Detection of Hazelnut and Almond Adulteration in Olive Oil: An Approach by qPCR
Abstract
:1. Introduction
2. Results and Discussion
2.1. DNA Extraction
2.2. Selection of a Specific System to Detect Almond or Hazelnut
2.3. Sensitivity in DNA Isolated from Vegetable Oils
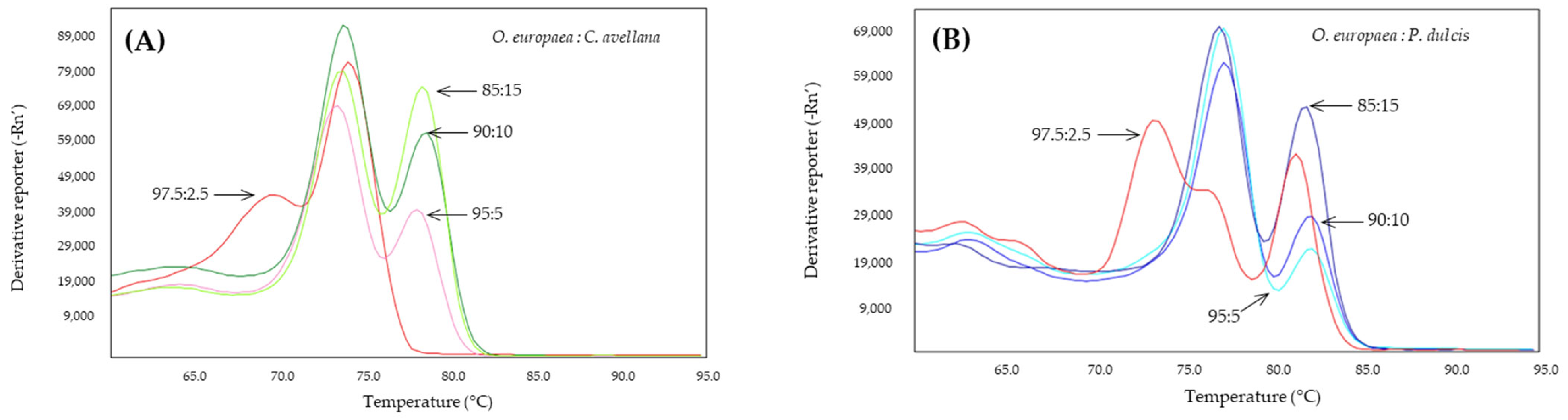
2.4. Detection of Almond and Hazelnut in Olive Oils
3. Materials and Methods
3.1. Plant Tissue and Oil Samples
3.2. DNA Extraction
3.3. Oligonucleotide Primers
3.4. qPCR Assay
3.5. Evaluation and Selection of the qPCR Systems
3.6. Detection of Olive Oil Adulteration
Nested qPCR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- International Olive Council (IOC). Olive World: Olive Oil. Available online: https://www.internationaloliveoil.org/olive-world/olive-oil/ (accessed on 9 November 2022).
- Aroca-Santos, R.; Lastra-Mejías, M.; Cancilla, J.C.; Torrecilla, J.S. Linear and non-linear quantification of extra virgin olive oil, soybean oil, and sweet almond oil in blends to assess their commercial labels. Food Compost. Anal. 2019, 75, 70–74. [Google Scholar] [CrossRef]
- Fernández-Lobato, L.; López-Sánchez, Y.; Blejman, G.; Jurado, F.; Moyano-Fuentes, J.; Vera, D. Life cycle assessment of the Spanish virgin olive oil production: A case study for Andalusian region. J. Clean. Prod. 2021, 290, 125677. [Google Scholar] [CrossRef]
- International Olive Council (IOC). World’s Olive Oil Production Has Tripled. 2021. Available online: https://www.internationaloliveoil.org/worlds-olive-oil-production-has-tripled/ (accessed on 9 November 2022).
- Azadmard-Damirchi, S. Review of the use of phytosterols as a detection tool for adulteration of olive oil with hazelnut oil. Food Addit. Contam. A 2010, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Salah, W.A.; Nofal, M. Review of some adulteration detection techniques of edible oils. J. Sci. Food Agric. 2021, 101, 811–819. [Google Scholar] [CrossRef]
- Christopoulou, E.; Lazaraki, M.; Komaitis, M.; Kaselimis, K. Effectiveness of determinations of fatty acids and triglycerides for the detection of adulteration of olive oils with vegetable oils. Food Chem. 2004, 84, 463–474. [Google Scholar] [CrossRef]
- Amereih, S.; Barghouthi, Z.; Marowan, O. Detection and quantification of adulteration in olive oil using a UV-spectrophotometric method. Palest. Tech. Univ. Res. J. 2014, 2, 14–19. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Torbati, M. Adulterations in some edible oils and fats and their detection methods. J. Food Qual. Hazards Control 2015, 2, 38–44. [Google Scholar]
- Carrillo, W.; Carpio, C.; Morales, D.; Vilcacundo, E.; Alvarez, M.; Silva, M. Content of fatty acids in corn (Zea mays L.) oil from Ecuador. Asian J. Pharm. Clin. Res. 2017, 10, 150–153. [Google Scholar] [CrossRef]
- Jabeur, H.; Zribi, A.; Makni, J.; Rebai, A.; Abdelhedi, R.; Bouaziz, M. Detection of chemlali extra-virgin olive oil adulteration mixed with soybean oil, corn oil, and sunflower oil by using GC and HPLC. J. Agric. Food Chem. 2014, 62, 4893–4904. [Google Scholar] [CrossRef]
- Zabaras, D. Olive oil adulteration with hazelnut oil and analytical approaches for its detection. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK, 2010; pp. 441–450. [Google Scholar]
- González-Domínguez, R.; Sayago, A.; Morales, M.T.; Fernández-Recamales, Á. Assessment of virgin olive oil adulteration by a rapid luminescent method. Foods 2019, 8, 287. [Google Scholar] [CrossRef]
- Blanch, G.P.; Caja, M.D.; del Castillo, M.L.R.; Herraiz, M. Comparison of different methods for the evaluation of the authenticity of olive oil and hazelnut oil. J. Agric. Food Chem. 1998, 46, 3153–3157. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Jeleń, H.H. The potential of different techniques for volatile compounds analysis coupled with PCA for the detection of the adulteration of olive oil with hazelnut oil. Food Chem. 2008, 110, 751–761. [Google Scholar] [CrossRef]
- Marigheto, N.A.; Kemsley, E.K.; Defernez, M.; Wilson, R.H. A comparison of mid-infrared and raman spectroscopies for the authentication of edible oils. J. Am. Oil Chem. Soc. 1998, 75, 987–992. [Google Scholar] [CrossRef]
- Baeten, V.; Fernández Pierna, J.A.; Dardenne, P.; Meurens, M.; García-González, D.L.; Aparicio-Ruiz, R. Detection of the presence of hazelnut oil in olive oil by FT-Raman and FT-MIR spectroscopy. J. Agric. Food Chem. 2005, 53, 6201–6206. [Google Scholar] [CrossRef]
- Maggio, R.M.; Cerretani, L.; Chiavaro, E.; Kaufman, T.S.; Bendini, A. A novel chemometric strategy for the estimation of extra virgin olive oil adulteration with edible oils. Food Control 2010, 21, 890–895. [Google Scholar] [CrossRef]
- Sayago, A.; Morales, M.T.; Aparicio, R. Detection of hazelnut oil in virgin olive oil by a spectrofluorimetric method. Eur. Food Res. Technol. 2004, 218, 480–483. [Google Scholar] [CrossRef]
- López-Díez, E.C.; Bianchi, G.; Goodacre, R. Rapid quantitative assessment of the adulteration of virgin olive oils with hazelnut oils using Raman spectroscopy and chemometrics. J. Agric. Food Chem. 2003, 51, 6145–6150. [Google Scholar] [CrossRef]
- Fauhl, C.; Reniero, F.; Guillou, C. 1H NMR as a tool for the analysis of mixtures of virgin olive oil with oils of different botanical origin. Mag. Reson. Chem. 2000, 38, 436–443. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P. High resolution NMR characterization of olive oils in terms of quality, authenticity and geographical origin. Mag. Reson. Chem. 2011, 49, 3–11. [Google Scholar] [CrossRef]
- Parker, T.; Limer, E.; Watson, A.D.; Defernez, M.; Williamson, D.; Kemsley, E.K. 60 MHz 1H NMR spectroscopy for the analysis of edible oils. TrAC Trends Anal. Chem. 2014, 57, 147–158. [Google Scholar] [CrossRef]
- Hou, X.; Wang, G.; Wang, X.; Ge, X.; Fan, Y.; Jiang, R.; Nie, S. Rapid screening for hazelnut oil and high-oleic sunflower oil in extra virgin olive oil using low-field nuclear magnetic resonance relaxometry and machine learning. J. Sci. Food Agric. 2021, 101, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Vigli, G.; Philippidis, A.; Spyros, A.; Dais, P. Classification of edible oils by employing 31P and 1H NMR spectroscopy in combination with multivariate statistical analysis. A proposal for the detection of seed oil adulteration in virgin olive oils. J. Agric. Food Chem. 2003, 51, 5715–5722. [Google Scholar] [CrossRef] [PubMed]
- Agiomyrgianaki, A.; Petrakis, P.V.; Dais, P. Detection of refined olive oil adulteration with refined hazelnut oil by employing NMR spectroscopy and multivariate statistical analysis. Talanta 2010, 80, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, G. 13C nuclear magnetic resonance spectroscopic detection of the adulteration of extra virgin olive oils extracted from different cultivars with cold-pressed hazelnut oil. J. AOAC Int. 2009, 92, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- García-González, D.L.; Mannina, L.; D’Imperio, M.; Segre, A.; Aparicio, R. Using 1H and 13C NMR techniques and artificial neural networks to detect the adulteration of olive oil with hazelnut oil. Eur. Food Res. Technol. 2004, 219, 545–548. [Google Scholar] [CrossRef]
- Ahmad, Z. The uses and properties of almond oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- Costa, J.; Mafra, I.; Oliveira, M.B. Advances in vegetable oil authentication by DNA-based markers. Trends Food Sci. Technol. 2012, 26, 43–55. [Google Scholar] [CrossRef]
- Ok, S. Detection of olive oil adulteration by low-field NMR relaxometry and UV-Vis spectroscopy upon mixing olive oil with various edible oils. Grasas Y Aceites 2017, 68, e173. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lapsley, K.; Blumberg, J. A nutrition and health perspective on almonds. J. Sci. Food Agric. 2006, 86, 2245–2250. [Google Scholar] [CrossRef]
- Masiri, J.; Benoit, L.; Meshgi, M.; Day, J.; Nadala, C.; Samadpour, M. A novel immunoassay test system for detection of modified allergen residues present in almond-, cashew-, coconut-, hazelnut-, and soy-based nondairy beverages. J. Food Prot. 2016, 79, 1572–1582. [Google Scholar] [CrossRef]
- Su, M.; Venkatachalam, M.; Liu, C.; Zhang, Y.; Roux, K.H.; Sathe, S.K. A murine monoclonal antibody based enzyme-linked immunosorbent assay for almond (Prunus dulcis L.) detection. J. Agric. Food Chem. 2013, 61, 10823–10833. [Google Scholar] [CrossRef]
- Heick, J.; Fischer, M.; Pöpping, B. First screening method for the simultaneous detection of seven allergens by liquid chromatography mass spectrometry. J. Chromatogr. A 2011, 1218, 938–943. [Google Scholar] [CrossRef]
- Prieto, N.; Iniesto, E.; Burbano, C.; Cabanillas, B.; Pedrosa, M.M.; Rovira, M.; Rodríguez, J.; Muzquiz, M.; Crespo, J.F.; Cuadrado, C.; et al. Detection of almond allergen coding sequences in processed foods by real time PCR. J. Agric. Food Chem. 2014, 62, 5617–5624. [Google Scholar] [CrossRef]
- Batrinou, A.; Strati, I.F.; Houhoula, D.; Tsaknis, J.; Sinanoglou, V.J. Authentication of olive oil based on DNA analysis. Grasas Y Aceites 2020, 71, e366. [Google Scholar] [CrossRef]
- Montemurro, C.; Miazzi, M.M.; Pasqualone, A.; Fanelli, V.; Sabetta, W.; di Rienzo, V. Traceability of PDO olive oil “Terra di Bari” using high resolution melting. J. Chem. 2015, 2015, 496989. [Google Scholar] [CrossRef]
- Bazakos, C.; Khanfir, E.; Aoun, M.; Spano, T.; Zein, Z.E.; Chalak, L.; Riachy, M.E.; Abou-Sleymane, G.; Ali, S.B.; Grati Kammoun, N.; et al. The potential of SNP-based PCR-RFLP capillary electrophoresis analysis to authenticate and detect admixtures of Mediterranean olive oils. Electrophoresis 2016, 37, 1881–1890. [Google Scholar] [CrossRef]
- Pasqualone, A.; Montemurro, C.; di Rienzo, V.; Summo, C.; Paradiso, V.M.; Caponio, F. Evolution and perspectives of cultivar identification and traceability from tree to oil and table olives by means of DNA markers. J. Sci. Food Agric. 2016, 96, 3642–3657. [Google Scholar] [CrossRef]
- Kalaitzis, P.; El-Zein, Z. Olive oil authentication, traceability and adulteration detection using DNA-based approaches. Lipid Technol. 2016, 28, 173–176. [Google Scholar] [CrossRef]
- Debode, F.; Janssen, E.; Marien, A.; Berben, G. DNA detection by conventional and real-time PCR after extraction from vegetable oils. J. Am. Oil Chem. Soc. 2012, 89, 1249–1257. [Google Scholar] [CrossRef]
- He, J.; Xu, W.; Shang, Y.; Zhu, P.; Mei, X.; Tian, W.; Huang, K. Development and optimization of an efficient method to detect the authenticity of edible oils. Food Control 2013, 31, 71–79. [Google Scholar] [CrossRef]
- Ramos-Gómez, S.; Busto, M.D.; Perez-Mateos, M.; Ortega, N. Development of a method to recovery and amplification DNA by real-time PCR from commercial vegetable oils. Food Chem. 2014, 158, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Rebollo, A.; Ramos-Gómez, S.; Busto, M.D.; Ortega, N. Development and optimization of an efficient qPCR system for olive authentication in edible oils. Food Chem. 2017, 232, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ganopoulos, I.; Bazakos, C.; Madesis, P.; Kalaitzis, P.; Tsaftaris, A. Barcode DNA high-resolution melting (Bar-HRM) analysis as a novel close-tubed and accurate tool for olive oil forensic use. J. Sci. Food Agric. 2013, 93, 2281–2286. [Google Scholar] [CrossRef]
- Vietina, M.; Agrimonti, C.; Marmiroli, N. Detection of plant oil DNA using high resolution melting (HRM) post PCR analysis: A tool for disclosure of olive oil adulteration. Food Chem. 2013, 141, 3820–3826. [Google Scholar] [CrossRef]
- Ramos-Gómez, S.; Busto, M.D.; Albillos, S.M.; Ortega, N. Novel qPCR systems for olive (Olea europaea L.) authentication in oils and food. Food Chem. 2016, 194, 447–454. [Google Scholar] [CrossRef]
- Uncu, A.T.; Uncu, A.O.; Frary, A.; Doganlar, S. Barcode DNA length polymorphisms vs fatty acid profiling for adulteration detection in olive oil. Food Chem. 2017, 221, 1026–1033. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Agrimonti, C.; Vietina, M.; Pafundo, S.; Marmiroli, N. The use of food genomics to ensure the traceability of olive oil. Trends Food Sci. Technol. 2011, 22, 237–244. [Google Scholar] [CrossRef]
- Arlorio, M.; Cereti, E.; Coïsson, J.D.; Travaglia, F.; Martelli, A. Detection of hazelnut (Corylus spp.) in processed foods using real-time PCR. Food Control 2007, 18, 140–148. [Google Scholar] [CrossRef]
- Iniesto, E.; Jiménez, A.; Prieto, N.; Cabanillas, B.; Burbano, C.; Pedrosa, M.M.; Rodríguez, J.; Muzquiz, M.; Crespo, J.F.; Cuadrado, C.; et al. Real time PCR to detect hazelnut allergen coding sequences in processed foods. Food Chem. 2013, 138, 1976–1981. [Google Scholar] [CrossRef]
- Schöringhumer, K.; Redl, G.; Cichna-Markl, M. Development and validation of a duplex real-time PCR method to simultaneously detect potentially allergenic sesame and hazelnut in food. J. Agric. Food Chem. 2009, 57, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Linacero, R.; Ballesteros, I.; Sanchiz, A.; Prieto, N.; Iniesto, E.; Martinez, Y.; Pedrosa, M.M.; Muzquiz, M.; Cabanillas, B.; Rovira, M.; et al. Detection by real time PCR of walnut allergen coding sequences in processed foods. Food Chem. 2016, 202, 334–340. [Google Scholar] [CrossRef]
- Consolandi, C.; Palmieri, L.; Severgnini, M.; Maestri, E.; Marmiroli, N.; Agrimonti, C.; Baldoni, L.; Donini, P.; De Bellis, G.; Gastiglioni, B. A procedure for olive oil traceability and authenticity: DNA extraction, multiplex PCR and LDR–universal array analysis. Eur. Food Res. Technol. 2008, 227, 1429–1438. [Google Scholar] [CrossRef]
- Raieta, K.; Muccillo, L.; Colantuoni, V. A novel reliable method of DNA extraction from olive oil suitable for molecular traceability. Food Chem. 2015, 172, 596–602. [Google Scholar] [CrossRef]
- Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Novel approach based on single-tube nested real-time PCR to detect almond allergens in foods. Food Res. Int. 2013, 51, 228–235. [Google Scholar] [CrossRef]
- Piknová, L.; Pangallo, D.; Kuchta, T. A novel real-time polymerase chain reaction (PCR) method for the detection of hazelnuts in food. Eur. Food Res. Technol. 2008, 226, 1155–1158. [Google Scholar] [CrossRef]
- D’Andrea, M.; Coïsson, J.D.; Locatelli, M.; Garino, C.; Cereti, E.; Arlorio, M. Validating allergen coding genes (Cor a 1, Cor a 8, Cor a 14) as target sequences for hazelnut detection via Real-Time PCR. Food Chem. 2011, 124, 1164–1171. [Google Scholar] [CrossRef]
- Köppel, R.; Dvorak, V.; Zimmerli, F.; Breitenmoser, A.; Eugster, A.; Waiblinger, H.-U. Two tetraplex real-time PCR for the detection and quantification of DNA from eight allergens in food. Eur. Food Res. Technol. 2010, 230, 367–374. [Google Scholar] [CrossRef]
- López-Calleja, I.M.; de la Cruz, S.; Pegels, N.; González, I.; Martín, R.; García, T. Sensitive and specific detection of almond (Prunus dulcis) in commercial food products by real-time PCR. LWT-Food Sci. Technol. 2014, 56, 31–39. [Google Scholar] [CrossRef]
- Brežná, B.; Šmíd, J.; Costa, J.; Radwanszky, J.; Mafra, I.; Kuchta, T. In silico and experimental evaluation of DNA-based detection methods for the ability to discriminate almond from other Prunus spp. Mol. Cell. Probes 2015, 29, 99–115. [Google Scholar] [CrossRef]
- Kubista, M.; Andrade, J.M.; Bengtsoon, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L.; et al. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, R.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bergerová, E.; Brežná, B.; Kuchta, T. A novel method with improved sensitivity for the detection of peanuts based upon single-tube nested real-time polymerase chain reaction. Eur. Food Res. Technol. 2011, 232, 1087–1091. [Google Scholar] [CrossRef]
- Christy, A.A.; Kasemsumran, S.; Du, Y.; Ozaki, Y. The detection and quantification of adulteration in olive oil by near-infrared spectroscopy and chemometrics. Anal. Sci. 2004, 20, 935–940. [Google Scholar] [CrossRef]
- Peña, F.; Cárdenas, S.; Gallego, M.; Valcárcel, M. Direct olive oil authentication: Detection of adulteration of olive oil with hazelnut oil by direct coupling of headspace and mass spectrometry, and multivariate regression techniques. J. Chromatogr. A 2005, 1074, 215–221. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Peptides and proteins in edible oils: Stability, allergenicity, and new processing trends. Trends Food Sci. Technol. 2006, 17, 56–63. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Identification of species-specific peptide markers in cold-pressed oils. Sci. Rep. 2020, 10, 19971. [Google Scholar] [CrossRef]

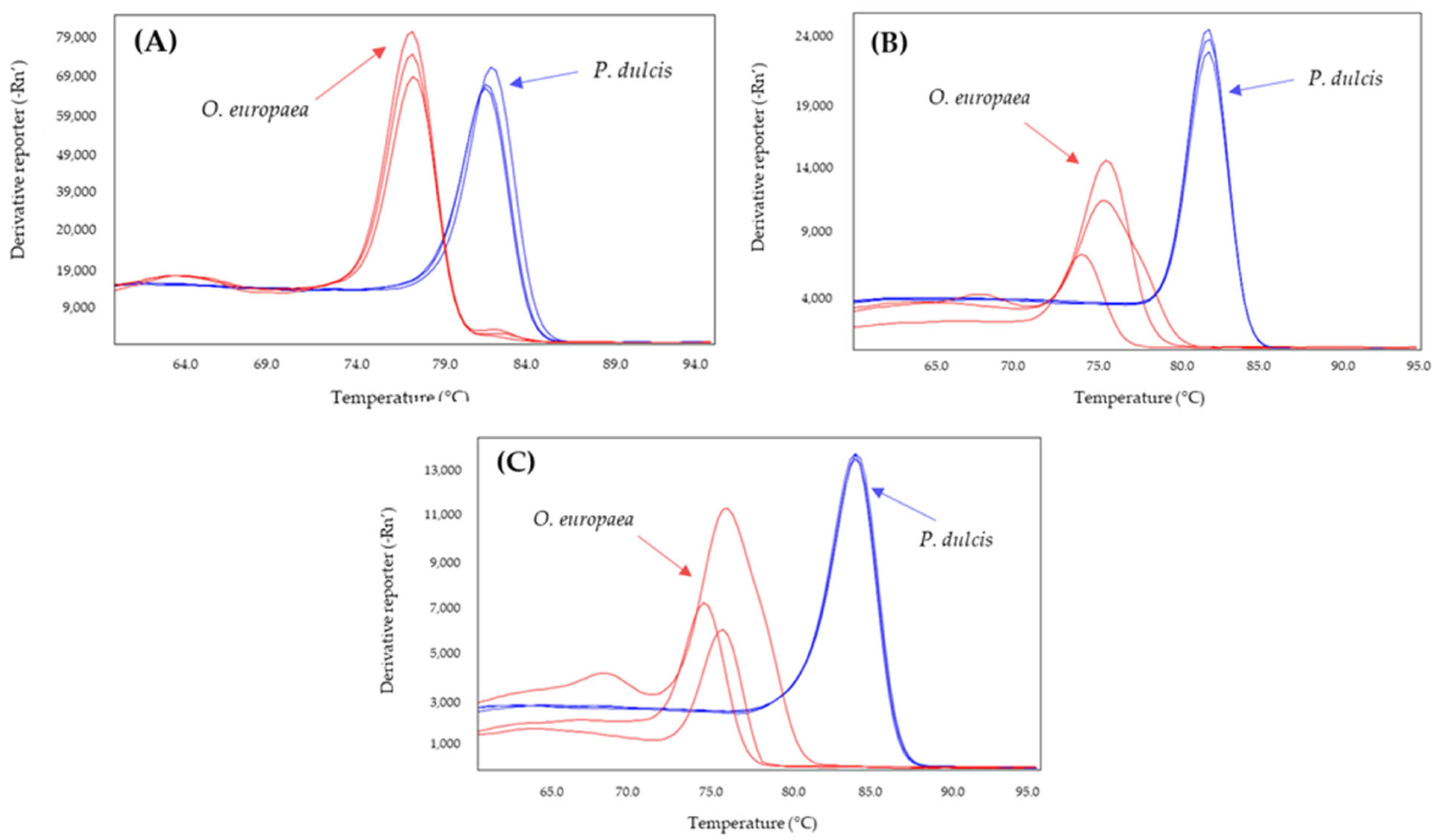
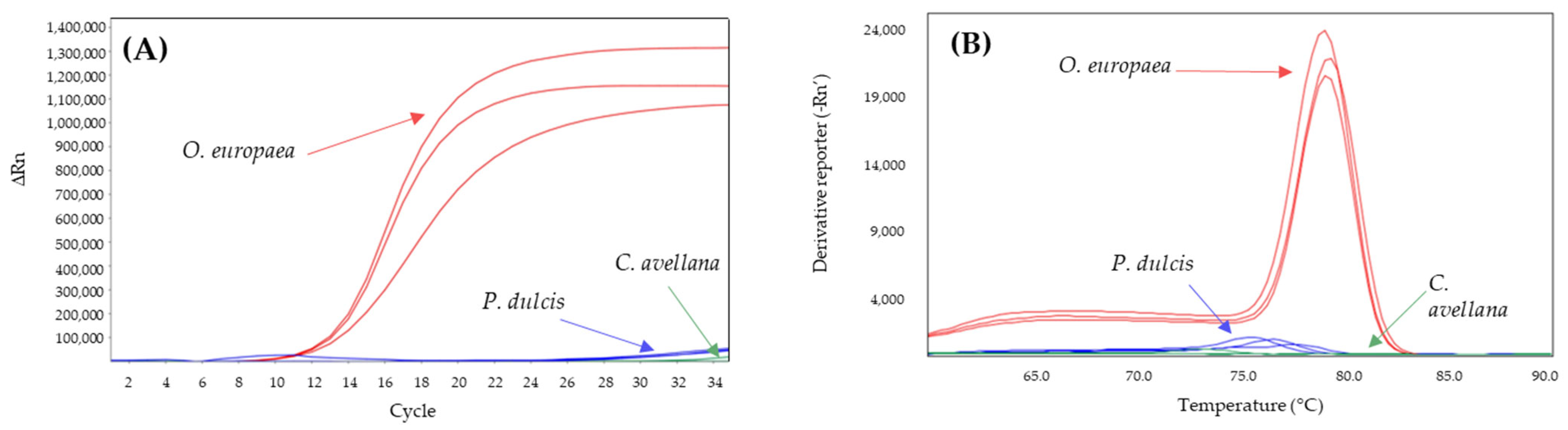
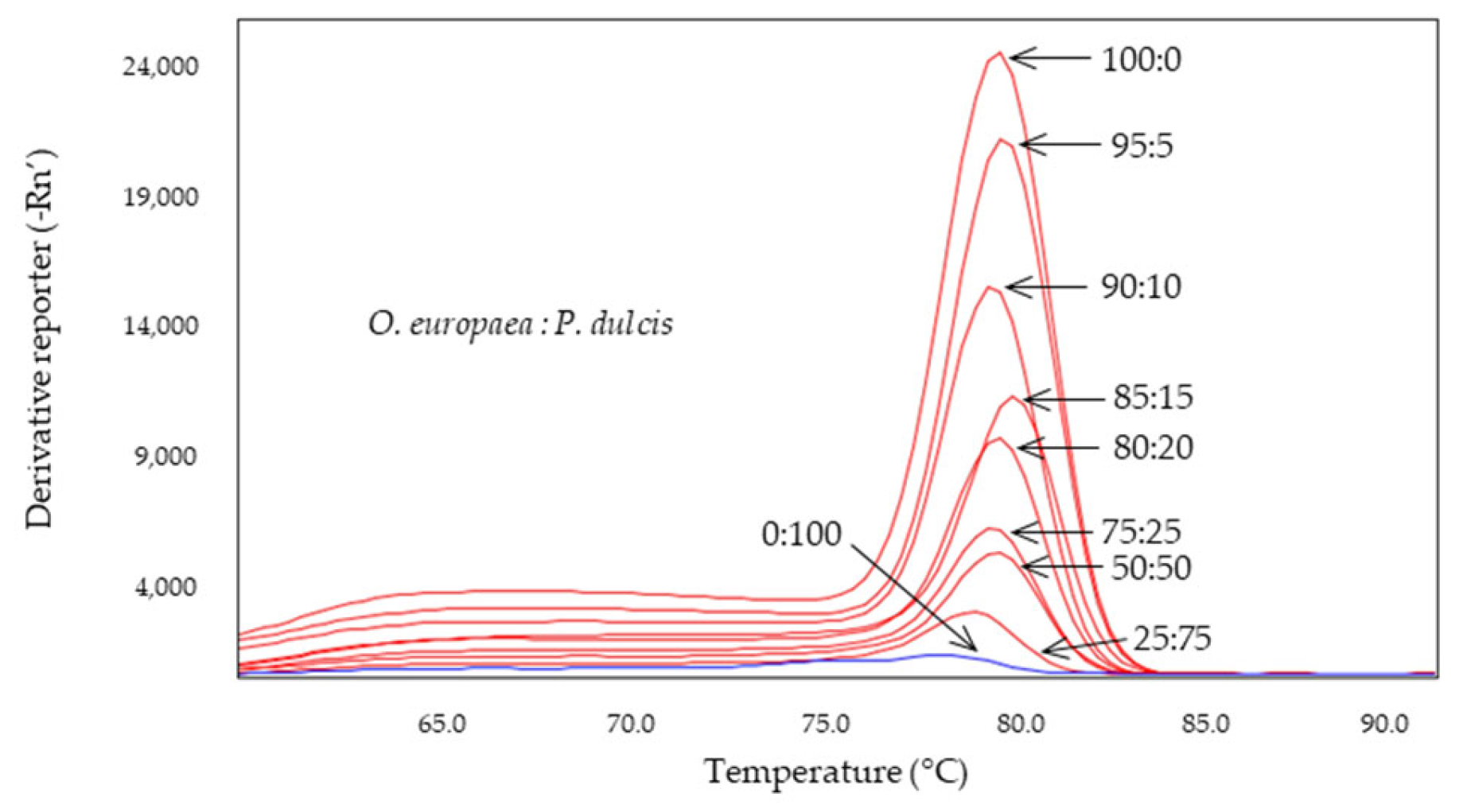
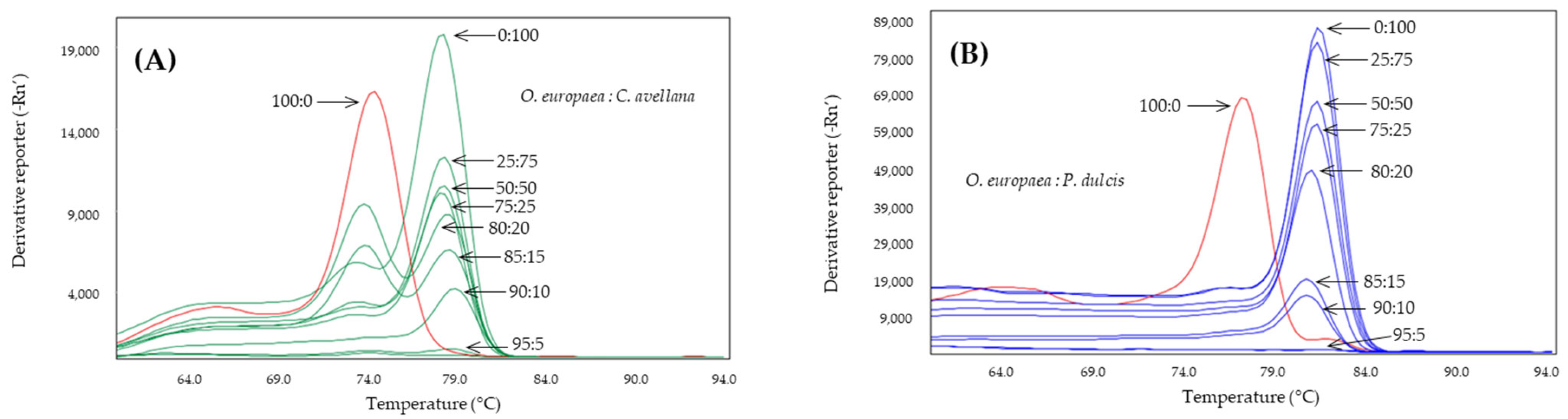
| Adulterant Specie | System | Tm Amplicon (°C) | |
|---|---|---|---|
| Adulterant | O. europaea | ||
| C. avellana | Hsp1 | 84.0 | 84.0 |
| Nocc1 | 84.5 | 77.0 | |
| Cora1FW/RS | 79.0 | 79.0 | |
| Cora1F2/R2 | 80.0 | 75.5 | |
| Cora13 | 86.0 | 86.0 | |
| P. dulcis | Madl | 83.5 | 78.0 |
| Prd6 | 86.0 | 86.0 | |
| Pru du 1 | 75.5 | 75.5 | |
| thau | 82.0 | 75.0 | |
| AlmondITS | 84.5 | 77.0 | |
| Adulterant Specie | System | Tm Amplicon (°C) a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adulterant | O. europaea | ||||||||
| 60 °C | 61 °C | 63 °C | 65 °C | 60 °C | 61 °C | 63 °C | 65 °C | ||
| C. avellana | Nocc1 | 84.5 | 84.5 | 84.5 | 84.5 | 78.0 | 84.5 | 84.5 | 84.5 |
| Cora1F2/R2 | 79.0 | 79.0 | 79.0 | N/A | 74.5 | 78.0 | 80.0 | 80.0 | |
| P. dulcis | Madl | 81.5 | 81.5 | N/A | N/A | 78.0 | 77.0 | 79.0 | 79.0 |
| thau | 82.0 | 82.0 | N/A | N/A | 75.0 | 75.0 | N/A | N/A | |
| AlmondITS | 84.5 | 84.0 | 83.5 | N/A | 77.0 | 75.0 | 82.5 | N/A | |
| Target | System | Calibration Curve | Accuracy and Efficiency | Analytical Sensitivity | |||
|---|---|---|---|---|---|---|---|
| Slope | Intercept | R2 a | E b (%) | LOD c (pg) | LOQ d (pg) | ||
| C. avellana | Nocc1 | −3.306 | 21.553 | 0.997 | 100.31 | 5 | 50 |
| Cora1F2/R2 | −3.372 | 19.016 | 0.997 | 97.95 | 5 | 5 | |
| P. dulcis | Madl | −3.390 | 24.702 | 0.995 | 97.25 | 5 | 5 |
| thau | −3.255 | 25.247 | 0.977 | 102.89 | 5 | 50 | |
| AlmondITS | −5.011 | 20.311 | 0.947 | 58.33 | 5 | - | |
| O. europaea | D-trnL | −3.322 | 14.499 | 0.999 | 100.01 | 0.5 | 0.5 |
| Name | Sequence (5′-3′) | PCR Conditions a | Target (bp) | GenBank Accesion | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Conc. (nM) | Den. t (s) | Ann. T (°C), t (s) | Ext. t (s) | Cycles | |||||
| Hsp1 | F: AGCGTCGAGAGTGGCAAGTTC R: CCTGCTCGCCTCCGCTTTC | 200 | 15 | 66, 45 | 60 | 50 | 126 | AF021807 | [58] |
| Nocc1 | F: GGCAAGTTCGTGAGCAGGTTC R: CTTTCGGAATAGTCACAGTGAG | 500 | 15 | 60, 60 | -- | 35 | 100 | AF021807 | [59] |
| Cora1FW/RS | F: GCTTTGTCCGACAAACTGGAG R: TCCTATGGTGTGGTACTTGCTG | 250 | 30 | 55, 30 | 40 | 35 | 105 | Z72440 | [60] |
| Cora1F2/R2 | F: ACTACATAAAGCAAAAGGTTGAAG R: TCGTAATTGATTTTCTCCAGTTTG | 800 | 15 | 60, 60 | -- | 35 | 109 | Z72440 | [54] |
| Cora13 | F: GCGGTCATCACAGTATCGCTT R: GTCACGTACCTGTAGATCCACGAC | 200 | 15 | 60, 60 | -- | 40 | 101 | AY224599 | [53] |
| Madl | F: CCTAGCGGAGGATCCATCATC R: GGTCTCAATGAGCTTGAAGAG | 160 | 15 | 60, 60 | 60 | 45 | 129 | BQ641046 | [61] |
| Prd6 | F: CCGCAGAACCAGTGCCAGCT R: CCCCGGCACACTGGAAGTCCT | 300 | 15 | 65, 45 | -- | -- | 121 | EU919663 | [58] |
| Pru du 1 | F: AGTGTATTGTGATTGGCTCCC R: AGTCTTTGGCTTGCATTTGG | 200 | 15 | 60, 60 | -- | 40 | 100 | KC969088 | [36] |
| thau | F: ACTGAGCACAACGGAATATC R: TAGGATGCCGTGCGTAGC | 500 | 15 | 58, 30 | 30 | 55 | 113 | EU424262 | [62] |
| AlmondITS | F: CTAGCCGAACGACCCGAGA R: CCGAGATAAAGGGGACGAG | 300F 900R | 5 | 60, 30 | -- | 55 | 76 | HF969276 | [63] |
| D-trnL | F: GGGCAATCCTGTAGCCAAA R: ACGCAGTCCACTCCATTTGT | 100 | 30 | 53, 30 | 60 | 40 | 110 | DQ131560 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Gómez, S.; Busto, M.D.; Ortega, N. Detection of Hazelnut and Almond Adulteration in Olive Oil: An Approach by qPCR. Molecules 2023, 28, 4248. https://doi.org/10.3390/molecules28104248
Ramos-Gómez S, Busto MD, Ortega N. Detection of Hazelnut and Almond Adulteration in Olive Oil: An Approach by qPCR. Molecules. 2023; 28(10):4248. https://doi.org/10.3390/molecules28104248
Chicago/Turabian StyleRamos-Gómez, Sonia, María D. Busto, and Natividad Ortega. 2023. "Detection of Hazelnut and Almond Adulteration in Olive Oil: An Approach by qPCR" Molecules 28, no. 10: 4248. https://doi.org/10.3390/molecules28104248






