Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers
Abstract
:1. Introduction
2. Photodynamic Therapy
2.1. A Piece of Photodynamic Therapy’s Origin and History
2.2. Principles of Photodynamic Therapy
2.3. Photodynamic Therapy Clinical Applications
2.4. Photodynamic Therapy Advantages and Disadvantages Compared to Other Strategies
- Double selectivity: It is spatially selective, as the light beam is focused on the precise location of the target tissue, and, as the PS preferentially accumulates and retains at the site to be treated, systemic toxicity is minimized [66]. Furthermore, as the half-life of the ROS produced, such as singlet oxygen and hydroxyl radicals, is less than a microsecond, reactions of an oxidative nature hardly occur in adjacent healthy tissues;
- Combinable with other therapies: If PDT does not produce the desired effect, it can be used in combination with other therapeutic modalities, such as chemotherapy, radiotherapy, surgery, and immunotherapy, among other more invasive means, since drug interactions do not occur as therapeutic targets are different from those of PDT, and irradiation treatment does not interfere with the activity of other antineoplastics [67,68]. Therapeutic targets differ, making their combination non-competitive and capable of producing improved effects compared to single treatments;
- Reduced recovery time [72];
- Repeatable in case of tumor recurrence: Photodynamic treatment can be repeated in the event of the appearance of a new primary tumor in a previously treated area without risk of damage to surrounding normal tissues or development of resistance to therapy, mechanisms recurrently observed and reported in the literature regarding conventional medicines, which limit their effectiveness [66,73,74];
- Lead to better aesthetic results: Especially in cases of skin cancer, good aesthetic results are observed compared to surgical methods [75]. Photodynamic treatment should not cause an increase in the temperature of the tissues, causing no destruction of the connective tissue and allowing the anatomical and functional integrity of the tissues to be maintained [76].
- Few commercially available PSs: Not many PS molecules are approved for clinical use. Furthermore, most PS currently applied are derived from porphyrins, a family of molecules whose molar absorptivity at higher wavelengths, in the regions of the electromagnetic spectrum where tissues are more transparent to light, is relatively low [77];
- Dependence on the presence of triplet molecular oxygen: PSs whose mechanisms of action are type I and type II reactions depend on triplet molecular oxygen since this is necessary for forming reactive species that induce cell death [80]. Developing new type III PSs could circumvent this limitation [43];
- Absence of contact with light sources for significant periods: Depending on the pharmacokinetic and pharmacodynamic properties of the PS, the time it takes to reach and accumulate preferentially in the target tissue is variable. However, during this period, designated by many as the “drug-light interval”, the patient cannot be exposed to any light, as this would activate the compound prematurely [26,81];
- Persistent cutaneous photosensitivity: Even after photodynamic treatment, the patient should not be exposed directly to light sources since the PS takes some time to be eliminated from the body. From light incidence until the patient can be exposed to radiation, it depends mainly on the photosensitizing molecule. Early exposure results in skin photosensitivity, an effect that may last over time [82];
- Dosimetry is difficult to prescribe because several parameters must be optimized before applying therapy. The PS dose to be administered, the “drug-light interval”, as well as the light source to be used, the irradiation area, and the dose of light to be applied must be determined considering factors such as the size and location of the tumor and the level of oxygenation of the tissues to be eliminated [83].
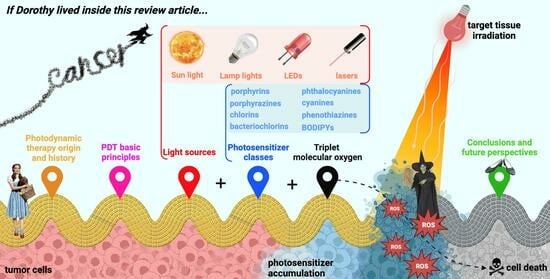
3. The “Three Elements” of Photodynamic Therapy
3.1. Light Source
3.2. Triplet Molecular Oxygen
3.3. Photosensitizer
4. N-Heterocyclic-Bearing Dyes as Photodynamic Therapy Photosensitizers
4.1. Most Recent Studies Regarding Photosensitizing Candidates’ Discovery
4.1.1. Porphyrin-Based Photosensitizers
Porphyrins
- Inferring the functioning of specific cell receptors, Manathanath et al. [144] synthesized a series of tetrahydroxiphenyl-derived porphyrins (THPP) appended with the 4,6-diamino-1,3,5-triazine group. This moiety was introduced since triazines are known for their attractive bioactivity due to their kinase receptor inhibitory nature, particularly their ability to inhibit the epidermal growth factor receptor-tyrosine kinase, overexpressed in tumor cells, which is involved in tumor proliferation, metastasis, and angiogenesis processes [145,146];
- Responsiveness to elements overexpressed in the tumor environment: Huang et al. [147] designed a “dual response” porphyrin-based PS capable of responding to the typical increase in glutathione (GSH) and hydrogen sulfide concentration in tumor cells. For this purpose, they prepared a reversible derivative of the already known THPP esterified with 2,4-dinitrobenzosulfonyl chloride, which presented low to moderate toxicity and zero formation of singlet oxygen. Furthermore, the investigators testified that only in the presence of GSH and hydrogen sulfide occurs the photosensitizing agent activation by the quenching group departure, stating that it has a high clinical potential in reducing the effect of cutaneous photosensitivity;
- Improvement of functional properties of this family of PSs: Certain functional groups can significantly contribute to refining physiological and chemical stability and biocompatibility [148]. The introduction of polyethylene glycol (PEG) moieties into porphyrin cores, for example, has been reported several times in the literature [149,150,151]. Lazewski et al. [151] demonstrated that, irrespective of the coordinated metal, short-PEGylated porphyrins reveal reduced dark cytotoxicity, increased ability to produce singlet oxygen, and that the structural location where the polymer is introduced can result in the variation of the biological effects;
- As an interface between single-PSs and nano-PSs, Li et al. [152] demonstrate that the chain conjugation of PEG-bearing porphyrins with perylene diimide units resulted, by self-assembly, in a kind of nanoparticle with attractive properties: (i) high biocompatibility; (ii) intense absorption in the visible and NIR regions; (iii) therapeutic efficiency in vitro and in vivo with reduced side effects; (iv) potential use as a theranostic agent by obtaining second near-infrared (NIR-II) fluorescence images.
Porphyrazines
Chlorins
Bacteriochlorins
Phthalocyanines
4.1.2. Cyanine-Based Photosensitizers
- Polymethine chain length: The number of carbons present in the polymethine chain is the property that defines which class cyanines belong to. Cyanine-based compounds with a single carbon in the methine chain are called monomethine cyanines (Cy1), with three trimethines (Cy3), five pentamethines (Cy5), and seven heptamethine cyanines (Cy7). Cy5 and Cy7 are the most noted in the literature in the study of their photobiology properties since they efficiently absorb at higher wavelengths: while Cy1 absorbs at around 400 nm, Cy7 sees its maximum absorption peaks around 800 nm [243];
- Heterocycle units: For PDT purposes, the preparation of cyanines bearing heterocycles derived from indolenine, benzoindole, benzothiazole, benzoselenazole, benzoxazole, and quinolines is the most common. Among those mentioned above, indolenine and benz[e]indole derivatives are the most highlighted in the recent literature. Furthermore, anthracene units serving as heterocyclic units have been reported [244,245]. The nature of the heterocycles may condition the biological activity of cyanines, varying, for example, the stability of dyes to light [246];
- Central ring: The introduction of cyclic structures in the center of cyanines is a strategy that allows for improving the rigidity and chemical stability of this class of molecules. It is especially common in the case of Cy5, where there are rings with four or five members (squaraine and croconaine dyes, respectively [247,248]) and, in the case of Cy7, cyclohexane rings or their derivatives (for example, a boron fluoride complex within the core structure [128]). The functionalization of Cy7’s cyclohexane ring with halogenated atoms such as bromine and chloride is common [249], which serve as excellent leaving groups for functionalization with other groups of interest [250,251,252];
- N-alkyl chains: Since cyanines are intrinsically lipophilic compounds, this characteristic can be modulated by increasing or decreasing the length of N-aliphatic chains. This modulation of biocompatibility is also carried out, for example, by introducing sulfonic groups [249,252], carboxylic acids [253,254], pyridines [255], or even more attractively, by functionalizing them with PEG chains [253] or HA units [256]. The conjugation of cyanines with porphyrin-based dyes, adding them via N-alkyl chains, is another recently reported strategy to formulate new promising PSs [257]. A further attractive approach is the dimerization of these dyes, building molecules with two covalently linked cyanine nuclei, these chains serving as bridges between units [258]. The targeting of these compounds can also be enhanced, for example, by introducing specific antibodies to these chains [259];
- Halogenation and the so-called “heavy atom effect”: The introduction of halogens and other heavy atoms, such as selenium [260,261], contributes above all to more efficient anticancer activity and, by increasing the lifetime of these dyes in the triplet state, to the improvement of their singlet oxygen production. The presence of iodine atoms is highlighted as the form of halogenation that most actively increases therapeutic activity [249,254,262]. Interestingly, Semenova et al. provide evidence that, effectively, there is no linearity between the therapeutic effects and the number of iodine atoms introduced, reporting that the “ideal” number for the Cy7 they prepared is two iodine atoms [263]. Despite this, for indolenine-based Cy5, bromination proved to be more advantageous [264]. However, for benz[e]indole-based brominated Cy5, the same research group shows that there is no advantage in these halogenated ones compared to non-halogenated ones, indicating that the heterocycle may play a role [265]. Intending to improve its bioavailability, Shi et al. introduce the trifluoromethyl group into the Cy7 N-alkyl chains, stating that this structural modification can improve the cellular uptake of the dye [266].
4.1.3. Phenothiazine-Based Photosensitizers
4.1.4. Boron-Dipyrromethene-Based Photosensitizers
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R. Photodynamic therapy: Oncologic horizons. Futur. Oncol. 2014, 10, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Song, Q.; Li, P.; Huang, W. Rejuvenated Photodynamic Therapy for Bacterial Infections. Adv. Healthc. Mater. 2019, 8, e1900608. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y. Photodynamic Therapy for the Treatment of Fungal Infections. Infect. Drug Resist. 2022, 15, 3251–3266. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Mouinga-Ondeme, A.G. Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia. Pharmaceuticals 2022, 15, 1273. [Google Scholar] [CrossRef]
- Conrado, P.C.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Gonçalves, R.S.; Lopes, L.D.; Lonardoni, M.V.; Teixeira, J.J.; Bonfim-Mendonça, P.S.; Kioshima, E.S. A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagnosis Photodyn. Ther. 2021, 34, 102221. [Google Scholar] [CrossRef]
- Wan, M.T.; Lin, J. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar] [CrossRef] [Green Version]
- Gensbittel, V.; Kräter, M.; Harlepp, S.; Busnelli, I.; Guck, J.; Goetz, J.G. Mechanical Adaptability of Tumor Cells in Metastasis. Dev. Cell 2021, 56, 164–179. [Google Scholar] [CrossRef]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef]
- Anisimov, V.N. The relationship between aging and carcinogenesis: A critical appraisal. Crit. Rev. Oncol. 2003, 45, 277–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, J.; Higham, C.; Langer, T.; Denzer, C.; Brabant, G. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr. Rev. 2019, 40, 711–767. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, D.C. Long-term toxicity of chemotherapy and radiotherapy in lymphoma survivors: Optimizing treatment for individual patients. Clin. Adv. Hematol. Oncol. 2015, 13, 103–112. [Google Scholar] [PubMed]
- Gu, B.; Wang, B.; Li, X.; Feng, Z.; Ma, C.; Gao, L.; Yu, Y.; Zhang, J.; Zheng, P.; Wang, Y.; et al. Photodynamic therapy improves the clinical efficacy of advanced colorectal cancer and recruits immune cells into the tumor immune microenvironment. Front. Immunol. 2022, 13, 1050421. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef]
- Abdel-Kader, M.H. (Ed.) History of Photodynamic Therapy. In Photodynamic Therapy; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–22. ISBN 978-3-642-39628-1. [Google Scholar]
- Abdel-kader, M.H. CHAPTER 1. The Journey of PDT Throughout History: PDT from Pharos to Present. In Comprehensive Series in Photochemical & Photobiological Sciences; Kostron, H., Hasan, T., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–21. ISBN 978-1-78262-451-6. [Google Scholar]
- Daniell, M.D.; Hill, J.S. A History of Photodynamic Therapy. ANZ J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef]
- Hashimoto, A.; Takamura-Enya, T.; Oda, Y. Synthesis and In Vitro Biological Evaluation of Psoralen-Linked Fullerenes. Photochem. Photobiol. 2019, 95, 1403–1411. [Google Scholar] [CrossRef]
- Hübinger, L.; Runge, R.; Rosenberg, T.; Freudenberg, R.; Kotzerke, J.; Brogsitter, C. Psoralen as a Photosensitizers for Photodynamic Therapy by Means of In Vitro Cherenkov Light. Int. J. Mol. Sci. 2022, 23, 15233. [Google Scholar] [CrossRef]
- Grzybowski, A.; Sak, J.; Pawlikowski, J. A brief report on the history of phototherapy. Clin. Dermatol. 2016, 34, 532–537. [Google Scholar] [CrossRef]
- Spikes, J.D. The origin and meaning of the term “photodynamic” (as used in “photodynamic therapy”, for example). J. Photochem. Photobiol. B Biol. 1991, 9, 369–371. [Google Scholar] [CrossRef]
- Moan, J.; Peng, Q. An Outline of the History of PDT. In Photodynamic Therapy; Patrice, T., Ed.; The Royal Society of Chemistry: London, UK, 2003; pp. 1–18. ISBN 978-0-85404-306-4. [Google Scholar]
- Szeimies, R.-M.; Dräger, J.; Abels, C.; Landthaler, M. Chapter 1 History of Photodynamic Therapy in Dermatology. In Comprehensive Series in Photosciences; Elsevier: Amsterdam, The Netherlands, 2001; Volume 2, pp. 3–15. ISBN 978-0-444-50828-7. [Google Scholar]
- Finsen, N.R. REMARKS on the RED-LIGHT TREATMENT of SMALL-POX: Is the Treatment of Small-pox Patients in Broad Daylight Warrantable? BMJ 1903, 1, 1297–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. Gynecol. Oncol. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236. [Google Scholar] [CrossRef] [PubMed]
- Wöhrle, D.; Hirth, A.; Bogdahn-Rai, T.; Schnurpfeil, G.; Shopova, M. Photodynamic therapy of cancer: Second and third generations of photosensitizers. Russ. Chem. Bull. 1998, 47, 807–816. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; InTech: London, UK, 2018; ISBN 978-1-78923-134-2. [Google Scholar]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [Green Version]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Greer, A.; Thomas, A.H. Practical Aspects in the Study of Biological Photosensitization Including Reaction Mechanisms and Product Analyses: A Do’s and Don’ts Guide. Photochem. Photobiol. 2023, 99, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.O.; Ha, K.S. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int. Rev. Cell. Mol. Biol. 2012, 295, 139–174. [Google Scholar]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Yi, Q.; Fang, W.; Zhang, J. A mini review of nanomaterials on photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2023, 54, 100568. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, J.; Long, S.; Zhao, X.; Li, H.; Du, J.; Shao, K.; Peng, X. The concept and examples of type-III photosensitizers for cancer photodynamic therapy. Chem 2022, 8, 197–209. [Google Scholar] [CrossRef]
- Nguyen, K.; Khachemoune, A. An update on topical photodynamic therapy for clinical dermatologists. J. Dermatol. Treat. 2019, 30, 732–744. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [Green Version]
- Lucena, S.R.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; Juarranz, Á.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Zhou, L.; Huang, C.; Li, Z.; Lu, T.; Cong, Q.; Liang, J.; Zhong, X.; Lu, L.; Jin, C. Twenty-year outcome in neovascular age-related macular degeneration treated with photodynamic therapy and intravitreal bevacizumab/ranibizumab injections: A case report. Photodiagnosis Photodyn. Ther. 2023, 42, 103349. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Williams, B.K. Current Indications for Photodynamic Therapy in Retina and Ocular Oncology. Curr. Ophthalmol. Rep. 2021, 9, 107–116. [Google Scholar] [CrossRef]
- Kubrak, T.; Karakuła, M.; Czop, M.; Kawczyk-Krupka, A.; Aebisher, D. Advances in Management of Bladder Cancer—The Role of Photodynamic Therapy. Molecules 2022, 27, 731. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, G.; Colin, P.; Betrouni, N.; Nevoux, P.; Ouzzane, A.; Puech, P.; Villers, A.; Mordon, S. Photodynamic therapy in urology: What can we do now and where are we heading? Photodiagnosis Photodyn. Ther. 2012, 9, 261–273. [Google Scholar] [CrossRef]
- Ibarra, A.M.C.; Cecatto, R.B.; Motta, L.J.; Franco, A.L.D.S.; Silva, D.D.F.T.D.; Nunes, F.D.; Hamblin, M.R.; Rodrigues, M.F.S.D. Photodynamic therapy for squamous cell carcinoma of the head and neck: Narrative review focusing on photosensitizers. Lasers Med. Sci. 2021, 37, 1441–1470. [Google Scholar] [CrossRef]
- Zhan, Q.; Wu, C.; Ding, H.; Huang, Y.; Jiang, Z.; Liao, N.; Wang, K.; Li, Y. Emerging trends in photodynamic therapy for head and neck cancer: A 10-year bibliometric analysis based on CiteSpace. Photodiagnosis Photodyn. Ther. 2022, 38, 102860. [Google Scholar] [CrossRef]
- Maździarz, A. Successful pregnancy and delivery following selective use of photodynamic therapy in treatment of cervix and vulvar diseases. Photodiagnosis Photodyn. Ther. 2019, 28, 65–68. [Google Scholar] [CrossRef]
- Matoba, Y.; Banno, K.; Kisu, I.; Aoki, D. Clinical application of photodynamic diagnosis and photodynamic therapy for gynecologic malignant diseases: A review. Photodiagnosis Photodyn. Ther. 2018, 24, 52–57. [Google Scholar] [CrossRef]
- Filonenko, E.V.; Trushina, O.I.; Novikova, E.G.; Zarochentseva, N.V.; Rovinskaya, O.V.; Ivanova-Radkevich, V.I.; Kaprin, A.D. Photodynamic therapy in the treatment of intraepithelial neoplasia of the cervix, vulva and vagina. Biomed. Photonics 2021, 9, 31–39. [Google Scholar] [CrossRef]
- Yano, T.; Minamide, T.; Takashima, K.; Nakajo, K.; Kadota, T.; Yoda, Y. Clinical Practice of Photodynamic Therapy Using Talaporfin Sodium for Esophageal Cancer. J. Clin. Med. 2021, 10, 2785. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Casas, A. Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef]
- Corrie, P.G. Cytotoxic chemotherapy: Clinical aspects. Medicine 2008, 36, 24–28. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Bhatia, K.; Bhumika; Das, A. Combinatorial drug therapy in cancer—New insights. Life Sci. 2020, 258, 118134. [Google Scholar] [CrossRef] [PubMed]
- de Crevoisier, R.; Lafond, C.; Mervoyer, A.; Hulot, C.; Jaksic, N.; Bessières, I.; Delpon, G. Image-guided radiotherapy. Cancer/Radiothérapie 2022, 26, 34–49. [Google Scholar] [CrossRef]
- Busk, M.; Overgaard, J.; Horsman, M.R. Imaging of Tumor Hypoxia for Radiotherapy: Current Status and Future Directions. Semin. Nucl. Med. 2020, 50, 562–583. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Veness, M.J. The role of radiotherapy in the management of non-melanoma skin cancer. Australas. J. Dermatol. 2019, 60, 265–272. [Google Scholar] [CrossRef]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Wilson, B.C. Photodynamic Therapy for Cancer: Principles. Can. J. Gastroenterol. 2002, 16, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.; Kim, H.; Li, X.; Yoon, J. Supramolecular agents for combination of photodynamic therapy and other treatments. Chem. Sci. 2021, 12, 7248–7268. [Google Scholar] [CrossRef]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, T.; Kahaleh, M. Comparing palliative treatment options for cholangiocarcinoma: Photodynamic therapy vs. radiofrequency ablation. Clin. Endosc. 2022, 55, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B.; Friedberg, J.S.; Glatstein, E.; Stevenson, J.P.; Sterman, D.H.; Hahn, S.M.; Cengel, K.A. Photodynamic therapy for the treatment of non-small cell lung cancer. J. Thorac. Dis. 2012, 4, 63–75. [Google Scholar] [CrossRef]
- Overholt, B.F.; Panjehpour, M.; Haydek, J.M. Photodynamic therapy for Barrett’s esophagus: Follow-up in 100 patients. Gastrointest. Endosc. 1999, 49, 1–7. [Google Scholar] [CrossRef]
- Singh, H.; Benn, B.S.; Jani, C.; Abdalla, M.; Kurman, J.S. Photodynamic therapy for treatment of recurrent adenocarcinoma of the lung with tracheal oligometastasis. Respir. Med. Case Rep. 2022, 37, 101620. [Google Scholar] [CrossRef]
- Succo, G.; Rosso, S.; Fadda, G.; Fantini, M.; Crosetti, E. Salvage photodynamic therapy for recurrent nasopharyngeal carcinoma. Photodiagnosis Photodyn. Ther. 2014, 11, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Queirós, C.; Garrido, P.M.; Silva, J.M.; Filipe, P. Photodynamic therapy in dermatology: Beyond current indications. Dermatol. Ther. 2020, 33, e13997. [Google Scholar] [CrossRef]
- Bown, S.G. Photodynamic therapy for photochemists. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120371. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef] [PubMed]
- Stolik, S.; Delgado, J.; Pérez, A.; Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J. Photochem. Photobiol. B Biol. 2000, 57, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Duan, L.; Wang, A.; Fei, J.; Li, J. Insight into the efficiency of oxygen introduced photodynamic therapy (PDT) and deep PDT against cancers with various assembled nanocarriers. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1583. [Google Scholar] [CrossRef] [PubMed]
- Ris, H.-B.; Altermatt, H.J.; Nachbur, B.; Stewart, J.C.M.; Wang, Q.; Lim, C.K.; Bonnett, R.; Althaus, U. Effect of drug-light interval on photodynamic therapy with meta-tetrahydroxyphenylchlorin in malignant mesothelioma. Int. J. Cancer 1993, 53, 141–146. [Google Scholar] [CrossRef]
- Trehan, M.; Taylor, C.R. Chapter 21 Cutaneous Photosensitivity and Photoprotection for Photodynamic Therapy Patients. In Comprehensive Series in Photosciences; Elsevier: Amsterdam, The Netherlands, 2001; Volume 2, pp. 321–337. ISBN 978-0-444-50828-7. [Google Scholar]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting photodynamic therapy dosimetry: Reductionist & surrogate approaches to facilitate clinical success. Phys. Med. Biol. 2016, 61, R57–R89. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Nichols, M.G.; Foster, T.H. Oxygen diffusion and reaction kinetics in the photodynamic therapy of multicell tumour spheroids. Phys. Med. Biol. 1994, 39, 2161–2181. [Google Scholar] [CrossRef]
- Seshadri, M.; Bellnier, D.; Vaughan, L.A.; Spernyak, J.A.; Mazurchuk, R.; Foster, T.; Henderson, B.W. Light Delivery over Extended Time Periods Enhances the Effectiveness of Photodynamic Therapy. Clin. Cancer Res. 2008, 14, 2796–2805. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.A.; Amorim, R.; Silva, M.; Baltazar, F.; Wolffenbuttel, R.; Correia, J.H. Photodynamic Therapy at Low-Light Fluence Rate: In vitro Assays on Colon Cancer Cells. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–6. [Google Scholar] [CrossRef]
- Cheng, Y.; Chang, Y.; Feng, Y.; Liu, N.; Sun, X.; Feng, Y.; Li, X.; Zhang, H. Simulated Sunlight-Mediated Photodynamic Therapy for Melanoma Skin Cancer by Titanium-Dioxide-Nanoparticle-Gold-Nanocluster-Graphene Heterogeneous Nanocomposites. Small 2017, 13, 1603935. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-N.; Hsu, R.; Chen, S.; Wong, T.-W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef] [PubMed]
- Erkiert-Polguj, A.; Halbina, A.; Polak-Pacholczyk, I.; Rotsztejn, H. Light-emitting diodes in photodynamic therapy in non-melanoma skin cancers—own observations and literature review. J. Cosmet. Laser Ther. 2016, 18, 105–110. [Google Scholar] [CrossRef]
- Neupane, J.; Ghimire, S.; Shakya, S.; Chaudhary, L.; Shrivastava, V.P. Effect of light emitting diodes in the photodynamic therapy of rheumatoid arthritis. Photodiagnosis Photodyn. Ther. 2010, 7, 44–49. [Google Scholar] [CrossRef]
- Mang, T.S. Lasers and light sources for PDT: Past, present and future. Photodiagnosis Photodyn. Ther. 2004, 1, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Lin, H.-P.; Chen, H.-M.; Yang, H.; Wang, Y.-P.; Chiang, C.-P. Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light-emitting diode or laser light. Lasers Surg. Med. 2009, 41, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef]
- Bessière, A.; Durand, J.-O.; Noûs, C. Persistent luminescence materials for deep photodynamic therapy. Nanophotonics 2021, 10, 2999–3029. [Google Scholar] [CrossRef]
- Tzani, M.A.; Gioftsidou, D.K.; Kallitsakis, M.G.; Pliatsios, N.V.; Kalogiouri, N.P.; Angaridis, P.A.; Lykakis, I.N.; Terzidis, M.A. Direct and Indirect Chemiluminescence: Reactions, Mechanisms and Challenges. Molecules 2021, 26, 7664. [Google Scholar] [CrossRef]
- Periyasami, G.; Martelo, L.; Baleizão, C.; Berberan-Santos, M.N. Strong green chemiluminescence from naphthalene analogues of luminol. New J. Chem. 2014, 38, 2258–2261. [Google Scholar] [CrossRef]
- Chen, T.-C.; Huang, L.; Liu, C.-C.; Chao, P.-J.; Lin, F.-H. Luminol as the light source for in situ photodynamic therapy. Process. Biochem. 2012, 47, 1903–1908. [Google Scholar] [CrossRef]
- Degirmenci, A.; Sonkaya, Ö.; Soylukan, C.; Karaduman, T.; Algi, F. BODIPY and 2,3-Dihydrophthalazine-1,4-Dione Conjugates as Heavy Atom-Free Chemiluminogenic Photosensitizers. ACS Appl. Bio Mater. 2021, 4, 5090–5098. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Correia, J.H. Enhanced Photodynamic Therapy: A Review of Combined Energy Sources. Cells 2022, 11, 3995. [Google Scholar] [CrossRef]
- Foglietta, F.; Macrì, M.; Panzanelli, P.; Francovich, A.; Durando, G.; Garello, F.; Terreno, E.; Serpe, L.; Canaparo, R. Ultrasound boosts doxorubicin efficacy against sensitive and resistant ovarian cancer cells. Eur. J. Pharm. Biopharm. 2023, 183, 119–131. [Google Scholar] [CrossRef]
- Nkune, N.W.; Abrahamse, H. Anti-Hypoxia Nanoplatforms for Enhanced Photosensitizer Uptake and Photodynamic Therapy Effects in Cancer Cells. Int. J. Mol. Sci. 2023, 24, 2656. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Wang, Y.; Cheng, H.; Li, S. Innovative strategies for photodynamic therapy against hypoxic tumor. Asian J. Pharm. Sci. 2023, 18, 100775. [Google Scholar] [CrossRef]
- Siemann, D.W.; Horsman, M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Ortel, B.J.; Pereira, S.P.; Hasan, T.; Maytin, E.V. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Woodhams, J.H.; MacRobert, A.J.; Bown, S.G. The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry. Photochem. Photobiol. Sci. 2007, 6, 1246–1256. [Google Scholar] [CrossRef]
- Zang, L.; Zhao, H. A strategy for monitoring oxygen concentration, oxygen consumption, and generation of singlet oxygen using a phosphorescent photosensitizer. J. Lumin. 2020, 224, 117282. [Google Scholar] [CrossRef]
- Hong, L.; Li, J.; Luo, Y.; Guo, T.; Zhang, C.; Ou, S.; Long, Y.; Hu, Z. Recent Advances in Strategies for Addressing Hypoxia in Tumor Photodynamic Therapy. Biomolecules 2022, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, D.; Wang, G.; Wang, Y.; Cao, L.; Sun, J.; Jiang, Q.; He, Z. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: Opportunities, challenges, and future development. Acta Pharm. Sin. B 2020, 10, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Rees, T.W.; Liao, X.; Ji, L.; Chao, H. Oxygen self-sufficient photodynamic therapy. Co-Ord. Chem. Rev. 2021, 432, 213714. [Google Scholar] [CrossRef]
- Maier, A.; Anegg, U.; Fell, B.; Rehak, P.; Ratzenhofer, B.; Tomaselli, F.; Sankin, O.; Pinter, H.; Smolle-Jüttner, F.M.; Friehs, G.B. Hyperbaric Oxygen and Photodynamic Therapy in the Treatment of Advanced Carcinoma of the Cardia and the Esophagus. Lasers Surg. Med. 2000, 26, 308–315. [Google Scholar] [CrossRef]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Khurana, B.; Ouk, T.-S.; Lucas, R.; Senge, M.O.; Sol, V. Photosensitizer-hyaluronic acid complexes for antimicrobial photodynamic therapy (aPDT). J. Porphyr. Phthalocyanines 2022, 26, 585–593. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Siewert, B. Does the chemistry of fungal pigments demand the existence of photoactivated defense strategies in basidiomycetes? Photochem. Photobiol. Sci. 2021, 20, 475–488. [Google Scholar] [CrossRef]
- Gill, M.; Giménez, A. Austrovenetin, the principal pigment of the toadstool Dermocybe austroveneta. Phytochemistry 1991, 30, 951–955. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Zhang, Z.; Fu, J.; You, L.; He, Y.; Hao, Y.; Gu, Z.; Yu, Z.; Qu, C.; et al. Hypericin-mediated photodynamic therapy for the treatment of cancer: A review. J. Pharm. Pharmacol. 2021, 73, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules 2022, 27, 5084. [Google Scholar] [CrossRef]
- Cieckiewicz, E.; Angenot, L.; Mathieu, V.; Pirotte, B.; de Tullio, P.; Frédérich, M. Hemisynthesis of glycosylated derivatives of pheophorbide a. Planta Med. 2014, 80, P2O2. [Google Scholar] [CrossRef]
- Martins, L.M.O.S.; Wang, X.; Silva, G.T.M.; Junqueira, H.C.; Fornaciari, B.; Lopes, L.F.; Silva, C.P.; Zhou, P.; Cavalcante, V.F.; Baptista, M.S.; et al. Red Wine Inspired Chromophores as Photodynamic Therapy Sensitizers. Photochem. Photobiol. 2022, 99, 732–741. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-H.; Yang, J.; Mueller, J.L.; Huang, H.-C. Intratumoral Photosensitizer Delivery and Photodynamic Therapy. Nano LIFE 2021, 11, 2130003. [Google Scholar] [CrossRef] [PubMed]
- Ivanova-Radkevich, V.I. Biochemical Basis of Selective Accumulation and Targeted Delivery of Photosensitizers to Tumor Tissues. Biochemistry 2022, 87, 1226–1242. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Bonnett, R.; Martínez, G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Yu, Y.; Guo, S.; Wang, W.; Zhu, S. A cyanine-derivative photosensitizer with enhanced photostability for mitochondria-targeted photodynamic therapy. Chem. Commun. 2019, 55, 13542–13545. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef] [Green Version]
- Bryden, F.; Boyle, R.W. Metalloporphyrins for Medical Imaging Applications. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 68, pp. 141–221. ISBN 978-0-12-803526-9. [Google Scholar]
- Tahoun, M.; Gee, C.T.; McCoy, V.E.; Sander, P.M.; Müller, C.E. Chemistry of porphyrins in fossil plants and animals. RSC Adv. 2021, 11, 7552–7563. [Google Scholar] [CrossRef]
- Park, J.M.; Hong, K.-I.; Lee, H.; Jang, W.-D. Bioinspired Applications of Porphyrin Derivatives. Acc. Chem. Res. 2021, 54, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Buglak, A.A.; Filatov, M.A.; Hussain, M.A.; Sugimoto, M. Singlet oxygen generation by porphyrins and metalloporphyrins revisited: A quantitative structure-property relationship (QSPR) study. J. Photochem. Photobiol. A Chem. 2020, 403, 112833. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [Green Version]
- Tope, W.D.; Martin, A.; Grevelink, J.M.; Starr, J.C.; Fewkes, J.L.; Flotte, T.J.; Deutsch, T.F.; Anderson, R.R. Lack of selectivity of protoporphyrin IX fluorescence for basal cell carcinoma after topical application of 5-aminolevulinic acid: Implications for photodynamic treatment. Arch. Dermatol. Res. 1995, 287, 665–674. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Zhang, X.; Liu, S.; Pei, Q.; Zheng, M.; Xie, Z. Porphyrin-Based Carbon Dots for Photodynamic Therapy of Hepatoma. Adv. Healthc. Mater. 2017, 6, 1600924. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Recent Advances in Porphyrin-Based Inorganic Nanoparticles for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 3358. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, W.; Li, C.; Sun, X.; Feng, X.; Liu, J. Synthesis and in vitro biological evaluation of novel water-soluble porphyrin complexes for cancer photodynamic therapy. Appl. Organomet. Chem. 2022, 36, e6598. [Google Scholar] [CrossRef]
- Wu, F.; Yang, M.; Zhang, J.; Zhu, S.; Shi, M.; Wang, K. Metalloporphyrin–indomethacin conjugates as new photosensitizers for photodynamic therapy. JBIC J. Biol. Inorg. Chem. 2019, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Couto, G.K.; Rodrigues, J.C.; Pacheco, B.S.; Simões, L.D.; Paschoal, J.D.; Seixas, F.K.; Acunha, T.V.; Iglesias, B.A.; Collares, T. Zinc(II), copper(II) and nickel(II) ions improve the selectivity of tetra-cationic platinum(II) porphyrins in photodynamic therapy and stimulate antioxidant defenses in the metastatic melanoma lineage (A375). Photodiagnosis Photodyn. Ther. 2020, 31, 101942. [Google Scholar] [CrossRef]
- Yang, M.; Deng, J.; Guo, D.; Sun, Q.; Wang, Z.; Wang, K.; Wu, F. Mitochondria-targeting Pt/Mn porphyrins as efficient photosensitizers for magnetic resonance imaging and photodynamic therapy. Dye. Pigment. 2019, 166, 189–195. [Google Scholar] [CrossRef]
- Frant, M.P.; Trytek, M.; Paduch, R. Assessing the In Vitro Activity of Selected Porphyrins in Human Colorectal Cancer Cells. Molecules 2022, 27, 2006. [Google Scholar] [CrossRef]
- Scoditti, S.; Chiodo, F.; Mazzone, G.; Richeter, S.; Sicilia, E. Porphyrins and Metalloporphyrins Combined with N-Heterocyclic Carbene (NHC) Gold(I) Complexes for Photodynamic Therapy Application: What Is the Weight of the Heavy Atom Effect? Molecules 2022, 27, 4046. [Google Scholar] [CrossRef]
- Manathanath, M.; Sasidharan, S.; Saudagar, P.; Panicker, U.G.; Sujatha, S. Photodynamic evaluation of triazine appended porphyrins as anti-leishmanial and anti-tumor agents. Polyhedron 2022, 217, 115711. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [Green Version]
- Ranson, M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br. J. Cancer 2004, 90, 2250–2255. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Xie, S.; Jiang, L.; Li, J.; Chen, J. A glutathione and hydrogen sulfide responsive photosensitizer for enhanced photodynamic therapy. Dye. Pigment. 2022, 205, 110529. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Choi, W.; Jangili, P.; Ge, Y.; Xu, Y.; Kang, J.; Liu, L.; Zhang, B.; Xie, Z.; et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem. Soc. Rev. 2021, 50, 9152–9201. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Jensen, T.J.; Vicente, M.G.H. Synthesis and cellular studies of PEG-functionalized meso-tetraphenylporphyrins. J. Photochem. Photobiol. B Biol. 2007, 86, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Li, X.; Huangfu, S.; Xia, K.; Yue, R.; Wu, H.; Song, W. Linear and high-molecular-weight poly-porphyrins for efficient photodynamic therapy. Biomater. Sci. 2021, 9, 4630–4638. [Google Scholar] [CrossRef] [PubMed]
- Lazewski, D.; Kucinska, M.; Potapskiy, E.; Kuzminska, J.; Tezyk, A.; Popenda, L.; Jurga, S.; Teubert, A.; Gdaniec, Z.; Kujawski, J.; et al. Novel Short PEG Chain-Substituted Porphyrins: Synthesis, Photochemistry, and In Vitro Photodynamic Activity against Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10029. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, Z.; Yang, L.; Chen, J.; Cheng, K.; Xue, Y.; Liu, G.; Luo, X.; Wu, F. Self-assembled porphyrin polymer nanoparticles with NIR-II emission and highly efficient photothermal performance in cancer therapy. Mater. Today Bio 2022, 13, 100198. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Yu, Q.; Liu, W.; Zhang, S.; Li, Y.; Chen, J.; Zhang, Z.; Lu, X. Enhanced porphyrin-based fluorescence imaging-guided photodynamic/photothermal synergistic cancer therapy by mitochondrial targeting. Sci. China Mater. 2022, 65, 527–535. [Google Scholar] [CrossRef]
- Jiao, J.; He, J.; Li, M.; Yang, J.; Yang, H.; Wang, X.; Yang, S. A porphyrin-based metallacage for enhanced photodynamic therapy. Nanoscale 2022, 14, 6373–6383. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Song, H.; Cai, Z.; Li, J.; Xiao, H.; Qi, R.; Zheng, M. Light triggered release of a triple action porphyrin-cisplatin conjugate evokes stronger immunogenic cell death for chemotherapy, photodynamic therapy and cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 329. [Google Scholar] [CrossRef]
- Magaela, N.B.; Matshitse, R.; Babu, B.; Managa, M.; Prinsloo, E.; Nyokong, T. Sn(IV) porphyrin-biotin decorated nitrogen doped graphene quantum dots nanohybrids for photodynamic therapy. Polyhedron 2022, 213, 115624. [Google Scholar] [CrossRef]
- Hou, M.; Chen, W.; Zhao, J.; Dai, D.; Yang, M.; Yi, C. Facile synthesis and in vivo bioimaging applications of porphyrin derivative-encapsulated polymer nanoparticles. Chin. Chem. Lett. 2022, 33, 4101–4106. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Jing, L.; Yue, X.; Dai, Z. Theranostic porphyrin dyad nanoparticles for magnetic resonance imaging guided photodynamic therapy. Biomaterials 2014, 35, 6379–6388. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Jenni, S.; Sour, A.; Heitz, V.; Bolze, F.; Pallier, A.; Bonnet, C.S.; Toth, E.; Ventura, B. A Porphyrin Dimer–GdDOTA Conjugate as a Theranostic Agent for One- and Two-Photon Photodynamic Therapy and MRI. Bioconjugate Chem. 2018, 29, 3726–3738. [Google Scholar] [CrossRef] [PubMed]
- Kopranenkov, V.N.; Luk’Yanets, E.A. Porphyrazines: Synthesis, properties, application. Russ. Chem. Bull. 1995, 44, 2216–2232. [Google Scholar] [CrossRef]
- Rodríguez-Morgade, M.S.; Stuzhin, P.A. The chemistry of porphyrazines: An overview. J. Porphyr. Phthalocyanines 2004, 8, 1129–1165. [Google Scholar] [CrossRef]
- Klein, T.; Ziegler, T. First example of an octa-glycoconjugated magnesium(II)porphyrazine. Tetrahedron Lett. 2016, 57, 495–497. [Google Scholar] [CrossRef]
- Wen, S.; Zhu, D.; Huang, P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 2013, 5, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci. Rep. 2021, 11, 7205. [Google Scholar] [CrossRef]
- Peskova, N.N.; Brilkina, A.A.; Gorokhova, A.A.; Shilyagina, N.Y.; Kutova, O.M.; Nerush, A.S.; Orlova, A.G.; Klapshina, L.G.; Vodeneev, V.V.; Balalaeva, I.V. The localization of the photosensitizer determines the dynamics of the secondary production of hydrogen peroxide in cell cytoplasm and mitochondria. J. Photochem. Photobiol. B Biol. 2021, 219, 112208. [Google Scholar] [CrossRef]
- Tasso, T.T.; Schlothauer, J.C.; Junqueira, H.C.; Matias, T.A.; Araki, K.; Liandra-Salvador, É.; Antonio, F.C.T.; Homem-De-Mello, P.; Baptista, M.S. Photobleaching Efficiency Parallels the Enhancement of Membrane Damage for Porphyrazine Photosensitizers. J. Am. Chem. Soc. 2019, 141, 15547–15556. [Google Scholar] [CrossRef] [PubMed]
- Yuzhakova, D.V.; Lermontova, S.A.; Grigoryev, I.S.; Muravieva, M.S.; Gavrina, A.I.; Shirmanova, M.V.; Balalaeva, I.V.; Klapshina, L.G.; Zagaynova, E.V. In vivo multimodal tumor imaging and photodynamic therapy with novel theranostic agents based on the porphyrazine framework-chelated gadolinium (III) cation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 3120–3130. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, L.N.; Lyubova, T.S.; Lermontova, S.A.; Belotelov, A.O.; Peskova, N.N.; Klapshina, L.G.; Balalaeva, I.V.; Shilyagina, N.Y. Comparative Analysis of Tetra(2-naphthyl)tetracyano-porphyrazine and Its Iron Complex as Photosensitizers for Anticancer Photodynamic Therapy. Pharmaceutics 2022, 14, 2655. [Google Scholar] [CrossRef] [PubMed]
- Balalaeva, I.V.; Mishchenko, T.A.; Turubanova, V.D.; Peskova, N.N.; Shilyagina, N.Y.; Plekhanov, V.I.; Lermontova, S.A.; Klapshina, L.G.; Vedunova, M.V.; Krysko, D.V. Cyanoarylporphyrazines with High Viscosity Sensitivity: A Step towards Dosimetry-Assisted Photodynamic Cancer Treatment. Molecules 2021, 26, 5816. [Google Scholar] [CrossRef]
- Mishchenko, T.A.; Turubanova, V.D.; Mitroshina, E.V.; Alzeibak, R.; Peskova, N.N.; Lermontova, S.A.; Klapshina, L.G.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Effect of novel porphyrazine photosensitizers on normal and tumor brain cells. J. Biophotonics 2020, 13, e201960077. [Google Scholar] [CrossRef]
- Shilyagina, N.Y.; Peskova, N.N.; Lermontova, S.A.; Brilkina, A.A.; Vodeneev, V.A.; Yakimansky, A.V.; Klapshina, L.G.; Balalaeva, I.V. Effective delivery of porphyrazine photosensitizers to cancer cells by polymer brush nanocontainers. J. Biophotonics 2017, 10, 1189–1197. [Google Scholar] [CrossRef]
- Yudintsev, A.V.; Shilyagina, N.Y.; Dyakova, D.V.; Lermontova, S.A.; Klapshina, L.G.; Guryev, E.L.; Balalaeva, I.V.; Vodeneev, V.A. Liposomal Form of Tetra(Aryl)Tetracyanoporphyrazine: Physical Properties and Photodynamic Activity In Vitro. J. Fluoresc. 2018, 28, 513–522. [Google Scholar] [CrossRef]
- Mlynarczyk, D.; Piskorz, J.; Popenda, L.; Stolarska, M.; Szczolko, W.; Konopka, K.; Jurga, S.; Sobotta, L.; Mielcarek, J.; Düzgüneş, N.; et al. S-seco-porphyrazine as a new member of the seco-porphyrazine family—Synthesis, characterization and photocytotoxicity against cancer cells. Bioorganic Chem. 2020, 96, 103634. [Google Scholar] [CrossRef]
- Yagodin, A.V.; Mikheev, I.A.; Bunin, D.A.; Sinelshchikova, A.A.; Martynov, A.G.; Gorbunova, Y.G.; Tsivadze, A.Y. Tetraquinoxalinoporphyrazine—π-extended NIR-absorbing photosensitizer with improved photostability. Dye. Pigment. 2023, 216, 111326. [Google Scholar] [CrossRef]
- Linares, I.A.; Martinelli, L.P.; Moritz, M.N.; Selistre-De-Araujo, H.S.; de Oliveira, K.T.; Perussi, J.R. Cytotoxicity of structurally-modified chlorins aimed for photodynamic therapy applications. J. Photochem. Photobiol. A Chem. 2022, 425, 113647. [Google Scholar] [CrossRef]
- Pereira, N.A.; Laranjo, M.; Pina, J.; Oliveira, A.S.; Ferreira, J.D.; Sánchez-Sánchez, C.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Piñeiro, M.; et al. Advances on photodynamic therapy of melanoma through novel ring-fused 5,15-diphenylchlorins. Eur. J. Med. Chem. 2018, 146, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, G.; Wang, L.; Cao, L.; Li, Y.; Zhao, W. Anti-tumor evaluation of a novel methoxyphenyl substituted chlorin photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 211, 112015. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.S.; Lee, T.H.; Liu, Y.; Han, K.-H.; Lee, W.K.; Yoon, I. Graphene Oxide Nanoparticles Having Long Wavelength Absorbing Chlorins for Highly-Enhanced Photodynamic Therapy with Reduced Dark Toxicity. Int. J. Mol. Sci. 2019, 20, 4344. [Google Scholar] [CrossRef] [Green Version]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, M.; Hu, X.; Du, Q.; Zhao, Z.; Jiang, Y.; Luan, Y. Nanoengineering of a newly designed chlorin e6 derivative for amplified photodynamic therapy via regulating lactate metabolism. Nanoscale 2021, 13, 11953–11962. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Ohdake, R.; Yamana, K.; Eto, T.; Sugikawa, K.; Ikeda, A. Photodynamic therapy using self-assembled nanogels comprising chlorin e6-bearing pullulan. J. Mater. Chem. B 2021, 9, 6357–6363. [Google Scholar] [CrossRef]
- Yang, J.; Teng, Y.; Fu, Y.; Zhang, C. Chlorins e6 loaded silica nanoparticles coated with gastric cancer cell membrane for tumor specific photodynamic therapy of gastric cancer. Int. J. Nanomed. 2019, 14, 5061–5071. [Google Scholar] [CrossRef] [Green Version]
- Sakamaki, Y.; Ozdemir, J.; Heidrick, Z.; Azzun, A.; Watson, O.; Tsuji, M.; Salmon, C.; Sinha, A.; Batta-Mpouma, J.; McConnell, Z.; et al. A Bioconjugated Chlorin-Based Metal–Organic Framework for Targeted Photodynamic Therapy of Triple Negative Breast and Pancreatic Cancers. ACS Appl. Bio Mater. 2021, 4, 1432–1440. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. [Google Scholar] [CrossRef]
- Yang, X.; Shi, X.; Zhang, Y.; Xu, J.; Ji, J.; Ye, L.; Yi, F.; Zhai, G. Photo-triggered self-destructive ROS-responsive nanoparticles of high paclitaxel/chlorin e6 co-loading capacity for synergetic chemo-photodynamic therapy. J. Control. Release 2020, 323, 333–349. [Google Scholar] [CrossRef]
- Laranjo, M.; Aguiar, M.C.; Pereira, N.A.; Brites, G.; Nascimento, B.F.; Brito, A.F.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Pineiro, M.; et al. Platinum(II) ring-fused chlorins as efficient theranostic agents: Dyes for tumor-imaging and photodynamic therapy of cancer. Eur. J. Med. Chem. 2020, 200, 112468. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.V.; Kustova, T.V.; Belykh, D.V.; Khudyaeva, I.S.; Berezin, D.B. Synthesis and investigation of novel chlorin sensitizers containing the myristic acid residue for antimicrobial photodynamic therapy. Dye. Pigment. 2020, 173, 107948. [Google Scholar] [CrossRef]
- Babu, B.; Sindelo, A.; Mack, J.; Nyokong, T. Thien-2-yl substituted chlorins as photosensitizers for photodynamic therapy and photodynamic antimicrobial chemotherapy. Dye. Pigment. 2020, 185, 108886. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Tanaka, M.; Sasaki, M.; Nomoto, A.; Osaki, T.; Okamoto, Y.; Yano, S. Potential of Photodynamic Therapy Based on Sugar-Conjugated Photosensitizers. J. Clin. Med. 2021, 10, 841. [Google Scholar] [CrossRef]
- Almeida, J.; Zhang, G.; Wang, M.; Queirós, C.; Cerqueira, A.F.R.; Tomé, A.C.; Barone, G.; Vicente, M.G.H.; Hey-Hawkins, E.; Silva, A.M.G.; et al. Synthesis, characterization, and cellular investigations of porphyrin– and chlorin–indomethacin conjugates for photodynamic therapy of cancer. Org. Biomol. Chem. 2021, 19, 6501–6512. [Google Scholar] [CrossRef]
- Hemelrijk, P.W.; Kwa, S.L.; van Grondelle, R.; Dekker, J.P. Spectroscopic properties of LHC-II, the main light-harvesting chlorophyll a/b protein complex from chloroplast membranes. Biochim. Biophys. Acta (BBA) Bioenerg. 1992, 1098, 159–166. [Google Scholar] [CrossRef]
- Bucks, R.R.; Boxer, S.G. Synthesis and spectroscopic properties of a novel cofacial chlorophyll-based dimer. J. Am. Chem. Soc. 1982, 104, 340–343. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Z.-L.; Chen, M. Spectroscopic Properties of Chlorophyll f. J. Phys. Chem. B 2013, 117, 11309–11317. [Google Scholar] [CrossRef]
- Niedzwiedzki, D.; Blankenship, R.E. Singlet and triplet excited state properties of natural chlorophylls and bacteriochlorophylls. Photosynth. Res. 2010, 106, 227–238. [Google Scholar] [CrossRef]
- Scherz, A.; Parson, W.W. Oligomers of bacteriochlorophyll and bacteriopheophytin with spectroscopic properties resembling those found in photosynthetic bacteria. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1984, 766, 653–665. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Co-Ord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Wu, M.; Liu, Z.; Zhang, W. An ultra-stable bio-inspired bacteriochlorin analogue for hypoxia-tolerant photodynamic therapy. Chem. Sci. 2021, 12, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Le, N.A.; Babu, V.; Kalt, M.; Schneider, L.; Schumer, F.; Spingler, B. Photostable Platinated Bacteriochlorins as Potent Photodynamic Agents. J. Med. Chem. 2021, 64, 6792–6801. [Google Scholar] [CrossRef] [PubMed]
- Otvagin, V.F.; Kuzmina, N.S.; Kudriashova, E.S.; Nyuchev, A.V.; Gavryushin, A.E.; Fedorov, A.Y. Conjugates of Porphyrinoid-Based Photosensitizers with Cytotoxic Drugs: Current Progress and Future Directions toward Selective Photodynamic Therapy. J. Med. Chem. 2022, 65, 1695–1734. [Google Scholar] [CrossRef]
- Pavlova, M.A.; Panchenko, P.A.; Alekhina, E.A.; Ignatova, A.A.; Plyutinskaya, A.D.; Pankratov, A.A.; Pritmov, D.A.; Grin, M.A.; Feofanov, A.V.; Fedorova, O.A. A New Glutathione-Cleavable Theranostic for Photodynamic Therapy Based on Bacteriochlorin e and Styrylnaphthalimide Derivatives. Biosensors 2022, 12, 1149. [Google Scholar] [CrossRef]
- Morozova, N.B.; Pavlova, M.A.; Plyutinskaya, A.D.; Pankratov, A.A.; Efendiev, K.T.; Semkina, A.S.; Pritmov, D.A.; Mironov, A.F.; Panchenko, P.A.; Fedorova, O.A. Photodiagnosis and photodynamic effects of bacteriochlorin-naphthalimide conjugates on tumor cells and mouse model. J. Photochem. Photobiol. B Biol. 2021, 223, 112294. [Google Scholar] [CrossRef]
- Zakharko, M.A.; Panchenko, P.A.; Zarezin, D.P.; Nenajdenko, V.G.; Pritmov, D.A.; Grin, M.A.; Mironov, A.F.; Fedorova, O.A. Conjugates of 3,4-dimethoxy-4-styrylnaphthalimide and bacteriochlorin for theranostics in photodynamic therapy. Russ. Chem. Bull. 2020, 69, 1169–1178. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Zakharko, M.A.; Grin, M.A.; Mironov, A.F.; Pritmov, D.A.; Jonusauskas, G.; Fedorov, Y.V.; Fedorova, O.A. Effect of linker length on the spectroscopic properties of bacteriochlorin—1,8-naphthalimide conjugates for fluorescence-guided photodynamic therapy. J. Photochem. Photobiol. A Chem. 2020, 390, 112338. [Google Scholar] [CrossRef]
- Lobo, A.C.S.; Gomes-Da-Silva, L.C.; Rodrigues-Santos, P.; Cabrita, A.; Santos-Rosa, M.; Arnaut, L.G. Immune Responses after Vascular Photodynamic Therapy with Redaporfin. J. Clin. Med. 2019, 9, 104. [Google Scholar] [CrossRef] [Green Version]
- Karwicka, M.; Pucelik, B.; Gonet, M.; Elas, M.; Dąbrowski, J.M. Effects of Photodynamic Therapy with Redaporfin on Tumor Oxygenation and Blood Flow in a Lung Cancer Mouse Model. Sci. Rep. 2019, 9, 12655. [Google Scholar] [CrossRef] [Green Version]
- Mendes, M.I.P.; Arnaut, L.G. Redaporfin Development for Photodynamic Therapy and its Combination with Glycolysis Inhibitors. Photochem. Photobiol. 2023, 99, 769–776. [Google Scholar] [CrossRef]
- Cheruku, R.R.; Cacaccio, J.; Durrani, F.A.; Tabaczynski, W.A.; Watson, R.; Siters, K.; Missert, J.R.; Tracy, E.C.; Dukh, M.; Guru, K.; et al. Synthesis, Tumor Specificity, and Photosensitizing Efficacy of Erlotinib-Conjugated Chlorins and Bacteriochlorins: Identification of a Highly Effective Candidate for Photodynamic Therapy of Cancer. J. Med. Chem. 2021, 64, 741–767. [Google Scholar] [CrossRef] [PubMed]
- Pratavieira, S.; Uliana, M.P.; Lopes, N.S.D.S.; Donatoni, M.C.; Linares, D.R.; Anibal, F.D.F.; de Oliveira, K.T.; Kurachi, C.; de Souza, C.W.O. Photodynamic therapy with a new bacteriochlorin derivative: Characterization and in vitro studies. Photodiagnosis Photodyn. Ther. 2021, 34, 102251. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Milanesio, M.E.; Fujita, H.; Lindsey, J.S.; Durantini, E.N. Bacteriochlorin-bis(spermine) conjugate affords an effective photodynamic action to eradicate microorganisms. J. Biophotonics 2020, 13, e201960061. [Google Scholar] [CrossRef]
- Tikhonov, S.; Ostroverkhov, P.; Suvorov, N.; Mironov, A.; Efimova, Y.; Plutinskaya, A.; Pankratov, A.; Ignatova, A.; Feofanov, A.; Diachkova, E.; et al. Tin Carboxylate Complexes of Natural Bacteriochlorin for Combined Photodynamic and Chemotherapy of Cancer è. Int. J. Mol. Sci. 2021, 22, 13563. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.L.M.; Barros, R.M.; Lima, D.P.D.S.; Nunes, A.M.A.; Sato, M.R.; Faccio, R.; Damasceno, B.P.G.D.L.; Oshiro-Junior, J.A. Prospective application of phthalocyanines in the photodynamic therapy against microorganisms and tumor cells: A mini-review. Photodiagnosis Photodyn. Ther. 2020, 32, 102032. [Google Scholar] [CrossRef]
- Lo, P.-C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. J. Photochem. Photobiol. B Biol. 2019, 191, 128–134. [Google Scholar] [CrossRef]
- Love, W.G.; Duk, S.; Biolo, R.; Jori, G.; Taylor, P.W. Liposome-Mediated Delivery of Photosensitizers: Localization of Zinc (11)-Phthalocyanine within Implanted Tumors after Intravenous Administration. Photochem. Photobiol. 1996, 63, 656–661. [Google Scholar] [CrossRef]
- De Simone, B.C.; Alberto, M.E.; Russo, N.; Toscano, M. Photophysical properties of heavy atom containing tetrasulfonyl phthalocyanines as possible photosensitizers in photodynamic therapy. J. Comput. Chem. 2021, 42, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Managa, M.; Nyokong, T. New type of metal-free and Zinc(II), In(III), Ga(III) phthalocyanines carrying biologically active substituents: Synthesis and photophysicochemical properties and photodynamic therapy activity. Inorg. Chim. Acta 2019, 491, 1–8. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.-G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef] [PubMed]
- Negri, L.B.; Martins, T.J.; da Silva, R.S.; Hamblin, M.R. Photobiomodulation combined with photodynamic therapy using ruthenium phthalocyanine complexes in A375 melanoma cells: Effects of nitric oxide generation and ATP production. J. Photochem. Photobiol. B Biol. 2019, 198, 111564. [Google Scholar] [CrossRef] [PubMed]
- Riega, S.D.E.; Valli, F.; Rodríguez, H.B.; Marino, J.; Roguin, L.P.; Lantaño, B.; Vior, M.C.G. Chalcogen bearing tetrasubstituted zinc (II) phthalocyanines for CT26 colon carcinoma cells photodynamic therapy. Dye. Pigment. 2022, 201, 110110. [Google Scholar] [CrossRef]
- Magadla, A.; Babu, B.; Mack, J.; Nyokong, T. Positively charged styryl pyridine substituted Zn(ii) phthalocyanines for photodynamic therapy and photoantimicrobial chemotherapy: Effect of the number of charges. Dalton Trans. 2021, 50, 9129–9136. [Google Scholar] [CrossRef]
- Ferreira, J.T.; Pina, J.; Ribeiro, C.A.F.; Fernandes, R.; Tomé, J.P.C.; Rodríguez-Morgade, M.S.; Torres, T. Highly Efficient Singlet Oxygen Generators Based on Ruthenium Phthalocyanines: Synthesis, Characterization and in vitro Evaluation for Photodynamic Therapy. Chem. A Eur. J. 2020, 26, 1789–1799. [Google Scholar] [CrossRef]
- Li, D.; Hu, Q.-Y.; Wang, X.-Z.; Li, X.; Hu, J.-Q.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. A non-aggregated silicon(IV) phthalocyanine-lactose conjugate for photodynamic therapy. Bioorganic Med. Chem. Lett. 2020, 30, 127164. [Google Scholar] [CrossRef]
- Li, K.; Dong, W.; Liu, Q.; Lv, G.; Xie, M.; Sun, X.; Qiu, L.; Lin, J. A biotin receptor-targeted silicon(IV) phthalocyanine for in vivo tumor imaging and photodynamic therapy. J. Photochem. Photobiol. B Biol. 2019, 190, 1–7. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.-Z.; Yang, L.-F.; Li, S.-C.; Hu, Q.-Y.; Li, X.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. Size-Tunable Targeting-Triggered Nanophotosensitizers Based on Self-Assembly of a Phthalocyanine–Biotin Conjugate for Photodynamic Therapy. ACS Appl. Mater. Interf. 2019, 11, 36435–36443. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Na, K. Glycyrrhetinic Acid-Modified Silicon Phthalocyanine for Liver Cancer-Targeted Photodynamic Therapy. Biomacromolecules 2021, 22, 811–822. [Google Scholar] [CrossRef]
- Openda, Y.I.; Babu, B.; Nyokong, T. Novel cationic-chalcone phthalocyanines for photodynamic therapy eradication of S. aureus and E. coli bacterial biofilms and MCF-7 breast cancer. Photodiagnosis Photodyn. Ther. 2022, 38, 102863. [Google Scholar] [CrossRef] [PubMed]
- Senapathy, G.J.; George, B.P.; Abrahamse, H. Enhancement of Phthalocyanine Mediated Photodynamic Therapy by Catechin on Lung Cancer Cells. Molecules 2020, 25, 4874. [Google Scholar] [CrossRef] [PubMed]
- Nkune, N.W.; Matlou, G.G.; Abrahamse, H. Photodynamic Therapy Efficacy of Novel Zinc Phthalocyanine Tetra Sodium 2-Mercaptoacetate Combined with Cannabidiol on Metastatic Melanoma. Pharmaceutics 2022, 14, 2418. [Google Scholar] [CrossRef] [PubMed]
- Doustvandi, M.A.; Mohammadnejad, F.; Mansoori, B.; Tajalli, H.; Mohammadi, A.; Mokhtarzadeh, A.; Baghbani, E.; Khaze, V.; Hajiasgharzadeh, K.; Moghaddam, M.M.; et al. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn. Ther. 2019, 28, 88–97. [Google Scholar] [CrossRef]
- Reis, S.R.R.D.; Helal-Neto, E.; Barros, A.O.D.S.D.; Pinto, S.R.; Portilho, F.L.; Siqueira, L.B.d.O.; Alencar, L.M.R.; Dahoumane, S.A.; Alexis, F.; Ricci-Junior, E.; et al. Dual Encapsulated Dacarbazine and Zinc Phthalocyanine Polymeric Nanoparticle for Photodynamic Therapy of Melanoma. Pharm. Res. 2021, 38, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, P.; Chen, Y.; Dong, E.; Feng, Z.; He, Z.; Zhou, C.; Wang, C.; Liu, Y.; Feng, C. Preparation of zinc phthalocyanine-loaded amphiphilic phosphonium chitosan nanomicelles for enhancement of photodynamic therapy efficacy. Colloids Surf. B Biointerfaces 2021, 202, 111693. [Google Scholar] [CrossRef]
- Rak, J.; Kabesova, M.; Benes, J.; Pouckova, P.; Vetvicka, D. Advances in Liposome-Encapsulated Phthalocyanines for Photodynamic Therapy. Life 2023, 13, 305. [Google Scholar] [CrossRef]
- Pinto, B.C.; Ambrósio, J.A.; Marmo, V.L.M.; Pinto, J.G.; Raniero, L.J.; Ferreira-Strixino, J.; Simioni, A.R.; Beltrame, M. Synthesis, characterization, and evaluation of chloroaluminium phthalocyanine incorporated in poly(ε-caprolactone) nanoparticles for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102850. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Vieira, L.G.; Horst, F.H.; de Araújo, E.C.; Ganassin, R.; Merker, C.; Meyer, T.; Böttner, J.; Venus, T.; Longo, J.P.F.; et al. Photodynamic therapy mediated by aluminium-phthalocyanine nanoemulsion eliminates primary tumors and pulmonary metastases in a murine 4T1 breast adenocarcinoma model. J. Photochem. Photobiol. B Biol. 2020, 204, 111808. [Google Scholar] [CrossRef]
- Matshitse, R.; Ngoy, B.P.; Managa, M.; Mack, J.; Nyokong, T. Photophysical properties and photodynamic therapy activities of detonated nanodiamonds-BODIPY-phthalocyanines nanoassemblies. Photodiagnosis Photodyn. Ther. 2019, 26, 101–110. [Google Scholar] [CrossRef]
- Matshitse, R.; Nwaji, N.; Managa, M.; Chen, Z.-L.; Nyokong, T. Photodynamic therapy characteristics of phthalocyanines in the presence of boron doped detonation nanodiamonds: Effect of symmetry and charge. Photodiagnosis Photodyn. Ther. 2022, 37, 102705. [Google Scholar] [CrossRef] [PubMed]
- Oshiro-Junior, J.A.; Sato, M.; Boni, F.I.; Santos, K.L.M.; de Oliveira, K.T.; de Freitas, L.M.; Fontana, C.R.; Nicholas, D.; McHale, A.P.; Callan, J.F.; et al. Phthalocyanine-loaded nanostructured lipid carriers functionalized with folic acid for photodynamic therapy. Mater. Sci. Eng. C 2020, 108, 110462. [Google Scholar] [CrossRef]
- Liang, X.; Xie, Y.; Wu, J.; Wang, J.; Petković, M.; Stepić, M.; Zhao, J.; Ma, J.; Mi, L. Functional titanium dioxide nanoparticle conjugated with phthalocyanine and folic acid as a promising photosensitizer for targeted photodynamic therapy in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2021, 215, 112122. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of Cyanine Derived Dyes in Photodynamic Therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Delaey, E.; van Laar, F.; De Vos, D.; Kamuhabwa, A.; Jacobs, P.; de Witte, P. A comparative study of the photosensitizing characteristics of some cyanine dyes. J. Photochem. Photobiol. B Biol. 2000, 55, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.L.; Maia, A.; Santos, A.O.; Lima, E.; Reis, L.V.; Nunes, M.J.; Boto, R.E.F.; Silvestre, S.; Almeida, P. An Insight into Symmetrical Cyanine Dyes as Promising Selective Antiproliferative Agents in Caco-2 Colorectal Cancer Cells. Molecules 2022, 27, 5779. [Google Scholar] [CrossRef]
- Ebaston, T.; Nakonechny, F.; Talalai, E.; Gellerman, G.; Patsenker, L. Iodinated xanthene-cyanine NIR dyes as potential photosensitizers for antimicrobial photodynamic therapy. Dye. Pigment. 2021, 184, 108854. [Google Scholar] [CrossRef]
- Thankarajan, E.; Walunj, D.; Bazylevich, A.; Prasad, C.; Hesin, A.; Patsenker, L.; Gellerman, G. A novel, dual action chimera comprising DNA methylating agent and near-IR xanthene-cyanine photosensitizer for combined anticancer therapy. Photodiagnosis Photodyn. Ther. 2022, 37, 102722. [Google Scholar] [CrossRef]
- Martins, T.D.; Lima, E.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Almeida, P.; Ferreira, L.F.V.; Silva, A.M.; Reis, L.V. Red and Near-Infrared Absorbing Dicyanomethylene Squaraine Cyanine Dyes: Photophysicochemical Properties and Anti-Tumor Photosensitizing Effects. Materials 2020, 13, 2083. [Google Scholar] [CrossRef]
- Lima, E.; Reis, L.V. ‘Lights, squaraines, action!’—The role of squaraine dyes in photodynamic therapy. Futur. Med. Chem. 2022, 14, 1375–1402. [Google Scholar] [CrossRef]
- Lynch, D.E.; Hamilton, D.G. Croconaine Dyes—The Lesser Known Siblings of Squaraines. Eur. J. Org. Chem. 2017, 2017, 3897–3911. [Google Scholar] [CrossRef]
- Usama, S.M.; Thavornpradit, S.; Burgess, K. Optimized Heptamethine Cyanines for Photodynamic Therapy. ACS Appl. Bio Mater. 2018, 1, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, T.; Zhang, H.; Xie, X.; Li, Z.; Chen, H.; Gao, Y. Folate and Heptamethine Cyanine Modified Chitosan-Based Nanotheranostics for Tumor Targeted Near-Infrared Fluorescence Imaging and Photodynamic Therapy. Biomacromolecules 2017, 18, 2146–2160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jin, H.; Gao, Y.; Lin, J.; Yang, H.; Yang, S.-P. Photostable Iridium(III)–Cyanine Complex Nanoparticles for Photoacoustic Imaging Guided Near-Infrared Photodynamic Therapy in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 15417–15425. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bai, J.; Qian, Y. The investigation of unique water-soluble heptamethine cyanine dye for use as NIR photosensitizer in photodynamic therapy of cancer cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117702. [Google Scholar] [CrossRef] [PubMed]
- Maltanava, H.; Belko, N.; Lugovski, A.; Brezhneva, N.; Bondarenko, E.; Chulkin, P.; Gusakov, G.; Vileishikova, N.; Samtsov, M.; Poznyak, S. Spectroelectrochemical and ESR investigation of free radicals derived from indotricarbocyanine dyes for photodynamic therapy. Dye. Pigment. 2022, 205, 110599. [Google Scholar] [CrossRef]
- Liu, H.; Yin, J.; Xing, E.; Du, Y.; Su, Y.; Feng, Y.; Meng, S. Halogenated cyanine dyes for synergistic photodynamic and photothermal therapy. Dye. Pigment. 2021, 190, 109327. [Google Scholar] [CrossRef]
- Thomas, A.P.; Palanikumar, L.; Jeena, M.T.; Kim, K.; Ryu, J.-H. Cancer-mitochondria-targeted photodynamic therapy with supramolecular assembly of HA and a water soluble NIR cyanine dye. Chem. Sci. 2017, 8, 8351–8356. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Zhang, C.; Tian, J.; Tian, Q.; Huang, G.; Zhang, W. A Cascade BIME-Triggered Near-IR Cyanine Nanoplatform for Enhanced Antibacterial Photodynamic Therapy. ACS Appl. Mater. Interfaces 2023, 15, 10520–10528. [Google Scholar] [CrossRef]
- James, N.S.; Cheruku, R.R.; Missert, J.R.; Sunar, U.; Pandey, R.K. Measurement of Cyanine Dye Photobleaching in Photosensitizer Cyanine Dye Conjugates Could Help in Optimizing Light Dosimetry for Improved Photodynamic Therapy of Cancer. Molecules 2018, 23, 1842. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ma, D.; Liu, Q.; Huang, D.; Zhao, X.; Yao, Q.; Xiong, T.; Long, S.; Du, J.; Fan, J.; et al. Enhancing Intersystem Crossing by Intermolecular Dimer-Stacking of Cyanine as Photosensitizer for Cancer Therapy. CCS Chem. 2022, 4, 3627–3636. [Google Scholar] [CrossRef]
- Kobzev, D.; Semenova, O.; Tatarets, A.; Bazylevich, A.; Gellerman, G.; Patsenker, L. Antibody-guided iodinated cyanine for near-IR photoimmunotherapy. Dye. Pigment. 2023, 212, 111101. [Google Scholar] [CrossRef]
- Debnath, S.; Agarwal, A.; Kumar, N.R.; Bedi, A. Selenium-Based Drug Development for Antioxidant and Anticancer Activity. Futur. Pharmacol. 2022, 2, 595–607. [Google Scholar] [CrossRef]
- Sun, J.; Feng, E.; Shao, Y.; Lv, F.; Wu, Y.; Tian, J.; Sun, H.; Song, F. A Selenium-Substituted Heptamethine Cyanine Photosensitizer for Near-Infrared Photodynamic Therapy. Chembiochem 2022, 23, e202200421. [Google Scholar] [CrossRef]
- Siriwibool, S.; Kaekratoke, N.; Chansaenpak, K.; Siwawannapong, K.; Panajapo, P.; Sagarik, K.; Noisa, P.; Lai, R.-Y.; Kamkaew, A. Near-Infrared Fluorescent pH Responsive Probe for Targeted Photodynamic Cancer Therapy. Sci. Rep. 2020, 10, 1283. [Google Scholar] [CrossRef] [Green Version]
- Semenova, O.; Kobzev, D.; Yazbak, F.; Nakonechny, F.; Kolosova, O.; Tatarets, A.; Gellerman, G.; Patsenker, L. Unexpected effect of iodine atoms in heptamethine cyanine dyes on the photodynamic eradication of Gram-positive and Gram-negative pathogens. Dye. Pigment. 2021, 195, 109745. [Google Scholar] [CrossRef]
- Pontremoli, C.; Chinigò, G.; Galliano, S.; Plata, M.M.; Dereje, D.; Sansone, E.; Gilardino, A.; Barolo, C.; Pla, A.F.; Visentin, S.; et al. Photosensitizers for photodynamic therapy: Structure-activity analysis of cyanine dyes through design of experiments. Dye. Pigment. 2023, 210, 111047. [Google Scholar] [CrossRef]
- Ciubini, B.; Visentin, S.; Serpe, L.; Canaparo, R.; Fin, A.; Barbero, N. Design and synthesis of symmetrical pentamethine cyanine dyes as NIR photosensitizers for PDT. Dye. Pigment. 2019, 160, 806–813. [Google Scholar] [CrossRef]
- Shi, H.; Tan, X.; Wang, P.; Qin, J. A novel near-infrared trifluoromethyl heptamethine cyanine dye with mitochondria-targeting for integration of collaborative treatment of photothermal and sonodynamic therapy. Mater. Today Adv. 2022, 14, 100251. [Google Scholar] [CrossRef]
- Li, M.; Sun, W.; Tian, R.; Cao, J.; Tian, Y.; Gurram, B.; Fan, J.; Peng, X. Smart J-aggregate of cyanine photosensitizer with the ability to target tumor and enhance photodynamic therapy efficacy. Biomaterials 2020, 269, 120532. [Google Scholar] [CrossRef]
- Lima, E.; Barroso, A.G.; Sousa, M.A.; Ferreira, O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Santos, A.O.; Reis, L.V. Picolylamine-functionalized benz[e]indole squaraine dyes: Synthetic approach, characterization and in vitro efficacy as potential anticancer phototherapeutic agents. Eur. J. Med. Chem. 2022, 229, 114071. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Barroso, A.G.; Ferreira, O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Santos, A.O.; Reis, L.V. Benz[e]indole-bearing aminosquaraine dyes: Which of the amines introduced into the squaric ring will be able to induce the best in vitro photodynamic effect? Dye. Pigment. 2023, 215, 111239. [Google Scholar] [CrossRef]
- Li, J.-H.; You, P.-D.; Lu, F.; Huang, J.-T.; Fu, J.-L.; Tang, H.-Y.; Zhou, C.-Q. Single aromatics sulfonamide substituted dibenzothiazole squaraines for tumor NIR imaging and efficient photodynamic therapy at low drug dose. J. Photochem. Photobiol. B Biol. 2023, 240, 112653. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Ferreira, O.; Gomes, V.S.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Synthesis and in vitro evaluation of the antitumoral phototherapeutic potential of squaraine cyanine dyes derived from indolenine. Dye. Pigment. 2019, 167, 98–108. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, Y.; Blum, N.T.; Huang, P.; Lin, J. Recent Advances in Croconaine Dyes for Bioimaging and Theranostics. Bioconjugate Chem. 2020, 31, 2072–2084. [Google Scholar] [CrossRef]
- Liu, N.; Gujrati, V.; Malekzadeh-Najafabadi, J.; Werner, J.P.F.; Klemm, U.; Tang, L.; Chen, Z.; Prakash, J.; Huang, Y.; Stiel, A.; et al. Croconaine-based nanoparticles enable efficient optoacoustic imaging of murine brain tumors. Photoacoustics 2021, 22, 100263. [Google Scholar] [CrossRef]
- Alejo, T.; Font, C.; Abad, M.; Andreu, V.; Sebastian, V.; Mendoza, G.; Arruebo, M. Spatiotemporal control of photothermal heating using pH sensitive near-infrared croconaine-based dyes. J. Photochem. Photobiol. A Chem. 2019, 382, 111936. [Google Scholar] [CrossRef]
- Wen, Y.; McGarraugh, H.H.; Schreiber, C.L.; Smith, B.D. Cell organelle targeting of near-infrared croconaine dye controls photothermal outcome. Chem. Commun. 2020, 56, 6977–6980. [Google Scholar] [CrossRef]
- Sun, R.; Ma, W.; Ling, M.; Tang, C.; Zhong, M.; Dai, J.; Zhu, M.; Cai, X.; Li, G.; Xu, Q.; et al. pH-activated nanoplatform for visualized photodynamic and ferroptosis synergistic therapy of tumors. J. Control. Release 2022, 350, 525–537. [Google Scholar] [CrossRef]
- Vara, J.; Gualdesi, M.S.; Bertolotti, S.G.; Ortiz, C.S. Two phenothiazine dyes as photosensitizers for the production of singlet oxygen. Photophysics, photochemistry and effects of aggregation. J. Mol. Struct. 2019, 1181, 1–7. [Google Scholar] [CrossRef]
- Padnya, P.; Khadieva, A.; Stoikov, I. Current achievements and perspectives in synthesis and applications of 3,7-disubstituted phenothiazines as Methylene Blue analogues. Dye. Pigment. 2023, 208, 110806. [Google Scholar] [CrossRef]
- Vara, J.; Gualdesi, M.S.; Aiassa, V.; Ortiz, C.S. Evaluation of physicochemical properties and bacterial photoinactivation of phenothiazine photosensitizers. Photochem. Photobiol. Sci. 2019, 18, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Dong, L.; He, X.; Zhao, C.; Zhang, W.; Li, X.; Lu, Y. Treatment of infected wounds with methylene blue photodynamic therapy: An effective and safe treatment method. Photodiagnosis Photodyn. Ther. 2020, 32, 102051. [Google Scholar] [CrossRef]
- Barbosa, A.F.S.; Santos, I.P.; Santos, G.M.P.; Bastos, T.M.; Rocha, V.P.C.; Meira, C.S.; Soares, M.B.P.; Pitta, I.R.; Pinheiro, A.L.B. Anti–Trypanosoma cruzi effect of the photodynamic antiparasitic chemotherapy using phenothiazine derivatives as photosensitizers. Lasers Med. Sci. 2019, 35, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, L.H.; da Rocha, T.E.; Wainwright, M.; Nuernberg, M.A.A.; Ervolino, E.; Souza, E.Q.M.; Brandini, D.A.; Garcia, V.G. Comparative effects of different phenothiazine photosensitizers on experimental periodontitis treatment. Photodiagnosis Photodyn. Ther. 2021, 34, 102198. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsieh, C.-F.; Wu, H.-J.; Ameen, M.; Hung, T.-P. Investigation of Sonosensitizers Based on Phenothiazinium Photosensitizers. Appl. Sci. 2022, 12, 7819. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wang, R.; Rong, X.; Liu, T.; Xia, X.; Fan, J.; Sun, W.; Peng, X. A glutathione activatable pro-drug-photosensitizer for combined chemotherapy and photodynamic therapy. Chin. Chem. Lett. 2022, 33, 4583–4586. [Google Scholar] [CrossRef]
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-Based Molecules, a Platform for Photonic and Solar Cells. Molecules 2020, 26, 153. [Google Scholar] [CrossRef]
- Ray, C.; Schad, C.; Moreno, F.; Maroto, B.L.; Bañuelos, J.; Arbeloa, T.; García-Moreno, I.; Villafuerte, C.; Muller, G.; de la Moya, S. BCl3-Activated Synthesis of COO-BODIPY Laser Dyes: General Scope and High Yields under Mild Conditions. J. Org. Chem. 2020, 85, 4594–4601. [Google Scholar] [CrossRef]
- Mei, Y.; Li, H.; Song, C.-Z.; Chen, X.-G.; Song, Q.-H. An 8-arylselenium BODIPY fluorescent probe for rapid and sensitive discrimination of biothiols in living cells. Chem. Commun. 2021, 57, 10198–10201. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, G.; Peng, C.; Han, X.; Duan, L.; Cheng, T. BODIPY-based fluorescent probe for selective detection of HSA in urine. Dye. Pigment. 2022, 197, 109915. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, G.; Hu, Z.; Zhu, C.; Chen, Y.; James, T.D.; Ma, L.; Wang, Z. A BODIPY-based probe for amyloid-β imaging in vivo. Org. Chem. Front. 2023, 10, 1903–1909. [Google Scholar] [CrossRef]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and controlled photosensitization with BODIPY dyes. Coordin. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Qi, S.; Kwon, N.; Yim, Y.; Nguyen, V.-N.; Yoon, J. Fine-tuning the electronic structure of heavy-atom-free BODIPY photosensitizers for fluorescence imaging and mitochondria-targeted photodynamic therapy. Chem. Sci. 2020, 11, 6479–6484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Zhang, D.; Wang, J.; Sun, C.; Cui, T.; Xu, Z.; Jiang, X.-D.; Du, J. Near-infrared upper phenyl-fused BODIPY as a photosensitizer for photothermal–photodynamic therapy. J. Mater. Chem. B 2022, 10, 3048–3054. [Google Scholar] [CrossRef]
- Gorbe, M.; Costero, A.M.; Sancenón, F.; Martínez-Máñez, R.; Ballesteros-Cillero, R.; Ochando, L.E.; Chulvi, K.; Gotor, R.; Gil, S. Halogen-containing BODIPY derivatives for photodynamic therapy. Dye. Pigment. 2018, 160, 198–207. [Google Scholar] [CrossRef]
- Miao, X.; Hu, W.; He, T.; Tao, H.; Wang, Q.; Chen, R.; Jin, L.; Zhao, H.; Lu, X.; Fan, Q.; et al. Deciphering the intersystem crossing in near-infrared BODIPY photosensitizers for highly efficient photodynamic therapy. Chem. Sci. 2019, 10, 3096–3102. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zhou, J.; Ji, X.; Lin, G.; Xu, S.; Dong, X.; Zhao, W. Discovery of a Monoiodo Aza-BODIPY Near-Infrared Photosensitizer: In vitro and in vivo Evaluation for Photodynamic Therapy. J. Med. Chem. 2020, 63, 9950–9964. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Zheng, X.; Yang, L.; Song, N.; Zhang, L.; Chen, L.; Xie, Z. Heavy atom substituted near-infrared BODIPY nanoparticles for photodynamic therapy. Dye. Pigment. 2020, 178, 108348. [Google Scholar] [CrossRef]
- Aksakal, N.E.; Eçik, E.T.; Kazan, H.H.; Çiftçi, G.Y.; Yuksel, F. Novel ruthenium(II) and iridium(III) BODIPY dyes: Insights into their application in photodynamic therapy in vitro. Photochem. Photobiol. Sci. 2019, 18, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Kundu, P.; Paul, S.; Kondaiah, P.; Chakravarty, A.R. Cobalt(III) Complexes for Light-Activated Delivery of Acetylacetonate-BODIPY, Cellular Imaging, and Photodynamic Therapy. Inorg. Chem. 2022, 61, 6837–6851. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kundu, P.; Kondaiah, P.; Chakravarty, A.R. BODIPY-Ruthenium(II) Bis-Terpyridine Complexes for Cellular Imaging and Type-I/-II Photodynamic Therapy. Inorg. Chem. 2021, 60, 16178–16193. [Google Scholar] [CrossRef]
- Callaghan, S.; Filatov, M.A.; Savoie, H.; Boyle, R.W.; Senge, M.O. In vitro cytotoxicity of a library of BODIPY-anthracene and -pyrene dyads for application in photodynamic therapy. Photochem. Photobiol. Sci. 2019, 18, 495–504. [Google Scholar] [CrossRef]
- Gündüz, E.Ö.; Gedik, M.E.; Günaydın, G.; Okutan, E. Amphiphilic Fullerene-BODIPY Photosensitizers for Targeted Photodynamic Therapy. ChemMedChem 2022, 17, e202100693. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lei, X.; Ren, H.; Zheng, S.; Qiang, J.; Zhang, Z.; Chen, Y.; Wei, T.; Wang, F.; Chen, X. PEGylated Dimeric BODIPY Photosensitizers as Nanocarriers for Combined Chemotherapy and Cathepsin B-Activated Photodynamic Therapy in 3D Tumor Spheroids. ACS Appl. Bio Mater. 2020, 3, 3835–3845. [Google Scholar] [CrossRef]
- Prieto-Castañeda, A.; García-Garrido, F.; Díaz-Norambuena, C.; Escriche-Navarro, B.; García-Fernández, A.; Bañuelos, J.; Rebollar, E.; García-Moreno, I.; Martínez-Máñez, R.; de la Moya, S.; et al. Development of Geometry-Controlled All-Orthogonal BODIPY Trimers for Photodynamic Therapy and Phototheragnosis. Org. Lett. 2022, 24, 3636–3641. [Google Scholar] [CrossRef]
- Badon, I.W.; Jee, J.-P.; Vales, T.P.; Kim, C.; Lee, S.; Yang, J.; Yang, S.K.; Kim, H.-J. Cationic BODIPY Photosensitizers for Mitochondrion-Targeted Fluorescence Cell-Imaging and Photodynamic Therapy. Pharmaceutics 2023, 15, 1512. [Google Scholar] [CrossRef]
- Badon, I.W.; Kim, C.; Lim, J.M.; Mai, D.K.; Vales, T.P.; Kang, D.; Cho, S.; Lee, J.; Kim, H.-J.; Yang, J. Mitochondrion-targeting PEGylated BODIPY dyes for near-infrared cell imaging and photodynamic therapy. J. Mater. Chem. B 2022, 10, 1196–1209. [Google Scholar] [CrossRef]
- Masood, M.A.; Wu, Y.; Chen, Y.; Yuan, H.; Sher, N.; Faiz, F.; Yao, S.; Qi, F.; Khan, M.I.; Ahmed, M.; et al. Optimizing the photodynamic therapeutic effect of BODIPY-based photosensitizers against cancer and bacterial cells. Dye. Pigment. 2022, 202, 110255. [Google Scholar] [CrossRef]
- Bai, J.; Qian, Y. Construction of an NIR and lysosome-targeted quinoline-BODIPY photosensitizer and its application in photodynamic therapy for human gastric carcinoma cells. Dye. Pigment. 2020, 181, 108615. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Li, H.; Yang, M.; Lv, J.; Zhou, Y.; Yuan, Z.; Li, X. A near-infrared and lysosome-targeted BODIPY photosensitizer for photodynamic and photothermal synergistic therapy. Org. Biomol. Chem. 2023, 21, 4672–4682. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, Y. A novel BODIPY-based photosensitizer with pH-active singlet oxygen generation for photodynamic therapy in lysosomes. Org. Biomol. Chem. 2019, 17, 8001–8007. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, W.; Liu, X.; Wang, S.; Wang, Y. BODIPY-Based Fluorescent Surfactant for Cell Membrane Imaging and Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Wang, H.; Chen, Z.; Dong, X.; Zhao, W.; Shi, Y.; Zhu, Q. Discovery of an Amino Acid-Modified Near-Infrared Aza-BODIPY Photosensitizer as an Immune Initiator for Potent Photodynamic Therapy in Melanoma. J. Med. Chem. 2022, 65, 3616–3631. [Google Scholar] [CrossRef]
- Li, C.; Lin, W.; Liu, S.; Zhang, W.; Xie, Z. Self-destructive PEG–BODIPY nanomaterials for photodynamic and photothermal therapy. J. Mater. Chem. B 2019, 7, 4655–4660. [Google Scholar] [CrossRef]
- Song, W.; Liu, H.; Wang, S.; Zhi, X.; Shen, Z. Photodynamically inactive prodrug based-on leuco-BODIPY: In vivo tumor targeting and microenvironment activated photodynamic therapy. J. Photochem. Photobiol. A Chem. 2023, 435, 114319. [Google Scholar] [CrossRef]
- Mai, D.K.; Kim, C.; Lee, J.; Vales, T.P.; Badon, I.W.; De, K.; Cho, S.; Yang, J.; Kim, H.-J. BODIPY nanoparticles functionalized with lactose for cancer-targeted and fluorescence imaging-guided photodynamic therapy. Sci. Rep. 2022, 12, 2541. [Google Scholar] [CrossRef]
- Khuong Mai, D.; Kang, B.; Pegarro Vales, T.; Badon, I.W.; Cho, S.; Lee, J.; Kim, E.; Kim, H.-J. Synthesis and Photophysical Properties of Tumor-Targeted Water-Soluble BODIPY Photosensitizers for Photodynamic Therapy. Molecules 2020, 25, 3340. [Google Scholar] [CrossRef]
- Durán-Sampedro, G.; Xue, E.Y.; Moreno-Simoni, M.; Paramio, C.; Torres, T.; Ng, D.K.P.; de la Torre, G. Glycosylated BODIPY- Incorporated Pt(II) Metallacycles for Targeted and Synergistic Chemo-Photodynamic Therapy. J. Med. Chem. 2023, 66, 3448–3459. [Google Scholar] [CrossRef]
- Chen, B.; Cao, J.; Zhang, K.; Zhang, Y.-N.; Lu, J.; Iqbal, M.Z.; Zhang, Q.; Kong, X. Synergistic photodynamic and photothermal therapy of BODIPY-conjugated hyaluronic acid nanoparticles. J. Biomater. Sci. Polym. Ed. 2021, 32, 2028–2045. [Google Scholar] [CrossRef]
- Sepehrpour, H.; Fu, W.; Sun, Y.; Stang, P.J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Miao, Y.; Zheng, D.; Li, X.; Liu, D.; Song, F. Self-Assembled Platinum Supramolecular Metallacycles Based on a Novel TADF Photosensitizer for Efficient Cancer Photochemotherapy. Mol. Pharm. 2021, 18, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, F.; Yu, X.; Wang, H.; Qiu, H.; Li, Y.; Yin, S.; Stang, P.J. Phenylthiol-BODIPY-based supramolecular metallacycles for synergistic tumor chemo-photodynamic therapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2203994119. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. Int. J. Mol. Sci. 2022, 23, 10198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ahmed, A.; Cong, H.; Wang, S.; Shen, Y.; Yu, B. Application of multifunctional BODIPY in photodynamic therapy. Dye. Pigment. 2020, 185, 108937. [Google Scholar] [CrossRef]
- Chang, Z.; Ye, J.-H.; Qi, F.; Fang, H.; Lin, F.; Wang, S.; Mu, C.; Zhang, W.; He, W. A PEGylated photosensitizer-core pH-responsive polymeric nanocarrier for imaging-guided combination chemotherapy and photodynamic therapy. New J. Chem. 2021, 45, 6180–6185. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, W.; Liu, S.; Li, Z.; Hu, X.; Xie, Z.; Duan, C.; Han, G. Engineering pH-Responsive BODIPY Nanoparticles for Tumor Selective Multimodal Imaging and Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 43928–43935. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-J.; Zhang, M.-S.; Li, X.-Q.; Yang, D.-C.; Xu, G.; Liu, J.-Y. A glutathione-responsive photosensitizer with fluorescence resonance energy transfer characteristics for imaging-guided targeting photodynamic therapy. Eur. J. Med. Chem. 2020, 193, 112203. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, N.; Chen, L.; Xie, Z. Reduction responsive BODIPY decorated mesoporous silica nanoscale platforms for photodynamic therapy. Microporous Mesoporous Mater. 2021, 311, 110689–110698. [Google Scholar] [CrossRef]
- Shi, H.; Sun, W.; Liu, C.; Gu, G.; Ma, B.; Si, W.; Fu, N.; Zhang, Q.; Huang, W.; Dong, X. Tumor-targeting, enzyme-activated nanoparticles for simultaneous cancer diagnosis and photodynamic therapy. J. Mater. Chem. B 2016, 4, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Shim, I.; An, J.; Ji, M.S.; Jangili, P.; Chi, S.-G.; Kim, J.S. Phenylthiourea-Conjugated BODIPY as an Efficient Photosensitizer for Tyrosinase-Positive Melanoma-Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 2120–2127. [Google Scholar] [CrossRef] [PubMed]











| Light Source | Description | References |
|---|---|---|
| Sun light | The use of sunlight as a treatment for dermatological conditions such as actinic keratosis is a cost-free and less painful option that can be easily performed at home. However, it is highly dependent on the weather conditions of the region, and there is no way to regulate the amount of energy emitted. | [88,89] |
| Lamp lights | Lamp lights are cheap, portable, easy to use, and emit at a wide range of wavelengths. In addition, filters eliminate radiation emitted without interest in the excitation of the photosensitizer, namely at short wavelengths. However, they have the disadvantage of losing energy in the form of heat, which is why they emit low-intensity light and are mainly limited to treating dermatological diseases. | [84] |
| Light-emitting diodes (LEDs) | LEDs are light sources with high power stability, capable of irradiating large areas, thermally non-destructive, inexpensive, small in size, and easily transportable. Combining lamps emitting at different wavelengths to excite several photosensitizers is possible with the same equipment. The light is incoherent and polychromatic, emitting in a narrow region of the electromagnetic spectrum. | [90,91] |
| Lasers | Lasers are currently the most commonly used clinically and produce high-intensity, coherent monochromatic light. The coherence contributes to the precise control of the fluence applied to the target tissue, which is more difficult to assess using incoherent sources. They can be coupled to optical fibers to deliver light to highly inaccessible tissues. It is the most expensive light source compared to those above. Comparative studies show comparable photodynamic efficacy using lasers or LEDs. | [78,84,92,93] |
| Property | Description | References |
|---|---|---|
| Easy synthesis | Optimizing PS synthesis is crucial for high yields and purity, affecting production scale and cost. | [3,113] |
| Absorption in the visible and near-infrared regions | Low-energy radiation reduces harm to healthy tissues and increases light permeability in biological tissues for deeper activation of PS. | |
| Amphiphilicity | Amphiphilic compounds are water-soluble and can easily cross the lipid membranes of cells, ensuring their availability and distribution, allowing the PS to target and accumulate in abnormal tissues more efficiently. | [123] |
| Various routes of administration | The PS or its formulation must allow safe and painless administration, whether performed orally, topically, or intravenously. In addition, several administration routes will enable the use of the same molecule in a broader range of diseases. | [124] |
| Selective tumor accumulation | It should be able to reach the neoplasia in a short time as well as selectively accumulate in tumor cells. | [125] |
| Harmless in the absence of light | Molecules that intrinsically have cytotoxicity should not be studied as potential PSs. To be applied in PDT, photosensitizing candidates must exhibit zero to low toxicity in the dark and significant cytotoxicity only when irradiated. | [123,126] |
| Resistance to photobleaching | The term “photobleaching” refers to the loss of the ability of the PS to absorb light due to its degradation during irradiation. Resistance to this phenomenon allows the use of higher energy light sources, which may result in deeper tissue penetration and greater therapeutic efficacy. | [127,128] |
| Long-term triplet state lifetime | The triplet state PS reacts with triplet molecular oxygen to produce ROS for type I and II PSs. Long-term triplet state lifetime enables a prolonged generation of cytotoxic species, which is crucial for the death of target cells. | [129] |
| Rapid clearance from the body | A rapidly cleared PS from the organism significantly decreases the duration of its presence in the body, thereby minimizing the risk of toxicity to healthy tissue and reducing the risk of side effects such as skin photosensitivity. | [126] |
| Structural Variation | Specification | Main Aspects and Conclusions | References |
|---|---|---|---|
| Metal coordination | Zn, In and GaPcs | Various metals were introduced into benzimidazole tetrasubstituted Pcs. The In-complexed one showed highly efficient singlet oxygen generation and in vitro phototoxicity. | [218] |
| RuPcs | Ruthenium complexation can create effective Pcs for PDT, known for its medicinal properties. In addition, loading NO+ and NO2− units onto metal allows the release of nitric oxide, boosting light toxicity and causing selective effects seen in vitro. | [219,220] | |
| Presence of chalcogen atoms | O, S, Se | Bathochromic shifts were observed for S and Se-bearing Pcs. The Se proved beneficial in increasing absorptivity and singlet oxygen production. PDT effects were observed for both derivatives, but the first derivative showed greater efficiency in vitro. | [221] |
| Charges number | 0, 4 or 8 positive charges | Improved properties (singlet oxygen production, water solubility, and cellular uptake) were perceived for cationic compounds compared to neutral ones, and lower half-maximal inhibitory values were observed for eight times positively charged compounds for MCF-7 cells. | [222] |
| Functionalization with biomolecules | Sugar units | Ruthenium-complexed Pcs were functionalized with glucose, galactose, and mannose. Adding sugar did not enhance cellular uptake, but one-bearing mannose proved better for PDT use. | [223] |
| Lactose Si-complexed Pcs proved to be biocompatible, stable in aqueous media, and efficient in ROS generation, showing in vitro high photocytotoxicity and selective tumor accumulation in vivo. | [224] | ||
| Biotin | Biotin-PEG-bearing SiPc has been shown to selectively accumulate in tumor tissue and reduce biotin receptor-overexpressed tumor growth progression in vivo. In addition, PEG units made the potential PS more soluble in water and less prone to aggregation. | [225] | |
| It was perceived that self-assembled biotin-amine SiPc of different sizes (10, 20, 40, and 90 nm), depending on the percentage of surfactant, exhibited targeting and improved photoactivity in vivo, especially for the 20 nm particles after avidin-presence disassembly. | [226] | ||
| Glycyrrhetinic acid | The interest in conjugating glycyrrhetinic acid to SiPc is due to its overexpressed receptors in liver cancer. The PS candidate was shown to effectively destroy the liver tumor tissue via necrosis and apoptosis. Side effects have not been observed in vivo. | [227] | |
| Chalcones | Chalcone-bearing cationic Zn- and In-complexed Pcs saw, for MCF-7 breast adenocarcinoma cells, half-maximal inhibitory concentration values reduced to less than half after irradiation. Despite its promise, mechanisms of action still need to be studied. | [228] |
| Aim | How? | Main Aspects and Conclusions | References |
|---|---|---|---|
| Long-wavelength absorption | Conjugated double bonds and heavy atom introduction | The length of π-conjugate bonds influences the ability to absorb at redshifted lengths, which is why authors have invested in the synthesis of thiophene- and phenyl-fused BODIPYs, in the introduction of pyrrole rings, and, alternatively, in heavy atoms. | [292,293,294] |
| Improve singlet oxygen production ability | Halogenation | The inefficient intersystem crossing (ISC) of BODIPYs limits their usefulness in PDT. As for most PS classes, halogenation is a way to improve this photophysical property. Introducing iodine atoms helps the ISC in a more pronounced way than bromination. | [295,296,297] |
| Organic metal complexes | Aksakal et al. examined new ruthenium and iridium BODIPY complexes. They discovered that singlet oxygen generation increased by twenty times with ruthenium. Meanwhile, Jana et al. achieved quantum yields of 67% for a cobalt-complexed dye. These and other findings suggest a connection between these complexes and the singlet oxygen production ability. | [298,299,300] | |
| Anthracene, pyrene, and fullerene conjugation | Callaghan et al. studied several BODIPY dyes with diverse anthracenes and the pyrene group, all showing greater ISC than the phenyl group bearing one. Fullerene derivatives are also effective PSs, producing ROS efficiently with good biocompatibility and easy body clearance. | [301,302] | |
| Sulfur core-fusion | Thiophene-fused BODIPYs have shown improved singlet oxygen quantum yields (from 4% to 85%). Oscillations in its ability to carry out type II reactions were due to different functional groups introduced in its meso position. | [292] | |
| BODIPY dimers and trimers | Combining several BODIPY units covalently linked in the same structure can influence properties, namely the increase in the production capacity of singlet oxygen. This effect is shown by Lu et al. for dimeric dyes and by Prieto-Castañeda et al., whose molecular geometry of analogous trimers is also shown to influence this photophysical property. | [303,304] | |
| Targeting specific organelles | Cationic character | Cationic BODIPYs can become more hydrophilic and gain the ability to target mitochondria. They are attracted to the negatively charged inner mitochondrial membrane, accumulating in the mitochondrial matrix through a concentration gradient. | [305,306,307] |
| Morpholine group introduction | The introduction of the morpholine into the core of BODIPYs induces their directing to the lysosome organelle. This finding was observed by several research groups in BODIPY dyes, as this group was introduced to the dyes through a linker. | [308,309,310] | |
| Amphiphilicity and biomolecule mimetize | Wang et al. have designed a phospholipid-mimetizing BODIPY-based fluorescent surfactant with two hydrophobic chains for cell membrane imaging and PDT. Concerning the latter, its effect is induced by damaging the tumor cells’ cytoplasmic membrane. | [311] | |
| Enhance biocompatibility | Aminoacid functionalization | Amino acid conjugation is a practical approach to improving aqueous solubility and ameliorating their biocompatibility and photobiological efficacy. For example, the aspartic acid-modified BODIPY reported by Yu et al. exhibited enhanced aqueous solubility, singlet oxygen generation ability, and a good phototoxicity ratio. | [312] |
| Polymer-junction self-assembly | To enhance the biocompatibility of BODIPYs, these molecules can be modified by attaching PEG derivatives or other hydrophilic polymers. This modification helps to reduce non-specific interactions with biomolecules and improves the stability of self-assembled BODIPY-based polymer-conjugated nanoparticles. | [306,313] | |
| Enhance tumor targeting | Polyamine chain introduction | Tumor cells require amine growth factors and regularly exhibit polyamine transporter overexpression. This makes it possible for BODIPY-polyamine molecules to be transported to cancerous cells, which can improve the effectiveness of these tumor-targeting drugs. In addition, these transporters tolerate the uptake of diverse amines, making them a valuable tool for drug delivery. | [314] |
| Sugar-conjugate structures | Recent research has revealed that galactose-containing macromolecules can successfully target various types of cancer. Combining lactose with BODIPYs efficiently targets and impacts tumor cells through enhanced recognition and interaction with overexpressed specific receptors. | [315,316] | |
| Glucose Transporter 1 is often overexpressed in cancer cells, increasing glucose intake and metabolism. As a result, glycosylated BODIPY triangular skeletons were designed by Durán-Sampedro et al. to promote cellular uptake through the Warburg effect. | [317] | ||
| Polysaccharide-conjugate structures | For example, hyaluronic acid (HA) is commonly used as a polysaccharide-based drug carrier since it is water-soluble, has excellent biocompatibility, and is harmless. Chen et al. prepared an HA-BODIPY through an azide linker group that only triggered phototherapeutics inside tumor cells. This behavior is due to its self-assembled form in the extracellular environment; therefore, ROS production is inhibited. | [318] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.; Reis, L.V. Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers. Molecules 2023, 28, 5092. https://doi.org/10.3390/molecules28135092
Lima E, Reis LV. Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers. Molecules. 2023; 28(13):5092. https://doi.org/10.3390/molecules28135092
Chicago/Turabian StyleLima, Eurico, and Lucinda V. Reis. 2023. "Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers" Molecules 28, no. 13: 5092. https://doi.org/10.3390/molecules28135092