Tannins Can Have Direct Interactions with Anthelmintics: Investigations by Isothermal Titration Calorimetry
Abstract
:1. Introduction
2. Results and Discussion
2.1. ITC Method Development for HT-Anthelmintic Interactions
2.2. Interactions between Tannin Monomers and Thiabendazole
2.3. Interactions of Hydrolysable Tannin Dimers and a Trimer to Thiabendazole
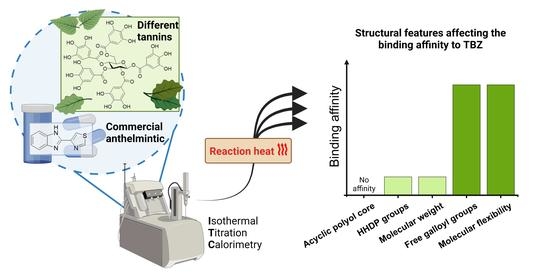
2.4. Structural Features and Bioactivities of HTs Associating with Their Interactions with TBZ
3. Materials and Methods
3.1. Reagents
3.2. Collection of Plant Material, Extraction, and Purification
3.3. UHPLC-DAD-ESI-HRMS and UHPLC-DAD-ESI-MS/MS Analyses
3.4. Isothermal Titration Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized Metabolites: Physiological and Biochemical Role in Stress Resistance, Strategies to Improve Their Accumulation, and New Applications in Crop Breeding and Management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.-P.; Karonen, M. Chemical Ecology of Tannins and Other Phenolics: We Need a Change in Approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Gontijo, D.C.; Gontijo, P.C.; Brandão, G.C.; Diaz, M.A.N.; de Oliveira, A.B.; Fietto, L.G.; Leite, J.P.V. Antioxidant Study Indicative of Antibacterial and Antimutagenic Activities of an Ellagitannin-Rich Aqueous Extract from the Leaves of Miconia latecrenata. J. Ethnopharmacol. 2019, 236, 114–123. [Google Scholar] [CrossRef]
- Moilanen, J.; Salminen, J.P. Ecologically Neglected Tannins and Their Biologically Relevant Activity: Chemical Structures of Plant Ellagitannins Reveal Their In Vitro Oxidative Activity at High pH. Chemoecology 2008, 18, 73–83. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jones, C.P.; Hagerman, A.E.; Karonen, M.; Salminen, J.P. Ellagitannins Have Greater Oxidative Activities than Condensed Tannins and Galloyl Glucoses at High pH: Potential Impact on Caterpillars. J. Chem. Ecol. 2006, 32, 2253–2267. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. Biomed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, V.; Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. NMR Metabolomics and DNA Sequencing of Escherichia coli and Staphylococcus aureus Cultures Treated with Hydrolyzable Tannins. Metabolites 2023, 13, 320. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.P.; Hoste, H. Ellagitannins Inhibit the Exsheathment of Haemonchus contortus and Trichostrongylus colubriformis Larvae: The Efficiency Increases Together with the Molecular Size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Direct Anthelmintic Effects of Condensed Tannins towards Different Gastrointestinal Nematodes of Sheep: In Vitro and in Vivo Studies. Vet. Parasitol. 2001, 99, 205–219. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.J.; Quijada, J.; Chan-Perez, I.; Dakheel, M.M.; Kommuru, D.S.; Mueller-Harvey, I.; Terrill, T.H. Interactions between Nutrition and Infections with Haemonchus contortus and Related Gastrointestinal Nematodes in Small Ruminants. Adv. Parasitol. 2016, 93, 239–351. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P. Tannins for Suppression of Internal Parasites. J. Anim. Sci. 2003, 81, E102–E109. [Google Scholar] [CrossRef]
- Engström, M.T.; Karonen, M.; Ahern, J.R.; Baert, N.; Payré, B.; Hoste, H.; Salminen, J.-P. Chemical Structures of Plant Hydrolyzable Tannins Reveal Their in Vitro Activity against Egg Hatching and Motility of Haemonchus contortus Nematodes. J. Agric. Food Chem. 2016, 64, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Concentration, and Diet Composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef] [Green Version]
- Baert, N.; Pellikaan, W.F.; Karonen, M.; Salminen, J.-P. A Study of the Structure-Activity Relationship of Oligomeric Ellagitannins on Ruminal Fermentation In Vitro. J. Dairy Sci. 2016, 99, 8041–8052. [Google Scholar] [CrossRef] [Green Version]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of Condensed Tannin Extract from Quebracho Trees to Reduce Methane Emissions from Cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, L.H.; Carvalho-Costa, F.A. Status of Benzimidazole Resistance in Intestinal Nematode Populations of Livestock in Brazil: A Systematic Review. BMC Vet. Res. 2017, 13, 358. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Valladares, M.; Geurden, T.; Bartram, D.J.; Martínez-Pérez, J.M.; Robles-Pérez, D.; Bohórquez, A.; Florez, E.; Meana, A.; Rojo-Vázquez, F.A. Resistance of Gastrointestinal Nematodes to the Most Commonly Used Anthelmintics in Sheep, Cattle and Horses in Spain. Vet. Parasitol. 2015, 211, 228–233. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Leathwick, D.M. Anthelmintic Resistance in Nematode Parasites of Cattle: A Global Issue? Trends Parasitol. 2011, 27, 176–181. [Google Scholar] [CrossRef]
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing Helminths and Their Economic Impact on Farmed Ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef]
- Learmount, J.; Stephens, N.; Boughtflower, V.; Barrecheguren, A.; Rickell, K. The Development of Anthelmintic Resistance with Best Practice Control of Nematodes on Commercial Sheep Farms in the UK. Vet. Parasitol. 2016, 229, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sangster, N.C.; Cowling, A.; Woodgate, R.G. Ten Events That Defined Anthelmintic Resistance Research. Trends Parasitol. 2018, 34, 553–563. [Google Scholar] [CrossRef]
- Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A. Direct and Indirect Effects of Bioactive Tannin-Rich Tropical and Temperate Legumes against Nematode Infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; Lifschitz, A.L.; Macedo, S.R.D.; Campos, N.R.C.L.; Viana-Filho, M.; Alcântara, A.C.S.; Araújo, J.G.; Alencar, L.M.R.; Costa-Junior, L.M. Combination of Synthetic Anthelmintics and Monoterpenes: Assessment of Efficacy, and Ultrastructural and Biophysical Properties of Haemonchus contortus Using Atomic Force Microscopy. Vet. Parasitol. 2021, 290, 109345. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.V.A.; Fryganas, C.; Acevedo, N.; Caraballo, L.; Thamsborg, S.M.; Mueller-Harvey, I.; Williams, A.R. Proanthocyanidins Inhibit Ascaris suum Glutathione-S-Transferase Activity and Increase Susceptibility of Larvae to Levamisole in Vitro. Parasitol. Int. 2016, 65, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.V.; Silva, C.R.; Viviani, P.; Luque, S.; Lloberas, M.; Costa-Júnior, L.M.; Lanusse, C.; Virkel, G.; Lifschitz, A. Combination of Bioactive Phytochemicals and Synthetic Anthelmintics: In Vivo and in Vitro Assessment of the Albendazole-Thymol Association. Vet. Parasitol. 2020, 281, 109121. [Google Scholar] [CrossRef]
- Whitney, T.R.; Wildeus, S.; Zajac, A.M. The Use of Redberry Juniper (Juniperus pinchotii) to Reduce Haemonchus contortus Fecal Egg Counts and Increase Ivermectin Efficacy. Vet. Parasitol. 2013, 197, 182–188. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Klein, D.R.; Whitney, T.R.; Scott, C.B.; Muir, J.P.; Lambert, B.D.; Craig, T.M. Effect of Using Redberry Juniper (Juniperus pinchotii) to Reduce Haemonchus contortus In Vitro Motility and Increase Ivermectin Efficacy. Vet. Parasitol. 2013, 197, 271–276. [Google Scholar] [CrossRef]
- Dupuy, J.; Larrieu, G.; Sutra, J.F.; Lespine, A.; Alvinerie, M. Enhancement of Moxidectin Bioavailability in Lamb by a Natural Flavonoid: Quercetin. Vet. Parasitol. 2003, 112, 337–347. [Google Scholar] [CrossRef]
- Gaudin, E.; Simon, M.; Quijada, J.; Schelcher, F.; Sutra, J.F.; Lespine, A.; Hoste, H. Efficacy of Sainfoin (Onobrychis viciifolia) Pellets against Multi Resistant Haemonchus contortus and Interaction with Oral Ivermectin: Implications for on-Farm Control. Vet. Parasitol. 2016, 227, 122–129. [Google Scholar] [CrossRef]
- Heckler, R.P.; Almeida, G.D.; Santos, L.B.; Borges, D.G.L.; Neves, J.P.L.; Onizuka, M.K.V.; Borges, F.A. P-Gp Modulating Drugs Greatly Potentiate the in Vitro Effect of Ivermectin against Resistant Larvae of Haemonchus Placei. Vet. Parasitol. 2014, 205, 638–645. [Google Scholar] [CrossRef]
- Miró, M.V.; Luque, S.; Cardozo, P.; Lloberas, M.; Sousa, D.M.; Soares, A.M.S.; Costa-Junior, L.M.; Virkel, G.L.; Lifschitz, A.L. Plant-Derived Compounds as a Tool for the Control of Gastrointestinal Nematodes: Modulation of Abamectin Pharmacological Action by Carvone. Front. Vet. Sci. 2020, 7, 601750. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, M.A.; Frazier, R.A.; Mueller-Harvey, I.; Clifton, L.A.; Gea, A.; Green, R.J. Binding of Pentagalloyl Glucose to Two Globular Proteins Occurs via Multiple Surface Sites. Biomacromolecules 2011, 12, 710–715. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Falconer, R.J.; Kennedy, J.A. Thermodynamics of Grape and Wine Tannin Interaction with Polyproline: Implications for Red Wine Astringency. J. Agric. Food Chem. 2010, 58, 12510–12518. [Google Scholar] [CrossRef]
- Frazier, R.A.; Papadopoulou, A.; Mueller-Harvey, I.; Kissoon, D.; Green, R.J. Probing Protein-Tannin Interactions by Isothermal Titration Microcalorimetry. J. Agric. Food Chem. 2003, 51, 5189–5195. [Google Scholar] [CrossRef]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Binding of an Oligomeric Ellagitannin Series to Bovine Serum Albumin (BSA): Analysis by Isothermal Titration Calorimetry (ITC). J. Agric. Food Chem. 2015, 63, 9. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, V.; Green, R.J.; Karonen, M. Interactions between Hydrolysable Tannins and Lipid Vesicles from Escherichia coli with Isothermal Titration Calorimetry. Molecules 2022, 27, 3204. [Google Scholar] [CrossRef]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of Tea Tannins and Condensed Tannins with Proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef]
- Aki, H.; Okamoto, Y.; Kimura, T. Compatibility and Stability Tests of Risperidone with Soft-Drinks by Isothermal Titration Microcalorimetry. J. Therm. Anal. Calorim. 2006, 85, 681–684. [Google Scholar] [CrossRef]
- Best Practices for Isothermal Titration Calorimetry to Study Binding Interactions—Part 1. Available online: https://www.materials-talks.com/best-practices-for-isothermal-titration-calorimetry-to-study-binding-interactions-part-1/ (accessed on 24 May 2023).
- Normal Rectal Temperature Ranges. Available online: https://www.msdvetmanual.com/multimedia/table/normal-rectal-temperature-ranges (accessed on 24 May 2023).
- MicroCal ITC200 System User Manual; Malvern Instruments Ltd.: Worcestershire, UK, 2015; p. 67.
- Wiseman, T.; Williston, S.; Brandts, J.F.; Lin, L.N. Rapid Measurement of Binding Constants and Heats of Binding Using a New Titration Calorimeter. Anal. Biochem. 1989, 179, 131–137. [Google Scholar] [CrossRef]
- Dobreva, M.A.; Green, R.J.; Mueller-Harvey, I.; Salminen, J.-P.; Howlin, B.J.; Frazier, R.A. Size and Molecular Flexibility Affect the Binding of Ellagitannins to Bovine Serum Albumin. J. Agric. Food Chem. 2014, 62, 9186–9194. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Ellagitannins with Glucopyranose Cores Have Higher Affinities to Proteins than Acyclic Ellagitannins by Isothermal Titration Calorimetry. J. Agric. Food Chem. 2019, 67, 12730–12740. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Hatano, T. Classification of Oligomeric Hydrolysable Tannins and Specificity of Their Occurrence in Plants. Phytochemistry 1993, 32, 507–521. [Google Scholar] [CrossRef]
- Engström, M.T.; Arvola, J.; Nenonen, S.; Virtanen, V.T.J.; Leppä, M.M.; Tähtinen, P.; Salminen, J.P. Structural Features of Hydrolyzable Tannins Determine Their Ability to Form Insoluble Complexes with Bovine Serum Albumin. J. Agric. Food Chem. 2019, 67, 6798–6808. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E. Fifty Years of Polyphenol-Protein Complexes. In Recent Advances in Polyphenol Research, Volume 3; Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 3, pp. 71–97. ISBN 9781444337464. [Google Scholar]
- Virtanen, V.; Räikkönen, S.; Puljula, E.; Karonen, M. Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy. Molecules 2021, 26, 373. [Google Scholar] [CrossRef]
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809. [Google Scholar] [CrossRef]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Structure–Activity Relationships in the Hydrophobic Interactions of Polyphenols with Cellulose and Collagen. Biopolymers 2003, 70, 403–413. [Google Scholar] [CrossRef]
- Phan, A.D.T.; D’Arcy, B.R.; Gidley, M.J. Polyphenol–Cellulose Interactions: Effects of pH, Temperature and Salt. Int. J. Food Sci. Technol. 2016, 51, 203–211. [Google Scholar] [CrossRef]
- Suominen, E.; Savila, S.; Sillanpää, M.; Damlin, P.; Karonen, M. Affinity of Tannins to Cellulose: A Simple Chromatographic Tool for Revealing the Structure-Activity Patterns. Molecules, 2023; manuscript submitted. [Google Scholar]
- Bai, J.; Zhao, S.; Fan, X.; Chen, Y.; Zou, X.; Hu, M.; Wang, B.; Jin, J.; Wang, X.; Hu, J.; et al. Inhibitory Effects of Flavonoids on P-Glycoprotein in Vitro and in Vivo: Food/Herb-Drug Interactions and Structure–Activity Relationships. Toxicol. Appl. Pharmacol. 2019, 369, 49–59. [Google Scholar] [CrossRef]
- von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Mendoza-de Gives, P.; Valles-de la Mora, B.; González-Cortazar, M.; Zamilpa, A.; Castillo Gallegos, E. Elucidation of Leucaena leucocephala Anthelmintic-like Phytochemicals and the Ultrastructural Damage Generated to Eggs of Cooperia Spp. Vet. Parasitol. 2015, 214, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic Inhibition of Haemonchus contortus Exsheathment by Flavonoid Monomers and Condensed Tannins. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Baert, N.; Karonen, M.; Salminen, J.P. Isolation, Characterisation and Quantification of the Main Oligomeric Macrocyclic Ellagitannins in Epilobium angustifolium by Ultra-High Performance Chromatography with Diode Array Detection and Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2015, 1419, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.P.; Karonen, M.; Sinkkonen, J. Chemical Ecology of Tannins: Recent Developments in Tannin Chemistry Reveal New Structures and Structure-Activity Patterns. Chem. Eur. J. 2011, 17, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.P.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Seasonal Variation in the Content of Hydrolysable Tannins in Leaves of Betula pubescens. Phytochemistry 2001, 57, 15–22. [Google Scholar] [CrossRef]
- Salminen, J.P.; Ossipov, V.; Loponen, J.; Haukioja, E.; Pihlaja, K. Characterisation of Hydrolysable Tannins from Leaves of Betula pubescens by High-Performance Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 1999, 864, 283–291. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Rice, M.E.; Ritchard, N.T. Mechanisms of Protein Precipitation for Two Tannins, Pentagalloyl Glucose and Epicatechin16 (4→8) Catechin (Procyanidin). J. Agric. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of Bioactive Plant Ellagitannins by Chromatographic, Spectroscopic and Mass Spectrometric Methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Rauha, J.P.; Wolfender, J.L.; Salminen, J.P.; Pihlaja, K.; Hostettmann, K.; Vuorela, H. Characterization of the Polyphenolic Composition of Purple Loosestrife (Lythrum salicaria). Z. Naturforsch. Sect. C J. Biosci. 2001, 56, 13–20. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic Compounds in the Fruits of Egyptian Medicinal Plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, Quantitation and Determination of Antioxidant Capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Moilanen, J.; Koskinen, P.; Salminen, J.-P.P. Distribution and Content of Ellagitannins in Finnish Plant Species. Phytochemistry 2015, 116, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.T.; Pälijärvi, M.; Salminen, J.P. Rapid Fingerprint Analysis of Plant Extracts for Ellagitannins, Gallic Acid, and Quinic Acid Derivatives and Quercetin-, Kaempferol- and Myricetin-Based Flavonol Glycosides by UPLC-QqQ-MS/MS. J. Agric. Food Chem. 2015, 63, 4068–4079. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillanpää, M.; Engström, M.T.; Tähtinen, P.; Green, R.J.; Käpylä, J.; Näreaho, A.; Karonen, M. Tannins Can Have Direct Interactions with Anthelmintics: Investigations by Isothermal Titration Calorimetry. Molecules 2023, 28, 5261. https://doi.org/10.3390/molecules28135261
Sillanpää M, Engström MT, Tähtinen P, Green RJ, Käpylä J, Näreaho A, Karonen M. Tannins Can Have Direct Interactions with Anthelmintics: Investigations by Isothermal Titration Calorimetry. Molecules. 2023; 28(13):5261. https://doi.org/10.3390/molecules28135261
Chicago/Turabian StyleSillanpää, Mimosa, Marica T. Engström, Petri Tähtinen, Rebecca J. Green, Jarmo Käpylä, Anu Näreaho, and Maarit Karonen. 2023. "Tannins Can Have Direct Interactions with Anthelmintics: Investigations by Isothermal Titration Calorimetry" Molecules 28, no. 13: 5261. https://doi.org/10.3390/molecules28135261