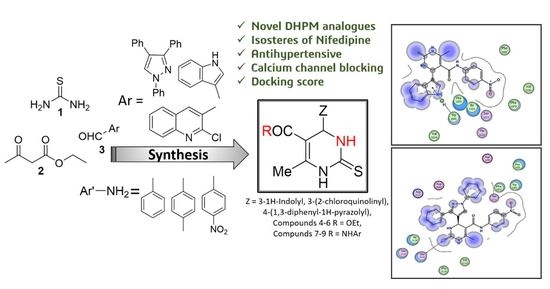
Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Pyrimidine Derivatives as Potential Calcium Channel Blockers
Abstract
:1. Introduction
2. Results and Discussions
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Evaluation of Antihypertensive Activity
2.2.2. Evaluation of Calcium Channel Blocking Activity
2.3. Molecular Modeling and Binding Mode Prediction
2.4. Structure–Activity Relationships
3. Materials and Methods
3.1. Chemistry
Materials and Methods
- 4a: Ethyl 4-(1H-indol-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate: Yield: 76%; m.p.: 252–254 °C; IR ν (KBr cm−1): 3426 (NH), 3333 (NH), 3319 (NH), 3173 (CH, aromatic), 2978 (CH, aliphatic), 1749 (C=O ester), 1683 (C=O), 1277 (C=S), 1220 (C–O). 1HNMR (DMSO-d6, 400 MHz) δ: 1.19 (t, 3H, CH3), 2.1 (s, 3H, CH3), 3.76 (q, 2H, CH3CH2-O), 5.67 (s, 1H, CH), 7.28–8.05 (m, 5H, aromatic), 10.34, 10.51, 11.12 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 177.4 (1C, s), 161.6 (1C, s), 154.9, 146.0 (1C, s), 141.0, 139.67 (1C, s), 130.6 (1C, s), 128.1–128.3 (2C, 128.2 (s), 128.2 (s)), (1C, s), 113.4 (1C, s), 60.0 (1C, s), 52.1, (1C, s), 16.7 (1C, s), 14.9 (1C, s); MS (EI) m/z: 315.11 (M+, 12.5%); Calcd./Anal., for C16H17N3O2S: C, 60.93; H, 5.43; N, 13.32. Found: C, 60.68; H, 5.27; N, 13.66.
- 4b: Ethyl 4-(2-chloroquinolin-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate: Yield: 79%; m.p.: 218–220 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 1.18 (t, 3H, CH3), 2.39 (s, 3H, CH3), 3.67 (q, 2H, CH3CH2-O), 5.4 (s, 1H, CH), 7.36–8.09 (m, 5H, aromatic), 10.09, 11.33 (2 s, 2H, 2NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 178.1 (1C, s), 161.6 (1C, s), 154.7 (1C, s), 147.6 (1C, s), 146.0 (1C, s),134.7 (1C, s), 128.5 (1C, s), 128.3 (1C, s), 127.4 (1C, s), 126.0 (1C, s), 125.5 (1C, s), 121.7 (1C, s), 113.4 (1C, s), 60.0 (1C, s), 55.6 (1C, s), 15.0 (1C, s), 14.5 (1C, s); Calcd./Anal., for C17H16ClN3O2S: C, 56.43; H, 4.46; N, 11.61. Found: C, 56.68; H, 4.27; N, 11.66.
- 4c: Ethyl 4-(1,3-diphenyl-1H-pyrazol-4-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate: Yield: 75%; m.p.: 218–220 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.23 (t, 3H, CH3), 2.50 (s, 3H, CH3), 3.92 (q, 2H, CH3CH2-O), 5.4 (s, 1H, CH), 7.16–8.11 (m, 11H, aromatic), 10.31, 11.49 (2 s, 2H, 2NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ ppm) 169.7 (1C, s), 162.0 (1C, s), 154.3 (1C, s), 146.1 (1C, s), 137.6 (1C, s), 130.6 (1C, s), 129.8 (2C, s), 128.2 (2C, s), 127.8-127.8 (2C, 127.8 (s), 127.8 (s)), 127.5 (1C, s), 127.3–127.8 (3C, 127.3 (s), 127.8 (s)), 121.8 (2C, s), 113.4 (1C, s), 60.2 (1C, s), 55.0 (1C, s), 15.1 (1C, s), 14.6 (1C, s), 14.2 (1C, s); Calcd./Anal., for C17H16ClN3O2S: C, 56.43; H, 4.46; N, 11.61. Found: C, 56.68; H, 4.27; N, 11.66.
- 5a: 4-(Indol-3yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid: Yield: 69%; m.p.: 233–235 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.23 (t, 3H, CH3), 5.53 (s, 1H, CH), 7.31–8.05 (m, 5H, aromatic), 10.32, 10.87, 11.19 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 177.5 (1C, s), 174.7 (1C, s), 154.9 (1C, s), 148.9 (1C, s), 128.1–128.3 (2C, 128.2 (s), 128.2 (s)), 128.4 (1C, s), 126.1 (1C, s), 125.8 (1C, s), 125.5 (1C, s), 121.7 (1C, s), 113.4 (1C, s), 51.9 (1C, s), 16.9 (1C, s); Calcd./Anal., for C14H13N3O3S: C, 55.43; H, 4.32; N, 13.85. Found: C, 55.66; H, 4.25; N, 13.55.
- 5b: 4-(2-Chloroquinolin-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid: Yield: 71%; m.p.: 244–246 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.44 (t, 3H, CH3), 5.47 (s, 1H, CH), 7.26–8.05 (m, 5H, aromatic), 10.35, 11.34 (2 s, 2H, 2NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 172.3 (1C, s), 165.3 (1C, s), 152.6 (1C, s), 146.0 (1C, s), 141.1 (1C, s), 130.6 (1C, s), 128.5 (1C, s), 128.3 (1C, s), 127.4 (1C, s), 126.0 (1C, s), 125.5 (1C, s), 121.8 (1C, s), 119.9 (1C, s), 55.8 (1C, s), 18.0 (1C, s); Calcd./Anal., for C15H12ClN3O3S: C, 51.51; H, 3.46; N, 12.01. Found: C, 51.60; H, 3.35; N, 12.25.
- 5c: 4-(1,3-Diphenyl-1H-pyrazol-4-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid: Yield: 68%; m.p.: 214–216 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.50 (t, 3H, CH3), 5.39 (s, 1H, CH), 7.1–7.91 (m, 11H, aromatic), 10.31, 11.39 (2 s, 2H, 2NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 169.2 (1C, s), 164.1 (1C, s), 154.3 (1C, s), 146.7 (1C, s), 138.1 (1C, s), 131.6 (1C, s), 130.0 (2C, s), 128.8 (2C, s), 126.0–127.5 (2C, 127.6 (s), 128.3 (s)), 125.5 (1C, s), 121.9 (2C, s), 114.6 (1C, s), 55.1 (1C, s), 16.8 (1C, s); Calcd./Anal., for C21H18N4O3S: C, 62.05; H, 4.46; N, 13.78. Found: C, 62.30; H, 4.45; N, 13.55.
- 6a: 4-(1H-indol-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonyl chloride: Yield: 68%; m.p.: 247–249 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.23 (t, 3H, CH3), 5.53 (s, 1H, CH), 7.41–8.07 (m, 5H, aromatic), 10.39, 10.67, 10.84 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 178.3 (1C, s), 173.4 (1C, s), 153.9, 149.0 (1C, s), 142.1(1C, s), 135.3 (1C, s), 129.3–128.3, (2C, 129.3 (s), 126.3 (s)) 127.4 (1C, s), 125.7 (1C, s), 122.0 (1C, s), 113.5 (1C, s), 110.9 (1C, s), 52.3 (1C, s), 17.8 (1C, s); Calcd./Anal., for C15H14ClN3OS: C, 56.33; H, 4.41; N, 13.14. Found: C, 56.28; H, 4.48; N, 13.22.
- 6b: 4-(2-Chloroquinolin-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonyl chloride: Yield: 73%; m.p.: 254–256 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.34 (t, 3H, CH3), 5.37 (s, 1H, CH), 7.26–8.05 (m, 5H, aromatic), 10.35, 10.94 (2 s, 2H, 2NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 178.1 (1C, s), 171.6 (1C, s), 152.7 (1C, s), 147.6 (1C, s), 146.1 (1C, s), 141.0 (1C, s), 130.6 (1C, s), 128.5 (1C, s), 128.2 (1C, s), 127.4 (1C, s), 126.0 (1C, s), 125.8 (1C, s), 121.8 (1C, s), 55.4 (1C, s), 16.8 (1C, s); Calcd./Anal., for C16H13Cl2N3OS: C, 52.47; H, 3.58; N, 11.47. Found: C, 52.29; H, 3.66; N, 11.25.
- 6c: 4-(Diphenyl-1H-pyrazol-4-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonyl chloride: Yield: 80%; m.p.: 235–237 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.34 (t, 3H, CH3), 5.32 (s, 1H, CH), 7.15–8.23 (m, 11H, aromatic), 10.41, 11.43 (2 s, 2H, 2NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 174.3 (1C, s), 167.0 (1C, s), 155.9 (1C, s), 148.0 (1C, s), 138.3 (1C, s), 130.6 (1C, s), 129.98 (2C, s), 128.8 (2C, s), 128.1–127.5 (1C, s), 127.5–121.8 (4C, 121.8 (s), 125.7 (s), 127.5 (s)), 112.8 (2C, s), 55.3 (1C, s), 17.9 (1C, s); Calcd./Anal., for C22H19ClN4OS: C, 62.48; H, 4.53; N, 13.25. Found: C, 62.21; H, 4.32; N, 13.25.
- 7a: 4-(1H-indoly-3-yl)-6-methyl-N-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 77%; m.p.: 266–268 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.36 (t, 3H, CH3), 5.19 (s, 1H, CH), 7.11–9.59 (m, 11H, aromatic), 10.65, 11.03, 11.37 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 167.6 (1C, s), 165.1 (1C, s), 153.9, 146.7 (1C, s), 139.1 (1C, s), 135.3 (1C, s), 130.5 (1C, s), 129.8-128.8 (4C, 128.8 (s), 129.0 (s), 129.8 (s)), 128.3 (1C, s), 127.5 (1C, s), 127.5 (1C, s), 125.4 (2C, s), 121.8 (1C, s), 113.4 (1C, s), 53.9 (1C, s), 17.3 (1C, s); Calcd./Anal., for C20H18N4OS: C, 66.28; H, 5.01; N, 15.46. Found: C, 66.45; H, 4.99; N, 15.35.
- 7b: 4-(2-Chloroquinolin-3-yl)-6-methyl-N-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 78%; m.p.: 260–262 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.24 (t, 3H, CH3), 5.3 (s, 1H, CH), 7.29–8.21 (m, 10H, aromatic), 9.89, 10.35, 11.39 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 177.9 (1C, s), 163.7 (1C, s), 155.9 (1C, s), 148.9 (1C, s), 145.1 (1C, s), 141.2 (1C, s), 139.7 (1C, s), 134.5 (1C, s), 130.6 (1C, s), 128.8 (2C, s), 128.6 (1C, s), 128.3–127.4 (2C, 128.3 (s), 127.4 (s)), 126.3 (1C, s), 125.8, 125.6 (1C, s), 121.7 (1C, s), 114.5 (2C, s), 57.1 (1C, s), 17.3 (1C, s); MS (EI) m/z: 408.11 (M+, 20.5%); Calcd./Anal., for C21H17ClN4OS: C, 61.68; H, 4.19; N, 13.70. Found: C, 61.69; H, 4.20; N, 13.46.
- 7c: 4-(1,3-Diphenyl-1H-pyrazol-4-yl)-6-methyl-N-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 73%; m.p.: 222–224 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.24 (t, 3H, CH3), 5.42 (s, 1H, CH), 7.25–8.18 (m, 10H, aromatic), 10.41, 10.73, 11.71 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 177.2 (1C, s), 161.62 (1C, s), 154.98 (1C, s), 148.93 (1C, s), 146.06 (1C, s), 141.07 (1C, s), 139.67 (1C, s), 134.53 (2C, s), 130.6–128.8 (4C, 130.61 (s), 128.8 (s)), 128.5–127.4 (3C, 128.5 (s), 128.3 (s), 127.4 (s)), 126.3 (1C, s), 126.0–125.5 (4C, 126.0 (s), 125.8 (s), 125.5 (s)), 121.75 (2C, s), 114.41(2C, s), 57.53 (1C, s), 17.38 (1C, s); Calcd./Anal., for C27H23N5OS: C, 69.65; H, 4.98; N, 15.04. Found: C, 69.59; H, 4.90; N, 14.98.
- 8a: 4-(1H-indol-3-yl)-6-methyl-thioxo-N-p-tolyl-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 76%; m.p.: 262–264 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.14 (t, 3H, CH3), 2.29 (t, 3H, CH3), 5.51 (s, 1H, CH), 7.31–8.19 (m, 9H, aromatic), 9.68, 10.36, 10.83, 11.35 (4 s, 4H, 4NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 167.6 (1C, s), 163.8 (1C, s), 153.9 (1C, s), 146.7 (1C, s), 140.9 (1C, s), 135.3 (1C, s), 130.5 (2C, s), 129.8 (1C, s), 129–128.2 (4C, 129.2 (s), 128.2 (s)), 127.5 (1C, s), 127.5 (1C, s), 126.0 (1C, s), 125.4 (1C, s), 121.8 (2C, s), 113.4 (1C, s), 53.9 (1C, s), 20.5 (1C, s), 17.6 (1C, s); Calcd./Anal., for C21H20N4OS: C, 67.00; H, 5.35; N, 14.88. Found: C, 67.36; H, 5.01; N, 14.84.
- 8b: 4-(2-Chloroquinolin-3-yl)-6-methyl-thioxo-N-p-tolyl-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 77%; m.p.: 282–284 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.13 (t, 3H, CH3), 2.25 (t, 3H, CH3), 5.47 (s, 1H, CH), 7.23–8.23 (m, 9H, aromatic), 10.15, 10.45, 11.5 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 172.3 (1C, s), 167.5 (1C, s), 152.6 (1C, s), 148.9 (1C, s), 144.2 (1C, s), 139.7 (1C, s), 130.6 (1C, s) (1C, s), 128.8 (2C, s), 128.6 (1C, s), 128.3 (1C, s), 127.4 (1C, s), 126.7 (1C, s), 126.3 (1C, s), 125.8 (1C, s), 121.7 (1C, s), 114.5 (2C, s), 57.4 (1C, s), 20.4 (1C, s), 17.3 (1C, s); Calcd./Anal., for C22H19ClN4OS: C, 62.48; H, 4.53; N, 13.25. Found: C, 62.55; H, 4.31; N, 13.36.
- 8c: 4-(1,3-Diphenyl-1H-pyrazol-4-yl)-6-methyl-2-thioxo-N-p-tolyl-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 77%; m.p.: 275–277 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.12 (t, 3H, CH3), 2.34 (t, 3H, CH3), 5.42 (s, 1H, CH), 7.15–8.19 (m, 14H, aromatic), 9.82, 10.7, 11.3 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 178.7 (1C, s), 168.1 (1C, s), 155.1 (1C, s), 151.0 (1C, s), 143.7 (1C, s), 142.0 (1C, s), 141.2 (1C, s), 139.7 (1C, s), 134.5 (2C, s), 130.6 (2C, s), 128.8 (2C, s), 128.6–128.3 (4C, 128.6 (s), 128.5 (s), 128.3 (s)), 127.5 (1C, s), 126.2–125.5 (4C, 126.2 (s), 126.2 (s), 125.5 (s)), 121.8 (2C, s), 113.1 (2C, s), 57.9 (1C, s), 23.0 (1C, s), 17.4 (1C, s); Calcd./Anal., for C28H25N5OS: C, 70.12; H, 5.25; N, 14.60. Found: C, 70.35; H, 5.11; N, 14.50.
- 9a: 4-(1H-indol-3-yl)-6-methyl-N-(4-nitrophenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 83%; m.p.: 263–265 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.29 (t, 3H, CH3), 5.5 (s, 1H, CH), 7.31–8.09 (m, 9H, aromatic), 9.89, 10.35, 10.8, 11.29 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 168.2 (1C, s), 164.0 (1C, s), 153.1 (1C, s), 148.5 (1C, s), 146.9 (1C, s), 139.3 (1C, s), 135.2 (1C, s), 129.2-128.3 (2C, 128.3 (s), 129.2 (s)), 128.4 (1C, s), 127.6 (1C, s), 127.5 (2C, s), 126.6 (1C, s), 125.4 (1C, s), 121.7 (2C, s), 114.0 (1C, s), 54.7 (1C, s), 17.6 (1C, s); Calcd./Anal., for C20H17N5O3S: C, 58.96; H, 4.21; N, 17.19. Found: C, 58.70; H, 4.06; N, 17.21.
- 9b: 4-(2-Chloroquinolin-3-yl)-6-methyl-N-(4-nitrophenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 83%; m.p.: 276–278 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.13 (t, 3H, CH3), 5.45 (s, 1H, CH), 7.17–8.19 (m, 9H, aromatic), 9.95, 10.86, 11.3 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 174.4 (1C, s), 168.3 (1C, s), 156.1 (1C, s), 149.0 (1C, s), 146.5 (1C, s), 143.6 (1C, s), 135.2 (1C, s), 130.7 (1C, s), 128.8 (1C, s), 128.5 (1C, s), 128.2 (1C, s), 127.4 (1C, s), 126.6 (1C, s), 126.3 (1C, s), 125.6 (2C, s), 121.7 (1C, s), 115.0 (2C, s), 57.5 (1C, s), 17.4 (1C, s); Calcd./Anal., for C21H16ClN5O3S: C, 55.57; H, 3.55; N, 15.43. Found: C, 55.67; H, 3.88; N, 15.26.
- 9c: 4-(1,3-Diphenyl-1H-pyrazol-4-yl)-6-methyl-N-(4-nitrophenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide: Yield: 83%; m.p.: 261–263 °C; 1HNMR (DMSO-d6, 400 MHz) δ: 2.4 (t, 3H, CH3), 5.59 (s, 1H, CH), 7.25–8.19 (m, 14H, aromatic), 9.58, 10.5, 11.59 (3 s, 3H, 3NH (D2O exchangeable)). 13C NMR: (DMSO-d6, 400 MHz) δ (ppm) 178.7 (1C, s), 168.5 (1C, s), 158.9 (1C, s), 155.1 (1C, s), 151.6, (1C, s) 143.9 (1C, s), 142.0 (1C, s), 141.1 (1C, s), 130.7 (2C, s), 128.8 (2C, s), 128.6–128.3 (2C, 128.86 (s), 128.3 (s)), 127.4 (1C, s), 126.2, 125.6 (4C, 126.2 (s), 126.8 (s), 125.6 (s)), 125.5 (2C, s), 121.1 (2C, s), 114.2 (2C, s), 57.8 (1C, s), 17.5 (1C, s); Calcd./Anal., for C27H22N6O3S: C, 63.52; H, 4.34; N, 16.46. Found: C, 63.87; H, 4.35; N, 16.66.
3.2. Biological Evaluation
3.2.1. Evaluation of In Vivo Antihypertensive Activity
3.2.2. Evaluation of Calcium Channel Blocking Activity
3.3. Molecular Modeling and Binding Mode Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Di Palo, K.E.; Barone, N.J. Hypertension and heart failure: Prevention, targets, and treatment. Heart Fail. Clin. 2020, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Ram, C.V.S. Beta-blockers in hypertension. Am. J. Cardiol. 2010, 106, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Matchar, D.B.; McCrory, D.C.; Orlando, L.A.; Patel, M.R.; Patel, U.D.; Patwardhan, M.B.; Powers, B.; Samsa, G.P.; Gray, R.N. Systematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann. Intern. Med. 2008, 148, 16–29. [Google Scholar] [CrossRef]
- Cutler, J.A.; Davis, B.R. Thiazide-type diuretics and β-adrenergic blockers as first-line drug treatments for hypertension. Circulation 2008, 117, 2691–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Chen, N.; Zhou, M.; Guo, J.; Zhu, C.; Zhou, J.; Ma, M.; He, L. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database Syst. Rev. 2021, 1, CD003654. [Google Scholar]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Poulter, N.R.; Sever, P.S. Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010, 9, 469–480. [Google Scholar] [CrossRef]
- Özkaya, E.; Yazganoğlu, K.D.; Özkaya, E.; Yazganoğlu, K.D. Calcium Channel Blockers. Advers. Cutan. Drug React. Cardiovasc. Drugs 2014, 129–142. [Google Scholar] [CrossRef]
- Chatki, P.K.; Tabassum, S. Analytical Methods of Dihydropyridines Based Calcium Channel Blockers-Amlodipine, Lacidipine, Isradipine, Nifedipine, Felodipine, Cilnidipine and its related formulations: A Review. Asian J. Res. Chem. 2021, 14, 221–234. [Google Scholar] [CrossRef]
- Loas, G.; Van de Borne, P.; Darquennes, G.; Le Corre, P. Association of amlodipine with the risk of in-hospital death in patients with COVID-19 and hypertension: A reanalysis on 184 COVID-19 patients with hypertension. Pharmaceuticals 2022, 15, 380. [Google Scholar] [CrossRef]
- Wang, A.L.; Iadecola, C.; Wang, G. New generations of dihydropyridines for treatment of hypertension. J. Geriatr. Cardiol. JGC 2017, 14, 67. [Google Scholar] [PubMed]
- Liang, L.; Kung, J.Y.; Mitchelmore, B.; Cave, A.; Banh, H.L. Comparative peripheral edema for dihydropyridines calcium channel blockers treatment: A systematic review and network meta-analysis. J. Clin. Hypertens. 2022, 24, 536–554. [Google Scholar] [CrossRef] [PubMed]
- van Zwieten, P.A.; Pfaffendorf, M. Pharmacology of the dihydropyridine calcium antagonists: Relationship between lipophilicity and pharmacodynamic responses. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1993, 11, S3–S8. [Google Scholar] [CrossRef]
- Farghaly, A.M.; AboulWafa, O.M.; Elshaier, Y.A.; Badawi, W.A.; Haridy, H.H.; Mubarak, H.A. Design, synthesis, and antihypertensive activity of new pyrimidine derivatives endowing new pharmacophores. Med. Chem. Res. 2019, 28, 360–379. [Google Scholar] [CrossRef]
- Alam, O.; Khan, S.A.; Siddiqui, N.; Ahsan, W.; Verma, S.P.; Gilani, S.J. Antihypertensive activity of newer 1, 4-dihydro-5-pyrimidine carboxamides: Synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2010, 45, 5113–5119. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Biginelli reaction mediated synthesis of antimicrobial pyrimidine derivatives and their therapeutic properties. Molecules 2021, 26, 6022. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. 4-Aryldihydropyrimidines via the Biginelli condensation: Aza-analogs of nifedipine-type calcium channel modulators. Molecules 1998, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rozzo, M. Development of a Green Method for the Production of Dihydropyrimidinones Through a Biginelli Reaction. 2014. Available online: https://digitalcommons.butler.edu/urc/2014/chemistry/1/ (accessed on 1 April 2023).
- Drapak, I.; Perekhoda, L.; Tsapko, T.; Berezniakova, N.; Tsapko, Y. Cardiovascular calcium channel blockers: Historical overview, development and new approaches in design. J. Heterocycl. Chem. 2017, 54, 2117–2128. [Google Scholar] [CrossRef]
- Zohny, Y.M.; Awad, S.M.; Rabie, M.A.; Al-Saidan, O.A. Synthesis of Dihydropyrimidines: Isosteres of Nifedipine and Evaluation of Their Calcium Channel Blocking Efficiency. Molecules 2023, 28, 784. [Google Scholar] [CrossRef]
- Şafak, C.; Gündüz, M.G.; İlhan, S.Ö.; Şimşek, R.; İşli, F.; Yıldırım, Ş.; Fincan, G.S.Ö.; Sarıoğlu, Y.; Linden, A. Synthesis and Myorelaxant Activity of Fused 1,4-Dihydropyridines on Isolated Rabbit Gastric Fundus. Drug Dev. Res. 2012, 73, 332–342. [Google Scholar] [CrossRef]
- Tu, S.; Miao, C.; Fang, F.; Youjian, F.; Li, T.; Zhuang, Q.; Zhang, X.; Zhu, S.; Shi, D. New potential calcium channel modulators: Design and synthesis of compounds containing two pyridine, pyrimidine, pyridone, quinoline and acridine units under microwave irradiation. Bioorg. Med. Chem. Lett. 2004, 14, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Lipkind, G.M.; Fozzard, H.A. Molecular modeling of interactions of dihydropyridines and phenylalkylamines with the inner pore of the L-type Ca2+ channel. Mol. Pharmacol. 2003, 63, 499–511. [Google Scholar] [CrossRef] [Green Version]
- El-Khouly, A.; Gündüz, M.; Cengelli, C.; Şimşek, R.; Erol, K.; Şafak, C.; Yıldırım, S.Ö.; Butcher, R. Microwave-assisted synthesis and spasmolytic activity of 4-indolylhexahydroquinoline derivatives. Drug Res. 2013, 63, 579–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisi, A.; Budriesi, R.; Rampa, A.; Fabbri, G.; Chiarini, A.; Valenti, P. Synthesis and pharmacological profile of some chloroxanthone-1, 4-dihydropyridine derivatives. Arzneimittelforschung 1996, 46, 848–851. [Google Scholar] [PubMed]
- Miri, R.; Javidnia, K.; Sarkarzadeh, H.; Hemmateenejad, B. Synthesis, study of 3D structures, and pharmacological activities of lipophilic nitroimidazolyl-1,4-dihydropyridines as calcium channel antagonist. Bioorg. Med. Chem. 2006, 14, 4842–4849. [Google Scholar] [CrossRef]
- Katouah, H.A.; Gaffer, H.E. Synthesis and docking study of pyrimidine derivatives scaffold for anti-hypertension application. ChemistrySelect 2019, 4, 6250–6255. [Google Scholar] [CrossRef]
- Matos, L.H.S.; Masson, F.T.; Simeoni, L.A.; Homem-de-Mello, M. Biological activity of dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789. [Google Scholar] [CrossRef]
- Jain, K.S.; Bariwal, J.B.; Kathiravan, M.K.; Phoujdar, M.S.; Sahne, R.S.; Chauhan, B.S.; Shah, A.K.; Yadav, M.R. Recent advances in selective α1-adrenoreceptor antagonists as antihypertensive agents. Bioorg. Med. Chem. 2008, 16, 4759–4800. [Google Scholar] [CrossRef]
- Prakash, G.S.; Lau, H.; Panja, C.; Bychinskaya, I.; Ganesh, S.K.; Zaro, B.; Mathew, T.; Olah, G.A. Synthesis of dihydropyrimidinones/thiopyrimidinones: Nafion-Ga, an efficient “green” lewis acid catalyst for the biginelli reaction. Catal. Lett. 2014, 144, 2012–2020. [Google Scholar] [CrossRef]
- Zorkun, I.S.; Saraç, S.; Çelebi, S.; Erol, K. Synthesis of 4-aryl-3,4-dihydropyrimidin-2 (1H)-thione derivatives as potential calcium channel blockers. Bioorg. Med. Chem. 2006, 14, 8582–8589. [Google Scholar] [CrossRef]
- Mahgoub, S.; El-Sayed, M.-I.K.; El-Shehry, M.F.; Awad, S.M.; Mansour, Y.E.; Fatahala, S.S. Synthesis of novel calcium channel blockers with ACE2 inhibition and dual antihypertensive/anti-inflammatory effects: A possible therapeutic tool for COVID-19. Bioorg. Chem. 2021, 116, 105272. [Google Scholar] [CrossRef] [PubMed]
- Teleb, M.; Zhang, F.-X.; Farghaly, A.M.; Wafa, O.M.A.; Fronczek, F.R.; Zamponi, G.W.; Fahmy, H. Synthesis of new N3-substituted dihydropyrimidine derivatives as L-/T-type calcium channel blockers. Eur. J. Med. Chem. 2017, 134, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Cassambai, S.; Mee, C.J.; Renshaw, D.; Hussain, A. Tiotropium bromide, a long acting muscarinic receptor antagonist triggers intracellular calcium signalling in the heart. Toxicol. Appl. Pharmacol. 2019, 384, 114778. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Pattanayek, R.; Potet, F.; Rebbeck, R.T.; Blackwell, D.J.; Nikolaienko, R.; Sequeira, V.; Le Meur, R.; Radwański, P.B.; Davis, J.P.J.C.C. The CaMKII inhibitor KN93-calmodulin interaction and implications for calmodulin tuning of NaV1. 5 and RyR2 function. Cell Calcium 2019, 82, 102063. [Google Scholar] [CrossRef]
- Tang, L.; Gamal El-Din, T.M.; Payandeh, J.; Martinez, G.Q.; Heard, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A.J.N. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 2014, 505, 56–61. [Google Scholar] [CrossRef] [Green Version]







| Compound Code | Dose (mL) | Control (mmHg) | Test (mmHg) | % Inhibition in Blood Pressure |
|---|---|---|---|---|
| Nifedipine | 0.3 | 29.07 | 20.02 | 31.32 |
| 0.3 | 28.35 | 20.78 | 26.54 | |
| 4a | 0.3 | 28.41 | 23.09 | 19.78 |
| 0.3 | 27.57 | 22.38 | 19.19 | |
| 4b | 0.3 | 28.34 | 23.5 | 18.53 |
| 0.3 | 28.38 | 24.18 | 18.79 | |
| 4c | 0.3 | 29.32 | 22.17 | 29.40 |
| 0.3 | 30.15 | 23.45 | 29.78 | |
| 5a | 0.3 | 29.27 | 25.07 | 15.82 |
| 0.3 | 29.13 | 25.63 | 15.12 | |
| 5b | 0.3 | 30.01 | 24.21 | 15.23 |
| 0.3 | 29.23 | 24.14 | 15.55 | |
| 5c | 0.3 | 28.19 | 23.43 | 16.32 |
| 0.3 | 28.32 | 23.54 | 16.09 | |
| 6a | 0.3 | 30.23 | 26.54 | 11.75 |
| 0.3 | 30.02 | 26.23 | 11.79 | |
| 6b | 0.3 | 28.90 | 26.85 | 11.09 |
| 0.3 | 28.38 | 26.92 | 11.07 | |
| 6c | 0.3 | 30.12 | 27.45 | 11.00 |
| 0.3 | 30.01 | 27.09 | 11.02 | |
| 7a | 0.3 | 29.34 | 20.19 | 31.42 |
| 0.3 | 30.18 | 21.42 | 27.58 | |
| 7b | 0.3 | 29.19 | 21.98 | 23.23 |
| 0.3 | 30.01 | 21.86 | 23.90 | |
| 7c | 0.3 | 29.33 | 22.50 | 27.32 |
| 0.3 | 28.45 | 22.21 | 28.41 | |
| 8a | 0.3 | 30.13 | 24.42 | 20.44 |
| 0.3 | 29.62 | 23.53 | 21.02 | |
| 8b | 0.3 | 30.51 | 25.61 | 18.21 |
| 0.3 | 30.00 | 25.03 | 19.81 | |
| 8c | 0.3 | 29.14 | 22.62 | 28.70 |
| 0.3 | 29.48 | 20.07 | 30.26 | |
| 9a | 0.3 | 29.15 | 23.15 | 24.30 |
| 0.3 | 30.17 | 22.54 | 26.86 | |
| 9b | 0.3 | 29.32 | 20.19 | 30.09 |
| 0.3 | 30.15 | 21.42 | 29.28 | |
| 9c | 0.3 | 29.71 | 21.42 | 27.44 |
| 0.3 | 30.43 | 20.53 | 31.02 |
| Compound Code | Dose (mL) | Control (cm) | Test (cm) | % Inhibition | IC50 (μg/L) |
|---|---|---|---|---|---|
| Nifedipine | 0.1 | 3.4 | 3.0 | 11.74 | 21 ± 0.28 |
| 0.2 | 3.4 | 2.7 | 20.47 | ||
| 0.3 | 3.4 | 2.3 | 32.26 | ||
| 0.4 | 3.3 | 2.1 | 35.76 | ||
| 0.5 | 3.3 | 1.7 | 48.38 | ||
| 0.6 | 3.3 | 1.2 | 61.56 | ||
| 4a | 0.1 | 3.4 | 2.9 | 17.24 | 23.07 ± 0.32 |
| 0.3 | 3.4 | 2.3 | 20.56 | ||
| 0.5 | 3.3 | 1.8 | 30.80 | ||
| 4b | 0.1 | 3.4 | 2.8 | 16.78 | 24.69 ± 0.22 |
| 0.3 | 3.4 | 2.3 | 19.36 | ||
| 0.5 | 3.3 | 1.6 | 29.83 | ||
| 4c | 0.1 | 3.3 | 2.4 | 16.25 | 22.42 ± 0.13 |
| 0.3 | 3.4 | 2.5 | 25.69 | ||
| 0.5 | 3.4 | 1.3 | 37.27 | ||
| 5a | 0.1 | 3.4 | 2.6 | 14.88 | 24.37 ± 0.41 |
| 0.3 | 3.3 | 2.1 | 19.71 | ||
| 0.5 | 3.4 | 1.6 | 35.23 | ||
| 5b | 0.1 | 3.3 | 2.7 | 14.87 | 25.25 ± 0.38 |
| 0.3 | 3.4 | 2.3 | 19.98 | ||
| 0.5 | 3.4 | 1.8 | 34.89 | ||
| 5c | 0.1 | 3.4 | 2.7 | 13.67 | 24.81 ± 0.43 |
| 0.3 | 3.4 | 2.3 | 18.96 | ||
| 0.5 | 3.3 | 1.5 | 32.96 | ||
| 6a | 0.1 | 3.3 | 2.6 | 8.56 | 26.84 ± 0.26 |
| 0.3 | 3.4 | 2.3 | 17.87 | ||
| 0.5 | 3.4 | 1.8 | 30.84 | ||
| 6b | 0.1 | 3.4 | 2.7 | 7.98 | 30.78 ± 0.24 |
| 0.3 | 3.3 | 2.1 | 16.98 | ||
| 0.5 | 3.4 | 1.8 | 30.09 | ||
| 6c | 0.1 | 3.3 | 2.7 | 8.98 | 29.73 ± 0.19 |
| 0.3 | 3.4 | 2.1 | 17.43 | ||
| 0.5 | 3.4 | 1.5 | 30.53 | ||
| 7a | 0.1 | 3.4 | 2.8 | 16.64 | 21.57 ± 0.12 |
| 0.3 | 3.4 | 2.2 | 28.59 | ||
| 0.5 | 3.4 | 1.7 | 39.10 | ||
| 7b | 0.1 | 3.4 | 2.6 | 15.09 | 22.11 ± 0.20 |
| 0.3 | 3.4 | 2.2 | 30.93 | ||
| 0.5 | 3.3 | 1.8 | 36.89 | ||
| 7c | 0.1 | 3.4 | 2.7 | 17.96 | 20.05 ± 0.05 |
| 0.3 | 3.4 | 2.2 | 29.97 | ||
| 0.5 | 3.3 | 1.5 | 44.98 | ||
| 8a | 0.1 | 3.3 | 2.7 | 16.76 | 22.63 ± 0.36 |
| 0.3 | 3.4 | 2.1 | 28.98 | ||
| 0.5 | 3.4 | 1.5 | 45.83 | ||
| 8b | 0.1 | 3.3 | 2.7 | 16.76 | 22.91 ± 0.29 |
| 0.3 | 3.4 | 2.1 | 29.56 | ||
| 0.5 | 3.4 | 1.5 | 44.87 | ||
| 8c | 0.1 | 3.4 | 2.7 | 16.24 | 19.83 ± 0.36 |
| 0.3 | 3.4 | 2.1 | 35.23 | ||
| 0.5 | 3.4 | 1.5 | 50.13 | ||
| 9a | 0.1 | 3.4 | 2.8 | 15.81 | 21.33 ± 0.21 |
| 0.3 | 3.4 | 2.1 | 34.53 | ||
| 0.5 | 3.3 | 1.3 | 45.82 | ||
| 9b | 0.1 | 3.4 | 2.6 | 17.12 | 20.45 ± 1.07 |
| 0.3 | 3.4 | 2.01 | 37.45 | ||
| 0.5 | 3.3 | 1.4 | 49.75 | ||
| 9c | 0.1 | 3.3 | 2.5 | 17.76 | 19.57 ± 0.18 |
| 0.3 | 3.4 | 2.1 | 36.20 | ||
| 0.5 | 3.4 | 1.6 | 50.86 |
| Ligands | Hydrogen Bonds between Atoms of Ligands and Amino Acids of Receptor | S-Score (Binding Energy) (kcal/mol) | |||||
|---|---|---|---|---|---|---|---|
| Ligands Atoms | Receptor | Type | Distance (Å) | Energy (kcal/mol) | |||
| Atoms | Residues | ||||||
| Ryanodine receptor | |||||||
| KN93 | C11 | OE1 | GLU 11 | H-donor | 3.15 | −0.7 | −7.11 |
| 8c | O11 | SD | MET 72 | H-donor | 3.47 | −0.5 | −7.04 |
| 9c | C20 | 6-ring | PHE 92 | H-pi | 4.47 | −0.7 | −8.41 |
| Nifedipine | N | O | GLU 11 | H-donor | 2.71 | −0.91 | −5.28 |
| dihydropyridine receptor | |||||||
| PX4 | O2 | NE1 | TRP 1076 | H-acceptor | 3.43 | −0.9 | −5.91 |
| 8c | 5-ring | NE1 | TRP 1076 | pi-H | 4.22 | −1.0 | −5.09 |
| 9c | S21 | CA | SER 1125 | H-acceptor | 4.13 | −0.8 | −6.89 |
| Nifedipine | CO | N | GLU 1096 | H-acceptor | 2.50 | −0.9 | −5.74 |
| CO | N | TYR 1193 | H-acceptor | 2.36 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zohny, Y.M.; Awad, S.M.; Rabie, M.A.; Alsaidan, O.A. Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Pyrimidine Derivatives as Potential Calcium Channel Blockers. Molecules 2023, 28, 4869. https://doi.org/10.3390/molecules28124869
Zohny YM, Awad SM, Rabie MA, Alsaidan OA. Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Pyrimidine Derivatives as Potential Calcium Channel Blockers. Molecules. 2023; 28(12):4869. https://doi.org/10.3390/molecules28124869
Chicago/Turabian StyleZohny, Yasser M., Samir M. Awad, Maha A. Rabie, and Omar Awad Alsaidan. 2023. "Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Pyrimidine Derivatives as Potential Calcium Channel Blockers" Molecules 28, no. 12: 4869. https://doi.org/10.3390/molecules28124869