyoxal in the Summertime Atmosphere at the Foot and Top of Mount Hua. Molecules 2023, 28, 5276. https://doi.org/10.3390/molecules28135276
Qi W, Zhang Y, Shen M, Li L, Dai W, Chen Y, Liu Y, Guo X, Cao Y, Wang X, et al. Comparison of Gas–Particle Partitioning of Glyoxal and Methylglyoxal in the Summertime Atmosphere at the Foot and Top of Mount Hua. Molecules. 2023; 28(13):5276. https://doi.org/10.3390/molecules28135276
Chicago/Turabian StyleQi, Weining, Yifan Zhang, Minxia Shen, Lu Li, Wenting Dai, Yukun Chen, Yali Liu, Xiao Guo, Yue Cao, Xin Wang, and et al. 2023. "Comparison of Gas–Particle Partitioning of Glyoxal and Methylglyoxal in the Summertime Atmosphere at the Foot and Top of Mount Hua" Molecules 28, no. 13: 5276. https://doi.org/10.3390/molecules28135276