Mössbauer and Structure-Magnetic Properties Analysis of AyB1−yCxFe2−xO4 (C=Ho,Gd,Al) Ferrite Nanoparticles Optimized by Doping
Abstract
:1. Introduction
2. Results and Analysis
2.1. X-ray Diffraction Characterisation
2.2. Scanning Electron Microscopy (SEM)
2.3. Magnetic Analysis
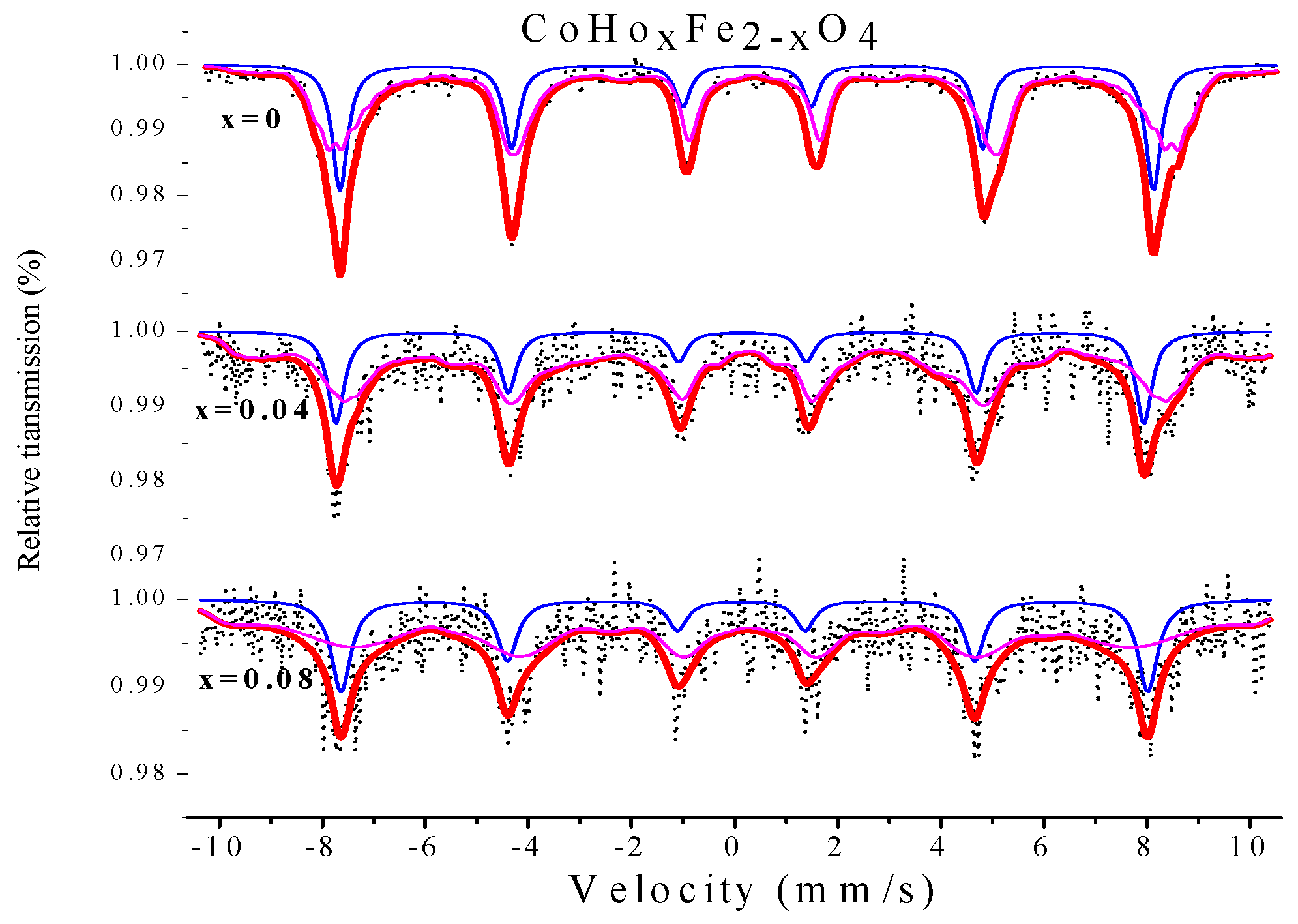
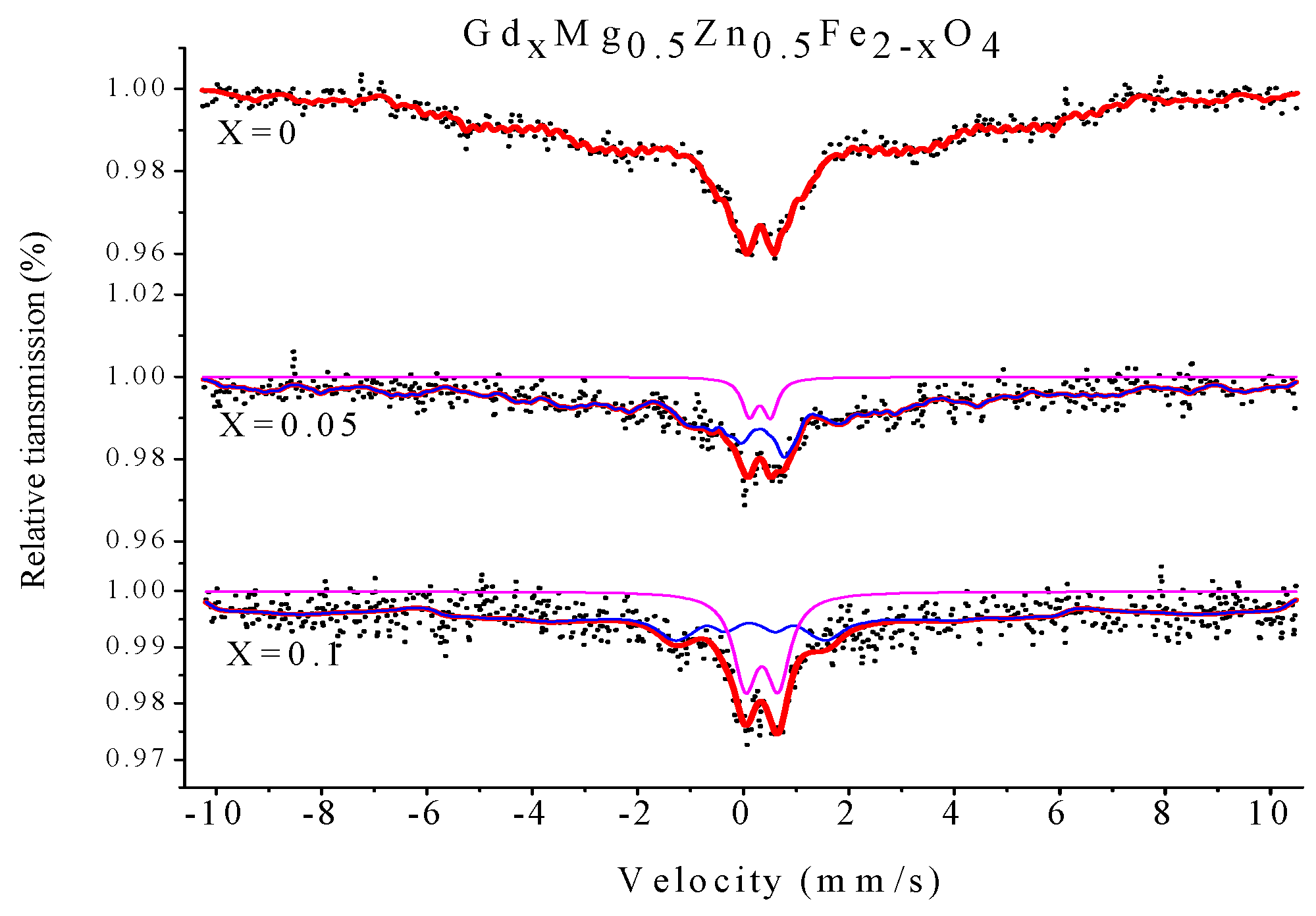
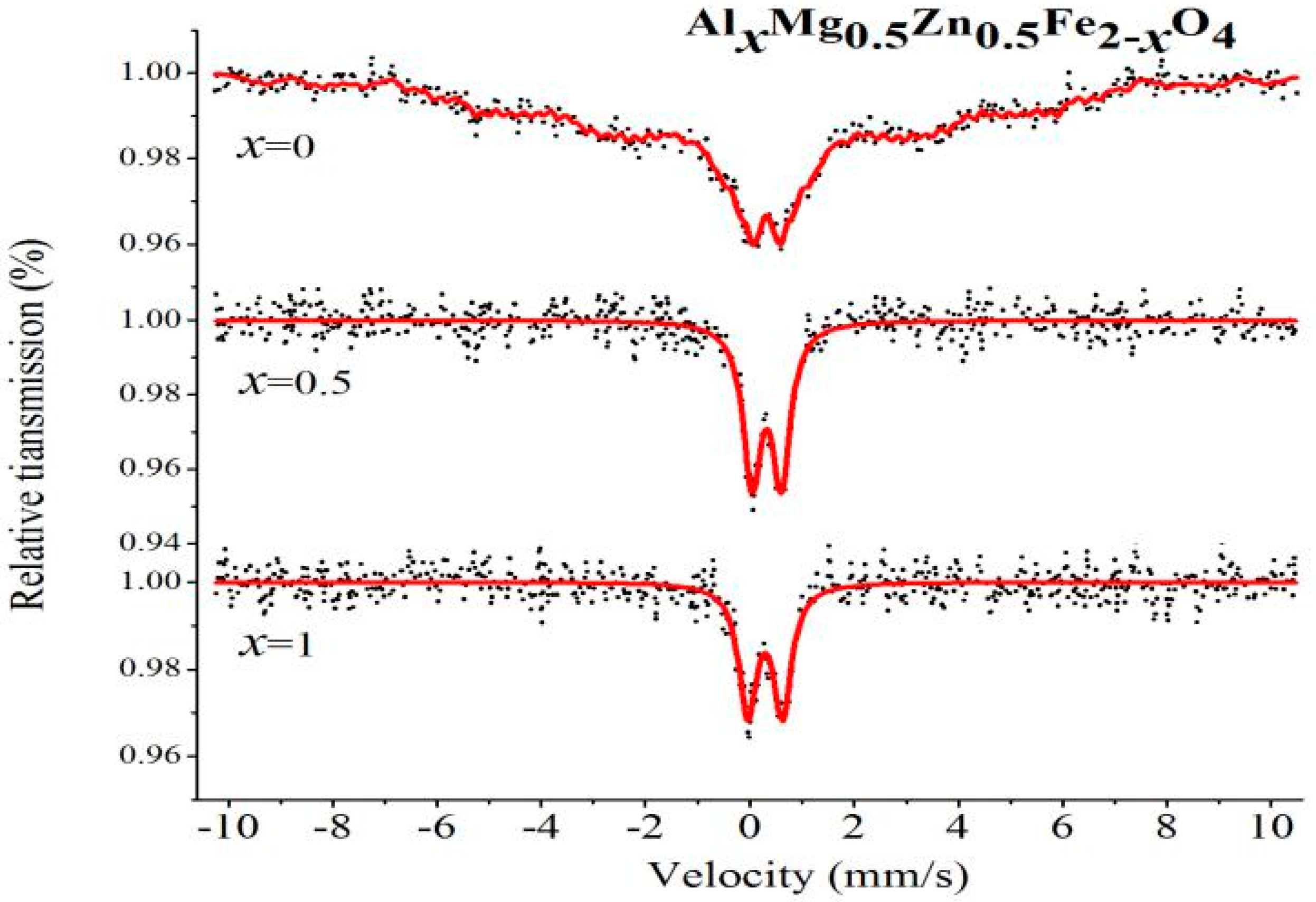
2.4. Mössbauer Spectroscopy
3. Experimental
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mohamed, R.M.; Rashada, M.M.; Haraz, F.A.; Sigmund, W. Structure and magnetic properties of nanocrystalline cobalt ferrite powders synthesized using organic acid precursor method. J. Magn. Magn. Mater. 2010, 322, 2058–2064. [Google Scholar] [CrossRef]
- Amiri, S.; Shokrollahi, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. C 2013, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanpo, N.; Berndt, C.C.; Wen, C.; Wang, J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013, 9, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.H. Experimental and modeling studies of the magnetomechanical effect in substituted cobalt ferrites for magnetoelastic stress sensors. J. Appl. Phys. 2010, 107, 5798. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Zhao, X.; Yu, L.; Cui, Y.; Feng, S. Magnetic properties of CoFe2O4 ferrite doped with rare earth ion. Mater. Lett. 2006, 60, 1–6. [Google Scholar] [CrossRef]
- Meng, X.; Li, H.; Chen, J.; Mei, L.; Wang, K.; Li, X. Mössbauer study of cobalt ferrite nanocrystals substituted with rare-earth Y3+ ions. J. Magn. Magn. Mater. 2009, 321, 1155–1158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, D. Infrared emission properties of RE (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, and Dy) and Mn co-doped Co0.6Zn0.4Fe2O4 ferrites. Mater. Chem. Phys. 2012, 131, 575–580. [Google Scholar] [CrossRef]
- Lohar, K.S.; Pachpinde, A.M.; Langade, M.M.; Kadam, R.H.; Shirsath, S.E. Self-propagating high temperature synthesis, structural morphology and magnetic interactions in rare earth Ho3+ doped CoFe2O4 nanoparticles. J. Alloys Compd. 2014, 604, 204–210. [Google Scholar] [CrossRef]
- Ali, I.; Islam, M.U.; Ishaque, M.; Khan, H.M.; Ashiq, M.N.; Rana, M.U. Structural and magnetic properties of holmium substituted cobalt ferrites synthesized by chemical co-precipitation method. J. Magn. Magn. Mater. 2012, 324, 3773–3777. [Google Scholar] [CrossRef]
- Pervaiz, E.; Gul, I.H. High frequency AC response, DC resistivity and magnetic studies of holmium substituted Ni-ferrite: A novel electromagnetic material. J. Magn. Magn. Mater. 2014, 349, 27–34. [Google Scholar] [CrossRef]
- Muthuselvam, I.P.; Bhowmik, R.N. Mechanical alloyed Ho3+ doping in CoFe2O4 spinel ferrite and understanding of magnetic nanodomains. J. Magn. Magn. Mater. 2010, 322, 767–776. [Google Scholar] [CrossRef]
- Amiri, S.; Shokrollahi, H. Magnetic and structural properties of RE doped Co-ferrite (RE = Nd, Eu, and Gd) nano-particles synthesized by co-precipitation. J. Magn. Magn. Mater. 2013, 345, 18–23. [Google Scholar] [CrossRef]
- Shinde, T.J.; Gadkari, A.B.; Vasambeka, P.N. Effect of Nd3+ substitution on structural and electrical properties of nanocrystalline zinc ferrite. J. Magn. Magn. Mater. 2010, 322, 2777–2781. [Google Scholar] [CrossRef]
- Panda, R.N.; Shih, J.C.; Chin, T.S. Magnetic properties of nano-crystalline Gd- or Pr-substituted CoFe2O4 synthesized by the citrate precursor technique. J. Magn. Magn. Mater. 2003, 257, 79–86. [Google Scholar] [CrossRef]
- Ruiz, M.M.; Mietta, J.L.; Antonel, P.S.; Pérez, O.E.; Negri, R.M.; Jorge, G. Structural and magnetic properties of Fe2-xCoSmxO4-nanoparticles and Fe2-xCoSmxO4-PDMS magnetoelastomers as a function of Sm content. J. Magn. Magn. Mater. 2013, 327, 11–19. [Google Scholar] [CrossRef]
- Tahar, L.B.; Smiri, L.S.; Artus, M.; Joudrier, A.-L.; Herbst, F.; Aulay, M.J.; Ammar, S.; Fiévet, F. Characterization and magnetic properties of Sm- and Gd-substituted CoFe2O4 nanoparticles prepared by forced hydrolysis in polyol. Mater. Res. Bull. 2007, 42, 1888–1896. [Google Scholar] [CrossRef]
- Borhan, A.I.; Slatineanu, T.; Iordan, A.R.; Palamaru, M.N. Influence of chromium ion substitution on the structure and properties of zinc ferrite synthesized by the sol-gel auto-combustion method. Polyhedron 2013, 56, 82–89. [Google Scholar] [CrossRef]
- Karimi, Z.; Mohammadifar, Y.; Shokrollahi, H.; Asl, S.K.; Yousefi, G.; Karimi, L. Magnetic and structural properties of nano sized Dy-doped cobalt ferrite synthesized by co-precipitation. J. Magn. Magn. Mater. 2014, 361, 150–156. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Yu, L.; Cui, Y.; Zhao, X.; Feng, S. Study on magnetic properties of nanocrystalline La-, Nd-, or Gd-substituted Ni-Mn ferrite at low temperatures. J. Magn. Magn. Mater. 2006, 305, 91–94. [Google Scholar] [CrossRef]
- Nikumbh, A.K.; Pawar, R.A.; Nighot, D.V.; Gugale, G.S.; Sangale, M.D.; Khanvilkar, M.B.; Nagawade, A.V. Structural, electrical, magnetic and dielectric properties of rare-earth substituted cobalt ferrites nanoparticles synthesized by the co-precipitation method. J. Magn. Magn. Mater. 2014, 355, 201–209. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Lin, J.; Lin, Q.; Dong, J. Mössbauer spectroscopy, Structural and magnetic studies of Zn2+ substituted magnesium ferrite nanomaterials prepared by Sol-Gel method. J. Nanomater. 2015, 2015, 854840. [Google Scholar] [CrossRef]
- Singhal, S.; Barthwal, S.K.; Chandra, K. XRD, magnetic and Mössbauer spectral studies of nano size aluminum substituted cobalt ferrites (CoAlxFe2-xO4). J. Magn. Magn. Mater. 2006, 306, 233–240. [Google Scholar] [CrossRef]
- Zhao, L.; Han, Z.; Yang, H.; Yu, L.; Cui, Y.; Jin, W.; Feng, S. Magnetic properties of nanocrystalline Ni0.7Mn0.3Gd0.1Fe1.9O4 ferrite at low temperatures. J. Magn. Magn. Mater. 2007, 309, 11–14. [Google Scholar] [CrossRef]
- Inbanathan, S.S.R.; Vaithyanathan, V.; Chelvane, J.A.; Markandeyulu, G.; Bharathi, K.K. Mössbauer studies and enhanced electrical properties of R (R = Sm, Gd and Dy) doped Ni ferrite. J. Magn. Magn. Mater. 2014, 353, 41–46. [Google Scholar] [CrossRef]
- He, Y.; Lei, C.; Lin, Q.; Dong, J.; Yu, Y.; Wang, L. Mössbauer and Structural properties of La-substituted Ni0.4Cu0.2Zn0.4Fe2O4 nanocrystalline ferrite. Sci. Adv. Mater. 2015, 7, 1809–1815. [Google Scholar] [CrossRef]
- Kumar, S.; Farea, A.M.M.; Batoo, K.M.; Lee, C.G.; Koo, B.H.; Yousef, A. Mössbauer studies of Co0.5CdxFe2.5-xO4 (0.0–0.5) ferrite. Phys. B Condens. Matter 2008, 403, 3604–3607. [Google Scholar] [CrossRef]
- Lin, J.; He, Y.; Lin, Q.; Wang, R.; Chen, H. Microstructural and Mössbauer spectroscopy Studies of Mg1-xZnxFe2O4(x = 0.5, 0.7) nanoparticles. J. Spectrosc. 2014, 2014, 540319. [Google Scholar] [CrossRef]
- Al-Maashani, M.; Gismelseed, A.M.; Khalaf, K.A.M.; Yousif, A.A.; Al-Rawas, A.D.; Widatallah, H.M.; Elzain, M.E. Structural and Mössbauer study of nanoparticles CoFe2O4, prepared by sol-gel auto-combustion and subsequent sintering. Hyperfine Interact. 2018, 239, 15. [Google Scholar] [CrossRef]
- Choodamani, C.; Nagabhushana, G.P.; Ashoka, S.; Prasad, B.D.; Rudraswamy, B.; Chandrappa, G.T. Structural and magnetic studies of Mg(1-x)ZnxFe2O4 nanoparticles prepared by a solution combustion method. J. Alloys Compd. 2013, 578, 103–109. [Google Scholar] [CrossRef]
- Mohseni, H.; Shokrollahi, H.; Sharifi, I.; Gheisari, K. Magnetic and structural studies of the Mn-doped Mg-Zn fer rite nanoparticles synthesized by the glycine nitrate process. J. Magn. Magn. Mater. 2012, 324, 3741–3747. [Google Scholar] [CrossRef]
- Haralkar, S.J.; Kadam, R.H.; More, S.S.; Shirsath, S.E.; Mane, M.L.; Patil, S.; Mane, D.R. Substitutional effect of Cr3+ ions on the properties of Mg-Zn ferrite nanoparticles. Phys. B Condens. Matter 2012, 407, 4338–4346. [Google Scholar] [CrossRef]
- Dascalu, G.; Popescu, T.; Feder, M.; Caltun, O.F. Structural, electric and magnetic properties of CoFe1.8RE0.2O4 (RE = Dy, Gd, La) bulk materials. J. Magn. Magn. Mater. 2013, 333, 69–74. [Google Scholar] [CrossRef]
- Chand, J.; Kumar, G.; Kumar, P.; Sharma, S.K.; Knobel, M.; Singh, M. Effect of Gd3+ doping on magnetic, electric and dielectric properties of MgGdxFe2-xO4 ferrites processed by solid state reaction technique. J. Alloys Compd. 2011, 509, 9638–9644. [Google Scholar] [CrossRef]
- Chae, K.P.; Lee, J.-G.; Kweon, H.S.; Lee, Y.B. Synthesis and magnetic properties of AlxCoFe2-xO4 ferrite powders. Phys. Status Solidi 2004, 201, 1883–1888. [Google Scholar] [CrossRef]
- Chandradass, J.; Jadhav, A.H.; Kim, K.H.; Kim, H. Influence of processing methodology on the structural and magnetic behavior of MgFe2O4 nanopowders. J. Alloys Compd. 2012, 517, 164–169. [Google Scholar] [CrossRef]
- Singhal, S.; Barthwal, S.K.; Chandra, K. Cation distribution in the nano size aluminium substituted cobalt ferrites using XRD, magnetic and Mössbauer spectral studies. Indian J. Pure Appl. Phys. 2007, 45, 821–825. [Google Scholar]
- Chandradass, J.; Jadhav, A.H.; Kim, H. Surfactant modified MgFe2O4 nanopowders by reverse micelle processing: Effect of water to surfactant ratio (R) on the particle size and magnetic property. Appl. Surf. Sci. 2012, 258, 3315–3320. [Google Scholar] [CrossRef]
- Peng, J.; Hojamberdiev, M.; Xu, Y.; Cao, B.; Wang, J.; Wu, H. Hydrothermal synthesis and magnetic properties of gadolinium-doped CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2011, 323, 133–138. [Google Scholar] [CrossRef]
- Gabal, M.A.; Abdel-Daiem, A.M.; Al Angari, Y.M.; Ismaeel, I.M. Influence of Al-substitution on structural, electrical and magnetic properties of Mn-Zn ferrites nanopowders prepared via the sol-gel auto-combustion method. Polyhedron 2013, 57, 105–111. [Google Scholar] [CrossRef]
- Lin, Q.; Lei, C.; He, Y.; Xu, J.; Wang, R. Mössbauer and XRD studies of Ni0.6Cu0.2Zn0.2CexFe2−xO4 ferrites By Sol-Gel auto-combustion. J. Nanosci. Nanotechnol. 2015, 15, 2997–3003. [Google Scholar] [CrossRef]
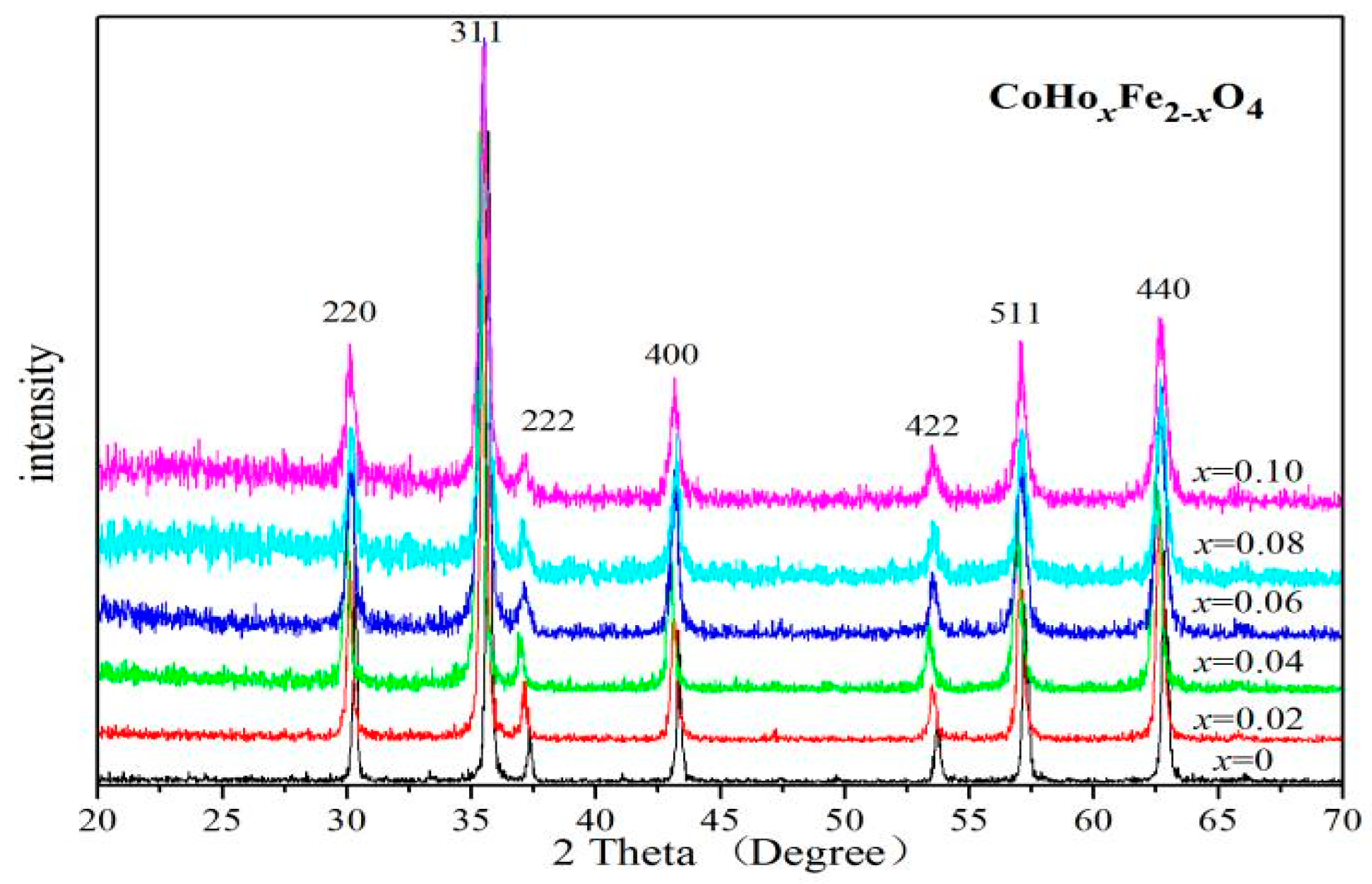




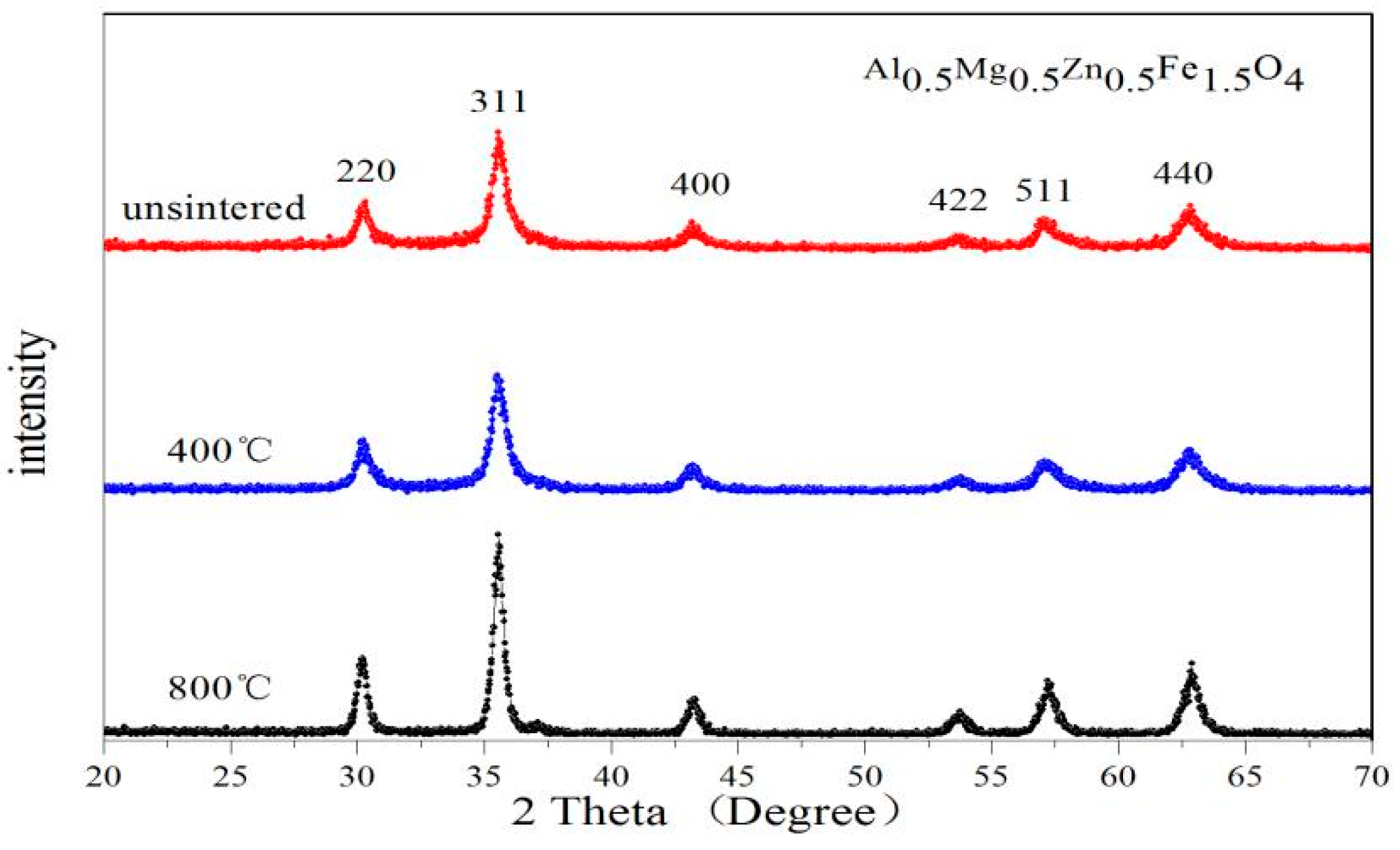
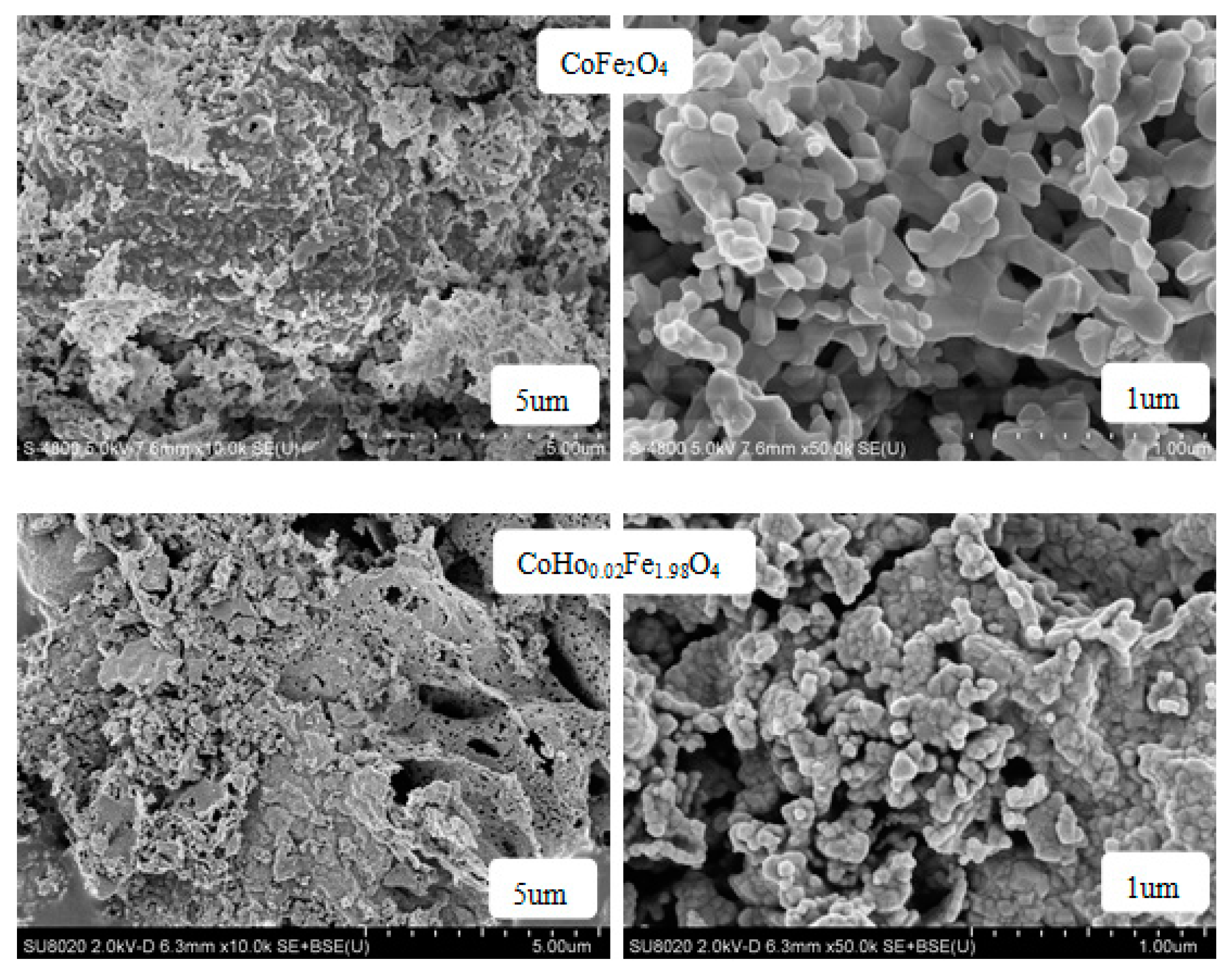

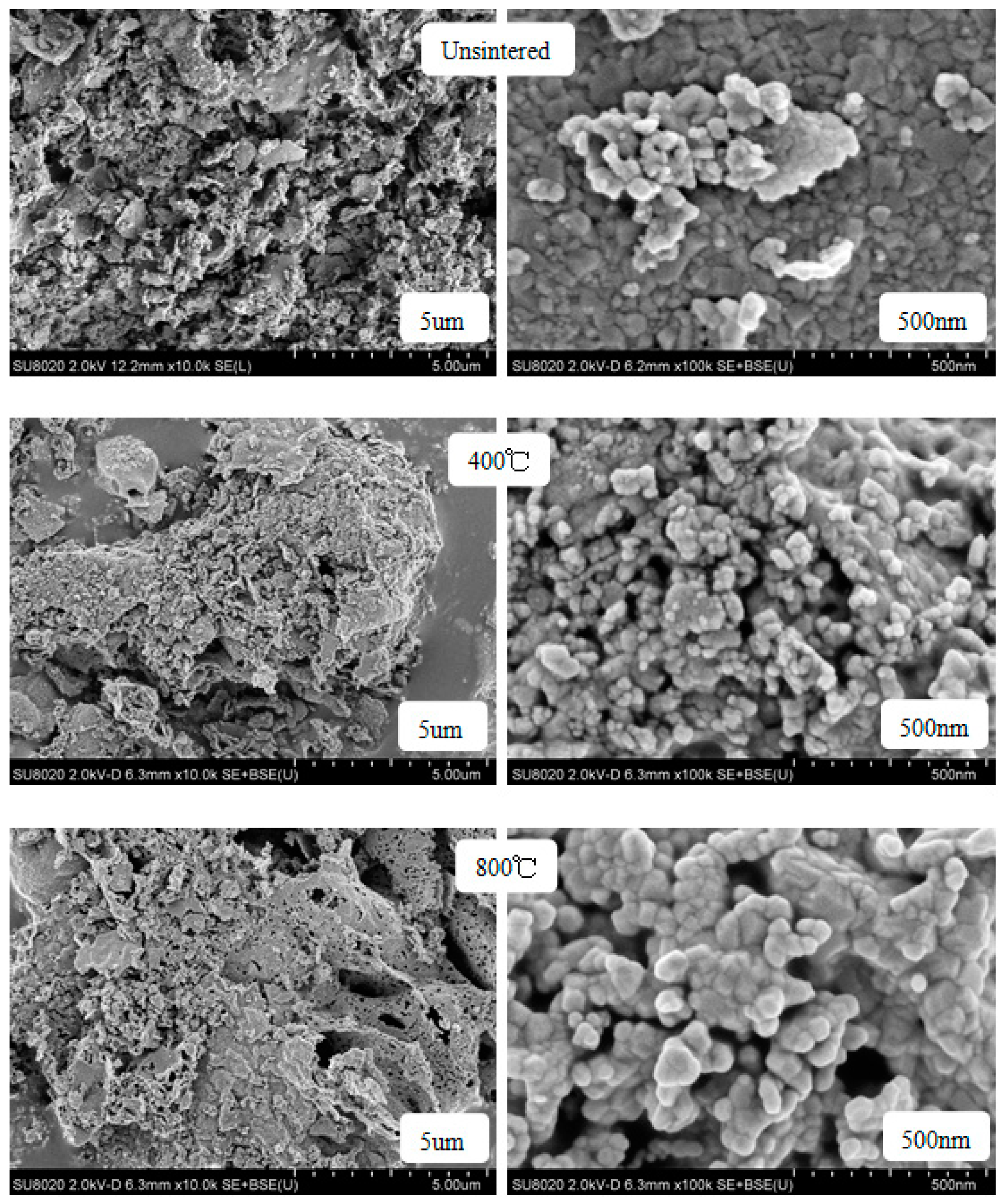

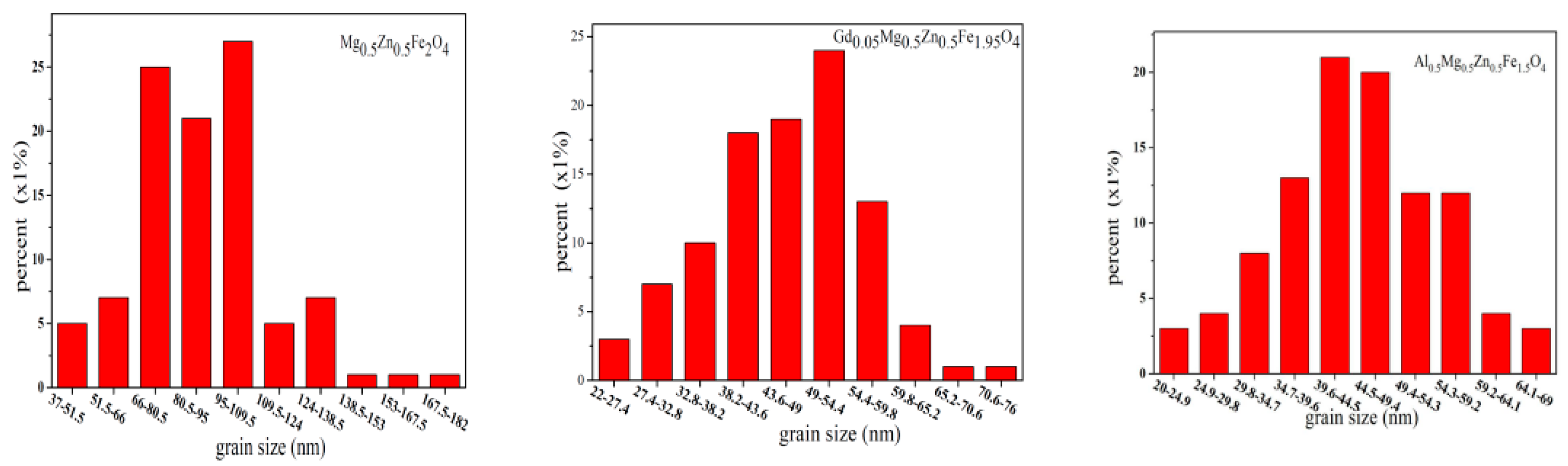
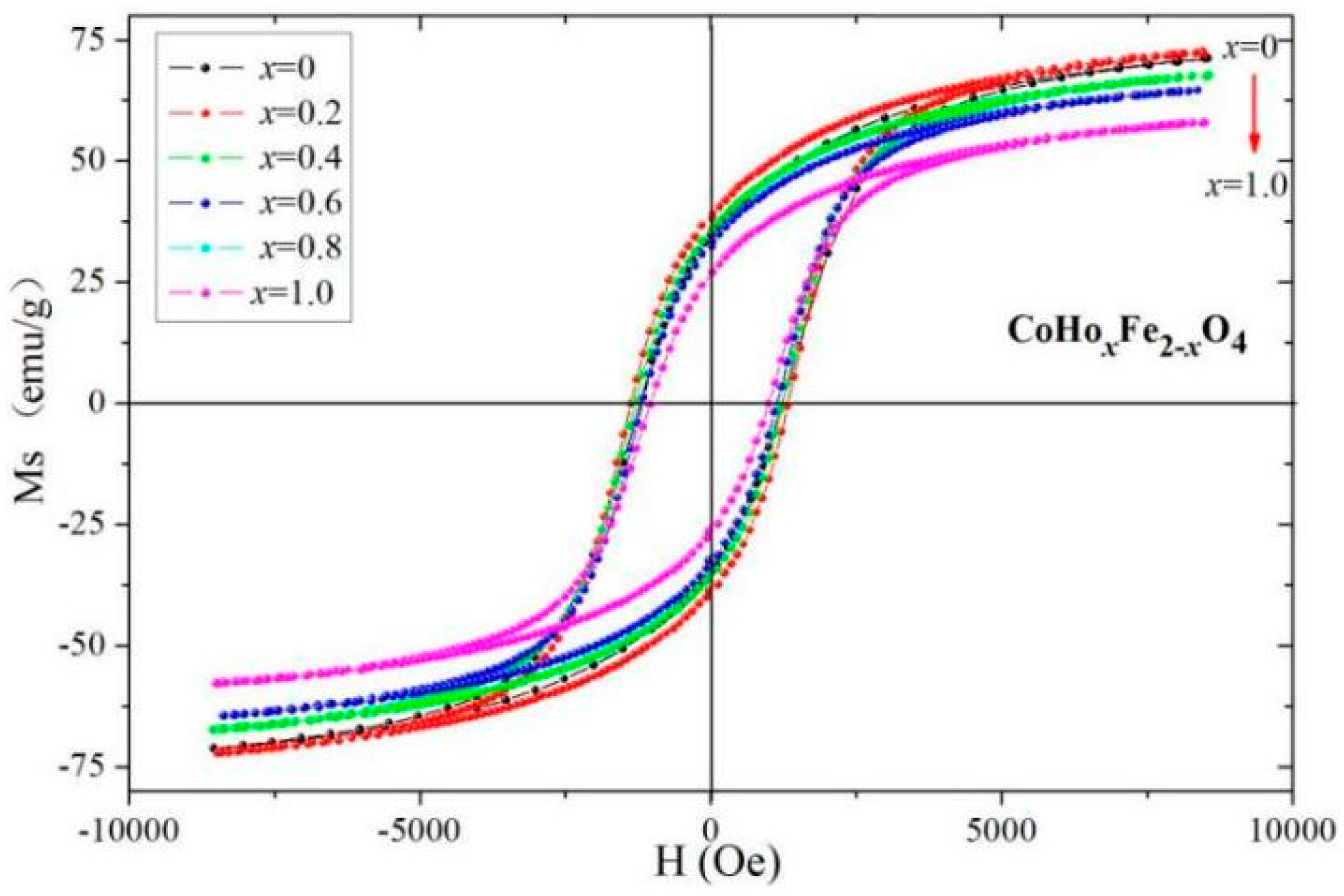


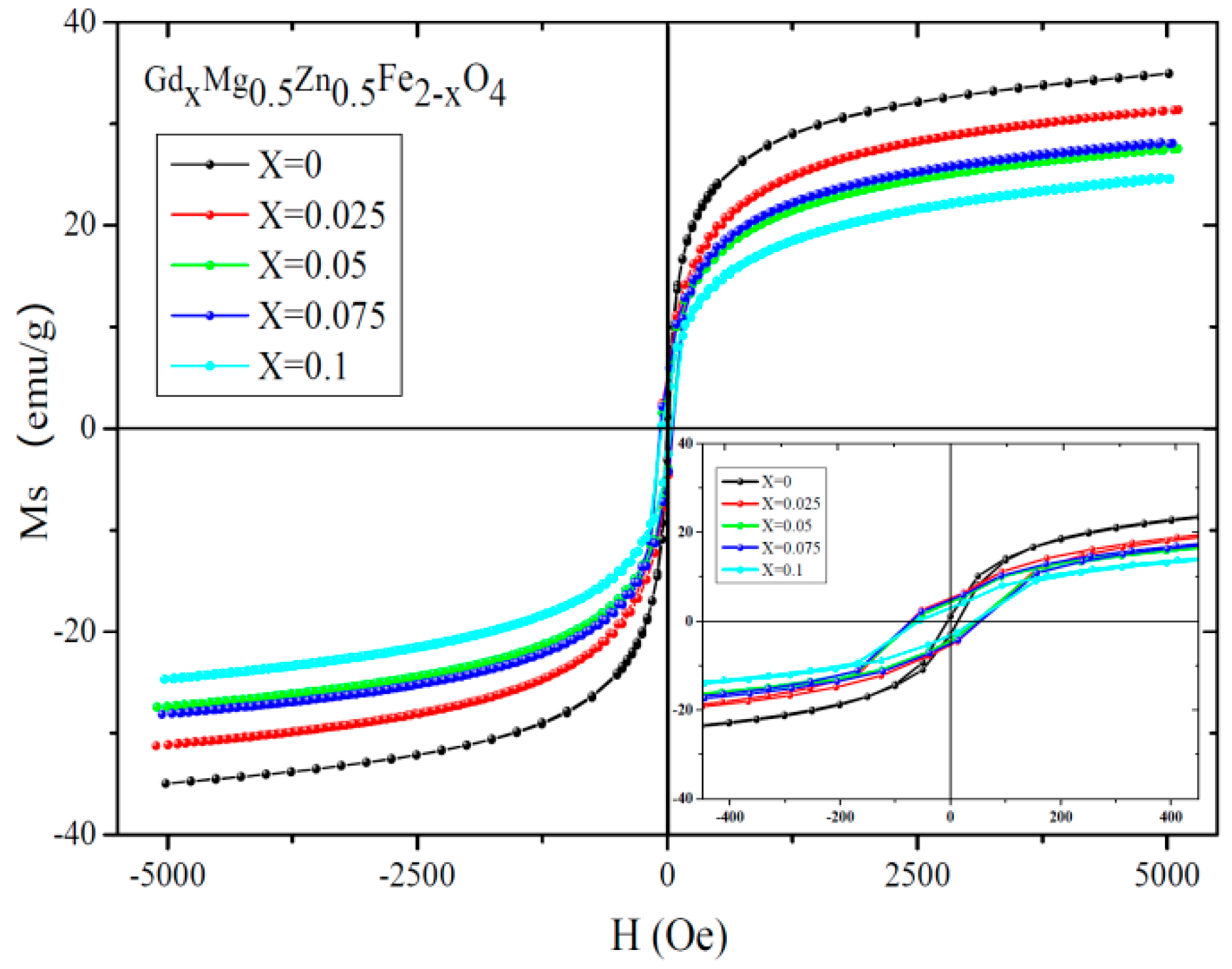
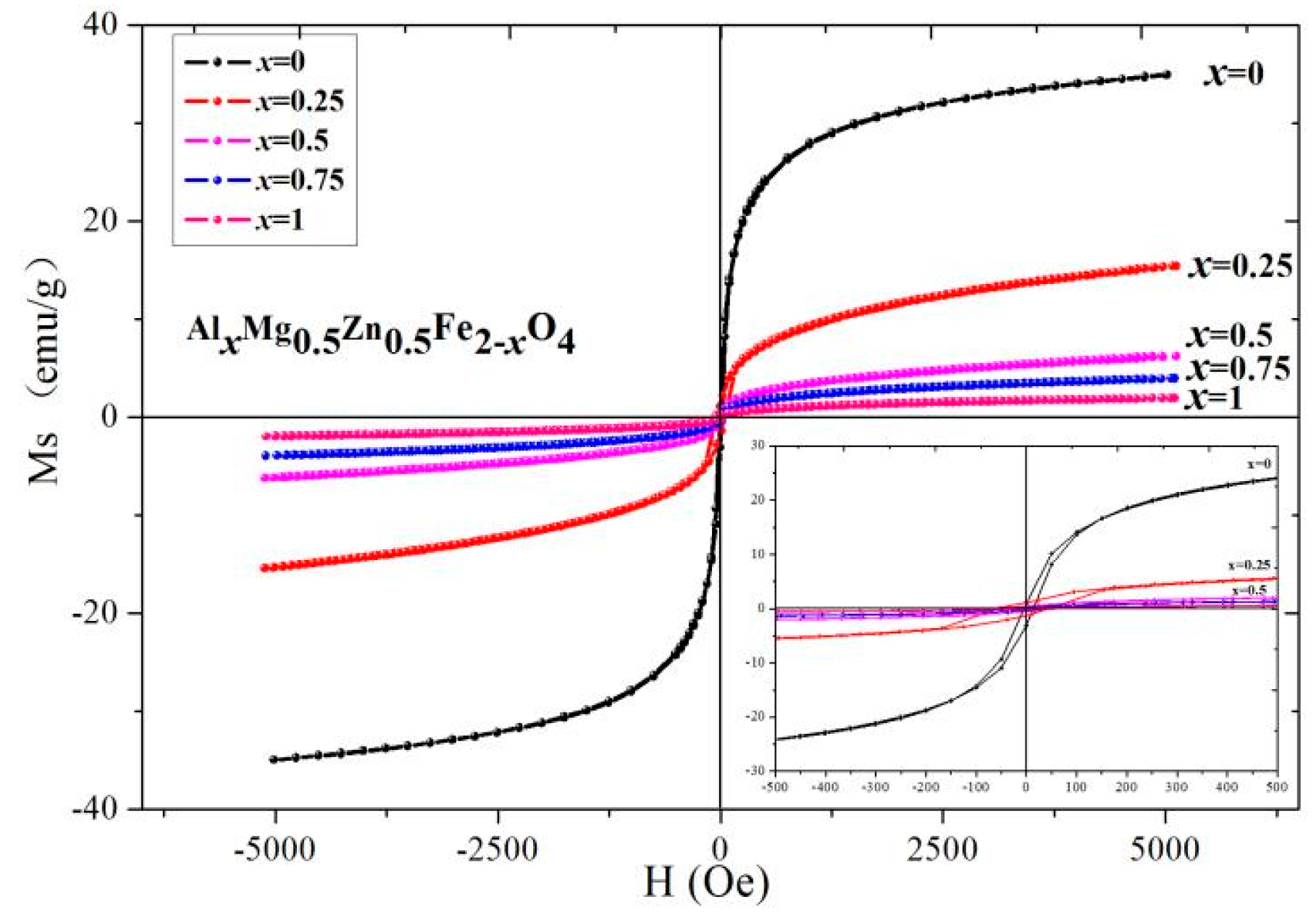

| Content (x) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| 0 | 8.35497 | 556 | 5.3468 |
| 0.02 | 8.38601 | 350 | 5.3342 |
| 0.04 | 8.40681 | 308 | 5.3434 |
| 0.06 | 8.38132 | 229 | 5.4415 |
| 0.08 | 8.37719 | 249 | 5.4989 |
| 0.10 | 8.38928 | 221 | 5.5243 |
| Temperature (°C) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| Un-sintered | 8.38379 | 249 | 5.3384 |
| 400 | 8.39279 | 293 | 5.3212 |
| 800 | 8.38601 | 350 | 5.3342 |
| Content (x) | Lattice Constant (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| 0 | 8.43133 | 377 | 4.8879 |
| 0.025 | 8.42094 | 344 | 4.9624 |
| 0.05 | 8.43047 | 301 | 5.0018 |
| 0.075 | 8.44593 | 290 | 5.0303 |
| 0.1 | 8.42142 | 214 | 5.1307 |
| Content (x) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| 0 | 8.43133 | 377 | 4.8879 |
| 0.25 | 8.39110 | 290 | 4.7963 |
| 0.5 | 8.36480 | 202 | 4.6779 |
| 0.75 | 8.31315 | 158 | 4.5988 |
| 1.0 | 8.25874 | 179 | 4.5201 |
| Temperature (°C) | Lattice Constant (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| Un-sintered | 8.41517 | 228 | 5.0292 |
| 400 °C | 8.41829 | 233 | 5.0236 |
| 800 °C | 8.43047 | 301 | 5.0018 |
| Temperature (°C) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| Un-sintered | 8.38226 | 165 | 4.6487 |
| 400 °C | 8.36985 | 137 | 4.6694 |
| 800 °C | 8.36480 | 202 | 4.6779 |
| Content (x) | MS (emu/g) | HC (Oe) | Mr (emu/g) |
|---|---|---|---|
| 0 | 70.58 | 1005 | 34.71 |
| 0.02 | 72.54 | 1351 | 37.77 |
| 0.04 | 67.58 | 1266 | 35.06 |
| 0.06 | 64.64 | 1158 | 31.90 |
| 0.08 | 61.82 | 1094 | 30.24 |
| 0.10 | 57.96 | 1016 | 26.28 |
| Temperature (°C) | MS (emu/g) | HC (Oe) | Mr (emu/g) |
|---|---|---|---|
| Un-sintered | 60.01 | 1301 | 29.98 |
| 400 | 55.80 | 2234 | 29.62 |
| 500 | 61.10 | 1944 | 32.37 |
| 600 | 63.52 | 1655 | 33.56 |
| 700 | 69.21 | 1426 | 36.91 |
| 800 | 72.54 | 1351 | 37.77 |
| 900 | 76.16 | 1152 | 40.99 |
| Temperature (°C) | MS (emu/g) | HC (Oe) | Mr (emu/g) |
|---|---|---|---|
| Un-sintered | 47.89 | 1290 | 20.68 |
| 400 | 39.75 | 2052 | 18.83 |
| 500 | 45.72 | 1533 | 20.42 |
| 600 | 50.30 | 1174 | 21.50 |
| 700 | 57.05 | 1014 | 25.13 |
| 800 | 57.96 | 1016 | 26.28 |
| 900 | 66.12 | 914 | 33.8 |
| Content (x) | MS (emu/g) | HC (Oe) | Mr (emu/g) |
|---|---|---|---|
| 0 | 34.98 | 13.64 | 1.10 |
| 0.025 | 31.38 | 62.52 | 4.67 |
| 0.05 | 27.54 | 55.79 | 3.79 |
| 0.075 | 28.17 | 62.99 | 4.36 |
| 0.1 | 24.67 | 50.52 | 2.61 |
| Content (x) | MS (emu/g) | HC (Oe) | Mr (emu/g) |
|---|---|---|---|
| 0 | 34.98 | 13.64 | 1.10 |
| 0.25 | 15.39 | 50.37 | 1.38 |
| 0.5 | 6.26 | 53.56 | 0.54 |
| 0.75 | 3.96 | 50.37 | 0.33 |
| 1 | 1.94 | 59.06 | 0.16 |
| Temperature (°C) | MS (emu/g) | HC (Oe) | Mr (emu/g) |
|---|---|---|---|
| Un-sintered | 25.29 | 71.92 | 4.39 |
| 400 °C | 22.93 | 69.58 | 3.73 |
| 800 °C | 27.54 | 55.79 | 3.79 |
| Content (x) | Component | I.S. (mm/s) | Q.S. (mm/s) | H (T) | Γ (mm/s) | A0 (%) |
|---|---|---|---|---|---|---|
| 0 | Sextet (A) | 0.237 | −0.004 | 48.946 | 0.360 | 32.4 |
| Sextet (B) | 0.375 | −0.024 | 45.695 | 0.322 | 67.6 | |
| 0.04 | Sextet (A) | 0.126 | −0.050 | 48.613 | 0.387 | 22.2 |
| Sextet (B) | 0.316 | 0.151 | 44.156 | 0.429 | 77.8 | |
| 0.08 | Sextet (A) | 0.157 | 0.061 | 48.546 | 0.463 | 24.5 |
| Sextet (B) | 0.224 | −0.137 | 43.641 | 0.740 | 75.5 |
| Content (x) | Component | I.S. (mm/s) | Q.S. (mm/s) | H (T) | Γ (mm/s) | A0 (mm/s) |
|---|---|---|---|---|---|---|
| 0 | Sextet (B) | 0.318 | 0.005 | 24.234 | 0.268 | 100 |
| 0.05 | Sextet (B) | 0.302 | −0.173 | 30.350 | 0.334 | 93.1 |
| Doublet | 0.301 | 0.409 | - | 0.327 | 6.9 | |
| 0.1 | Sextet (B) | 0.116 | −0.029 | 38.679 | 0.528 | 79.4 |
| Doublet | 0.348 | 0.627 | - | 0.537 | 20.6 |
| Content (x) | Component | I.S. (mm/s) | Q.S. (mm/s) | H (T) | Γ (mm/s) | A0 (mm/s) |
|---|---|---|---|---|---|---|
| 0 | Sextet (B) | 0.318 | 0.005 | 24.234 | 0.268 | 100 |
| 0.5 | Double | 0.322 | 0.568 | - | 0.417 | 100 |
| 1 | Double | 0.292 | 0.672 | - | 0.414 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Q.; Yang, F.; Zhang, Q.; Su, K.; Xu, H.; He, Y.; Lin, J. Mössbauer and Structure-Magnetic Properties Analysis of AyB1−yCxFe2−xO4 (C=Ho,Gd,Al) Ferrite Nanoparticles Optimized by Doping. Molecules 2023, 28, 4226. https://doi.org/10.3390/molecules28104226
Lin Q, Yang F, Zhang Q, Su K, Xu H, He Y, Lin J. Mössbauer and Structure-Magnetic Properties Analysis of AyB1−yCxFe2−xO4 (C=Ho,Gd,Al) Ferrite Nanoparticles Optimized by Doping. Molecules. 2023; 28(10):4226. https://doi.org/10.3390/molecules28104226
Chicago/Turabian StyleLin, Qing, Fang Yang, Qian Zhang, Kaimin Su, Huiren Xu, Yun He, and Jinpei Lin. 2023. "Mössbauer and Structure-Magnetic Properties Analysis of AyB1−yCxFe2−xO4 (C=Ho,Gd,Al) Ferrite Nanoparticles Optimized by Doping" Molecules 28, no. 10: 4226. https://doi.org/10.3390/molecules28104226





