Synthesis, Bioactivity and Molecular Docking of Nereistoxin Derivatives Containing Phosphonate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activity
2.2.1. Acetylcholinesterase (AChE) Assay (in vivo)
2.2.2. Insecticidal Activity against M. separata
2.2.3. Insecticidal Activity against M. persicae Sulzer and R. padi
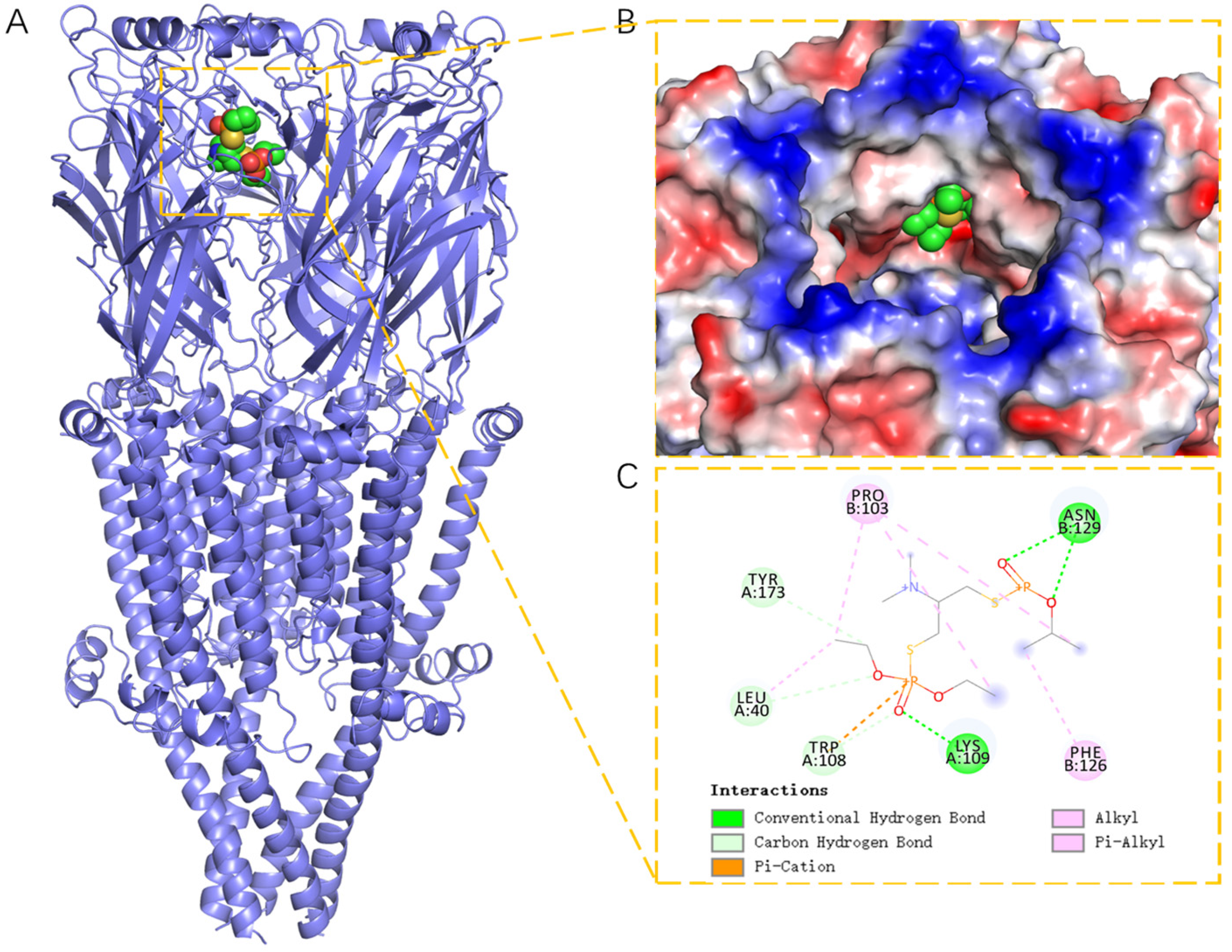
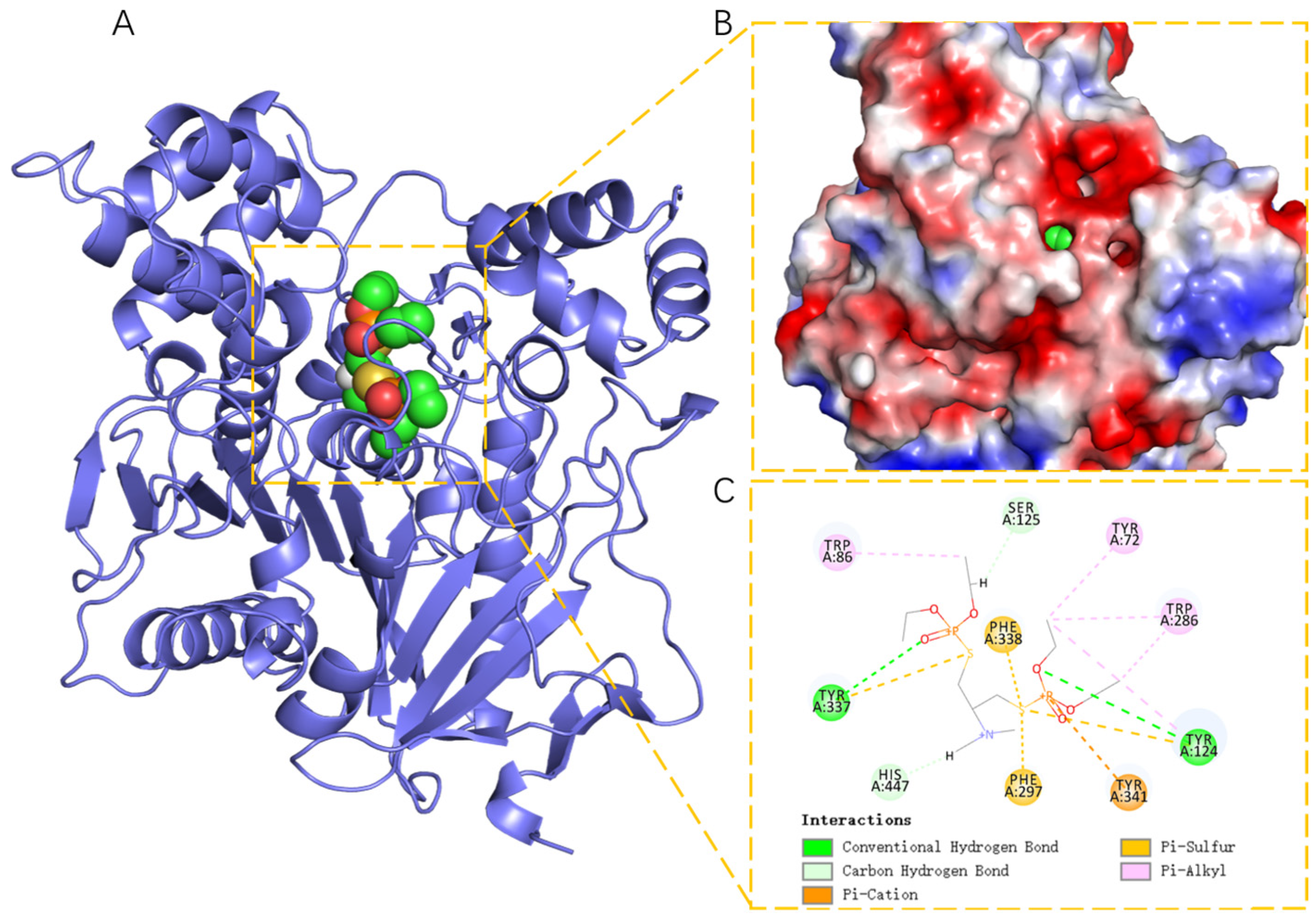
2.3. Structural Analysis of Docking
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedures
3.1.2. General Procedure for the Preparation of 2
3.1.3. General Procedure for the Preparation of 3
3.1.4. General Procedure for the Preparation of 5
3.1.5. General Procedure for the Preparation of 6
3.1.6. General Procedure for the Preparation of 7a–7h
3.2. Biological Tests
3.2.1. Bioassay Methods
3.2.2. Inhibitory Activity of Acetylcholinesterase (AChE)
3.2.3. Insecticidal Activity against M. separata
3.2.4. Insecticidal Activity against M. persicae
3.2.5. Insecticidal Activity against R. padi
3.3. Molecular Docking
3.3.1. Preparation of Target Proteins and Small-Molecular Structures
3.3.2. Analysis of Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emmel, T.C.; Scoble, N.J. The Lepidoptera: Form, Function and Diversity; Oxford University Press: Oxford, UK, 1995; pp. 4–5. [Google Scholar]
- Sharma, S.; Kooner, R.; Arora, R. Insect pests and crop losses. In Breeding Insect Resistant Crops for Sustainable Agriculture; Springer: Singapore, 2017; pp. 45–66. [Google Scholar]
- Chabert, A.; Sarthou, J.P. Practices of conservation agriculture prevail over cropping systems and landscape heterogeneity in understanding the ecosystem service of aphid biocontrol. Agric. Ecosyst. Environ. 2017, 249, 70–79. [Google Scholar] [CrossRef]
- Yi, X.U.; Gray, S.M. Aphids and their transmitted potato viruses: A continuous challenges in potato crops. J. Integr. Agric. 2020, 19, 367–375. [Google Scholar]
- Naveen, N.C.; Chaubey, R.; Kumar, D.; Rebijith, K.B.; Rajagopal, R.; Subrahmanyam, B.; Subramanian, S. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 2017, 7, 40634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, L.M.S.; Wanderley-Teixeira, V.; Ferreira, H.N.; Teixeira, Á.A.C.; Siqueira, H.A.A. Fitness costs associated with field–evolved resistance to chlorantraniliprole in Plutella xylostella (Lepidoptera: Plutellidae). Entomol. Res. 2014, 104, 88–96. [Google Scholar] [CrossRef]
- Lorsbach, B.A.; Sparks, T.C.; Cicchillo, R.M.; Garizi, N.V.; Hahn, D.R.; Meyer, K.G. Natural products: A strategic lead generation approach in crop protection discovery. Pest Manage. Sci. 2019, 75, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S. Nereistoxin, a poisonous constituent of Lumbriconereis heteropoda Marenz (Eunicidae). Yakugaku Zasshi 1934, 54, 648–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, K. Organic insecticides. Part X. Synthesis of nereistoxin and related compounds III. Agric. Biol. Chem. 1968, 32, 1199–1204. [Google Scholar]
- Konishi, K. Organic insecticides. Part XI. Synthesis of nereistoxin and related compounds IV. Agric. Biol. Chem. 1970, 34, 926–934. [Google Scholar]
- Konishi, K. Organic insecticides. Part XIII. Synthesis of nereistoxin andrelated compounds VI. Agric. Biol. Chem. 1970, 34, 1549–1560. [Google Scholar]
- Konishi, K. Pesticide Chemistry: Proceedings of the Second International IUPAC Congress of Pesticide Chemistry; Volume V: Herbicides, Fungicides, Formulation Chemistry, Israel, February 1971; Tahori, A.S., Ed.; Gordon and Breach, Science Publishers, Inc.: New York, NY, USA, 1972; pp. 179–189. [Google Scholar]
- Gholivand, K.; Mohammadpanah, F.; Pooyan, M.; Valmoozi, A.A.E.; Sharifi, M.; Mani-Varnosfaderani, A.; Hosseini, Z. Synthesis, crystal structure, insecticidal activities, molecular docking and QSAR studies of some new phospho guanidines and phospho pyrazines as cholinesterase inhibitors. Pestic. Biochem. Physiol. 2019, 157, 122–137. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Yan, X.; Wang, Y.; Liao, C.; Jiang, G. A review of organophosphate flame retardants and plasticizers in the environment: Analysis, occurrence and risk assessment. Sci. Total Environ. 2020, 731, 139071. [Google Scholar] [CrossRef]
- Li, A.; Fan, Y.; Cao, X.; Chen, L.; Wang, L.; Alves, C.S.; Mignani, S.; Majoral, J.P.; Tomas, H.; Shi, X. Morpholino-functionalized phosphorus dendrimers for precision regenerative medicine: Osteogenic differentiation of mesenchymal stem cells. Nanoscale 2019, 11, 17230–17234. [Google Scholar] [CrossRef] [PubMed]
- Mchardy, S.F.; Wang, H.Y.L.; Mccowen, S.V.; Valdez, M.C. Recent advances in acetylcholinesterase Inhibitors and Reactivators: An update on the patent literature (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar] [CrossRef]
- Baran, P.; Knouse, K.W.; Huang, Y.; Qiu, S.; Mcdonald, I. A P(V)-platform for oligonucleotide synthesis. Science 2021, 373, 1265–1270. [Google Scholar]
- Oswaldo, P.; Nicolaas, S.; Martin, B. Preparative Synthesis of an RP-Guanosine-3′,5′-Cyclic Phosphorothioate Analogue, a Drug Candidate for the Treatment of Retinal Degenerations. Org. Process Res. Dev. 2021, 25, 2453–2460. [Google Scholar]
- Crooke, S.T.; Bennett, C.F. Progress in antisense oligonucleotide therapeutics. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 107. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Yu, L.; Xi, Z.; Lan, M. Synthesis of Shacanlin Compounds. CN 1197074A, 28 October 1998. [Google Scholar]
- Yu, L.; Xi, Z.; Lan, M.; Chang, Z. Synthesis and biological activity tests of Shacanlin. Pesticides 1998, 37, 10–12. [Google Scholar]
- Zhao, H.; Yu, L. Toxicity and control efficacy of O, O, O′, O′-Tetramethyl-S, S′-(2-N,N-dimethylamino-trimethylene)-bis-dithiophosphate against Wheat Aphid. Pesticides 1998, 37, 33–34. [Google Scholar]
- Yurugi, S.; Sakai, M.; Kato, M.; Hagiwara, H.; Koniski, K.; Harukawa, T. Dithio Amino Alkanes. GB 976730A, 2 December 1964. [Google Scholar]
- Milan, J. Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 2018, 410, 125–131. [Google Scholar]
- Sakai, M. Studies on the insecticidal action of nereistoxin.IV. Role of the anticholinesterase activity in the insecticideal action to housefly Musca domestica L. (Diptera: Musicidae). Appl. Ent. Zool. 1966, 1, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Ivana, C.; Anita, V. Phytochemical composition, antiradical and anticholinesterase potentials of Centaurea alba and Centaurea jacea volatile oils. Croat. Chem. Acta. 2019, 92, 11–16. [Google Scholar]
- Santschi, N.; Togni, A. Electrophilic trifluoromethylation of S-hydrogen phosphorothioates. J. Org. Chem. 2011, 76, 4189–4193. [Google Scholar] [CrossRef]
- Franz, W.; Horst, T.; Ladislaus, S.; Eyer, P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem. Pharmacol. 2004, 68, 2237–2248. [Google Scholar]
- Sachiyo, S.; Masaya, M. Effect of acetone solution in a topical application method on mortality of rice planthoppers, Nilaparvata lugens, Sogatella furcifera, and Laodelphax striatellus (Homoptera: Delphacidae). Appl. Entomol. Zool. 2011, 46, 443–447. [Google Scholar]
- Shi, M.; Wei, J.; Qi, L.; Tian, L.; Wen, W.; Hang, B.; Bao, S. Design, Synthesis, and Study of the Insecticidal Activity of Novel Steroidal 1,3,4-Oxadiazoles. J. Agric. Food Chem. 2021, 69, 11572–11581. [Google Scholar]
- Che, Z.; Yu, X.; Zhi, X.; Fan, L.; Yao, X.; Xu, H. Synthesis of novel 4α-(acyloxy)-2′(2′,6′)-(di)halogenopodophyllotoxin derivatives as insecticidal agents. J. Agric. Food Chem. 2013, 61, 8148–8155. [Google Scholar] [CrossRef] [PubMed]


| Compound | R | AChE Inhibition Rate (%) | ||
|---|---|---|---|---|
| 5 min | 20 min | 30 min | ||
| 7a | Me | 90.78 | 96.10 | 96.26 |
| 7b | Et | 91.90 | 96.82 | 97.11 |
| 7c | nPr | 92.71 | 97.13 | 97.39 |
| 7d | iPr | 92.73 | 97.10 | 97.43 |
| 7e | nBu | 92.87 | 97.21 | 97.48 |
| 7f | tBu | 89.56 | 94.58 | 94.51 |
| 7g | Ph | 72.84 | 66.73 | 60.64 |
| 7h | Bn | 92.59 | 97.08 | 97.35 |
| Nereistoxin | / | 66.02 | 53.98 | 41.27 |
| Compound | R | IC50 (μM) | Final Concentration (μM) a | Ki (mM−1⋅min−1) |
|---|---|---|---|---|
| 7a | Me | 3.332 | 3.125 | 115.86 ± 64.99 |
| 7b | Et | 3.422 | 3.125 | 83.83 ± 3.12 |
| 7c | nPr | 10.28 | 12.5 | 33.95 ± 5.62 |
| 7d | iPr | 37.83 | 50 | 7.92 ± 3.73 |
| 7e | nBu | 179.3 | 200 | 0.94 ± 0.21 |
| 7f | tBu | — b | — | — |
| 7g | Ph | — c | — | — |
| 7h | Bn | 61.4 | 50 | 3.67 ± 0.14 |
| Nereistoxin | / | — d | — | — |
| Compound | R | Corrected Mortality Rate (%) | |
|---|---|---|---|
| 24 h | 48 h | ||
| 7a | Me | 100 | 100 |
| 7b | Et | 100 | 100 |
| 7c | nPr | 100 | 100 |
| 7d | iPr | 70 | 62.5 |
| 7e | nBu | 70 | 62.5 |
| 7f | tBu | 100 | 100 |
| 7g | Ph | 100 | 100 |
| 7h | Bn | 40 | 25 |
| Nereistoxin | / | 100 | 100 |
| Chlorpyrifos | / | 100 | 100 |
| Compound | y = ax + b | LC50 (μg/mL) | R2 | CI (95%) |
|---|---|---|---|---|
| 7a | y = 1.0818x + 1.8613 | 796.91 | 0.9796 | 336.36–1888.06 |
| 7b | y = 1.3741x + 0.9845 | 836.34 | 0.9583 | 408.44–1712.54 |
| 7c | y = 1.6902x + 0.0770 | 817.84 | 0.9653 | 448.10–1492.67 |
| 7f | y = 1.3767x + 2.0590 | 136.86 | 0.9864 | 73.56–254.45 |
| 7g | y = 1.7743x + 1.0230 | 174.33 | 0.9979 | 102.15–297.51 |
| Nereistoxin | y = 1.8644x + 0.9391 | 150.71 | 0.9784 | 92.25–246.21 |
| Chlorpyrifos | y = 1.6690x + 2.9764 | 16.31 | 0.9829 | 9.44–28.20 |
| Compound | R | Corrected Mortality Rate (%) | |||
|---|---|---|---|---|---|
| M. persicae | R. padi | ||||
| 24 h | 48 h | 24 h | 48 h | ||
| 7a | Me | 21.71 | 32.71 | 20.86 | 44.56 |
| 7b | Et | 20.65 | 57.38 | 30.20 | 83.57 |
| 7c | nPr | 23.16 | 33.45 | 21.32 | 42.43 |
| 7d | iPr | 15.51 | 17.68 | 24.63 | 27.50 |
| 7e | nBu | 42.93 | 40.25 | 43.01 | 43.12 |
| 7f | tBu | 13.03 | 80.03 | 19.92 | 89.97 |
| 7g | Ph | 3.96 | 3.61 | 7.15 | 25.45 |
| 7h | Bn | −0.63 | 23.27 | 0.48 | 32.23 |
| Nereistoxin | \ | 14.25 | 34.19 | 12.71 | 40.22 |
| Thiamethoxam | \ | 70.01 | 94.12 | — | — |
| Flunicotamid | \ | — | — | 93.23 | |
| Compound | M. persicae | |||
|---|---|---|---|---|
| y = ax + b | LC50 (μg/mL) | R2 | CI (95%) | |
| 7b | y = 0.0014x + 0.4399 | 42.93 | 0.9540 | 41.37–67.58 |
| 7f | y = 0.0019x + 0.2371 | 138.37 | 0.9662 | 20.08–57.26 |
| Thiamethoxam | y = 0.0194x + 0.2725 | 11.73 | 0.9684 | 21.80–77.11 |
| Compound | R. padi | |||
|---|---|---|---|---|
| y = ax + b | LC50 (μg/mL) | R2 | CI (95%) | |
| 7b | y = 1.6584x + 2.0732 | 58.19 | 0.9839 | 50.62–66.88 |
| 7f | y = 1.245x + 2.3602 | 131.64 | 0.9858 | 101.15–171.32 |
| Flunicotamid | y = 2.2140x + 2.7094 | 10.83 | 0.9726 | 6.67–12.12 |
| Compound | R | AChE (kcal/mol) | α7-nAchR (kcal/mol) | ||
|---|---|---|---|---|---|
| AChE-1 | AChE-2 | α7-nAchR-1 | α7-nAchR-2 | ||
| 7a | Me | −6.2 | −6.1 | −5.4 | −4.8 |
| 7b | Et | −6.6 | −6.4 | −5.7 | −4.9 |
| 7c | nPr | −7.0 | −7.0 | −5.9 | −5.0 |
| 7d | iPr | −7.4 | −6.9 | −6.0 | −5.5 |
| 7e | nBu | −7.2 | −5.0 | −5.9 | −5.0 |
| 7f | tBu | −6.7 | −5.4 | −6.2 | −5.6 |
| 7g | Ph | −9.2 | −9.1 | −8.1 | −7.4 |
| 7h | Bn | −9.0 | −6.2 | −8.5 | −6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Lu, X.; Zhang, Z.; Jin, Q.; Gao, R.; Li, L.; Wang, H. Synthesis, Bioactivity and Molecular Docking of Nereistoxin Derivatives Containing Phosphonate. Molecules 2023, 28, 4846. https://doi.org/10.3390/molecules28124846
Yan Q, Lu X, Zhang Z, Jin Q, Gao R, Li L, Wang H. Synthesis, Bioactivity and Molecular Docking of Nereistoxin Derivatives Containing Phosphonate. Molecules. 2023; 28(12):4846. https://doi.org/10.3390/molecules28124846
Chicago/Turabian StyleYan, Qiaoli, Xiaogang Lu, Zixuan Zhang, Qian Jin, Runli Gao, Liqin Li, and Hongmei Wang. 2023. "Synthesis, Bioactivity and Molecular Docking of Nereistoxin Derivatives Containing Phosphonate" Molecules 28, no. 12: 4846. https://doi.org/10.3390/molecules28124846





