Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory
Abstract
:1. Introduction
2. Results and Discussion
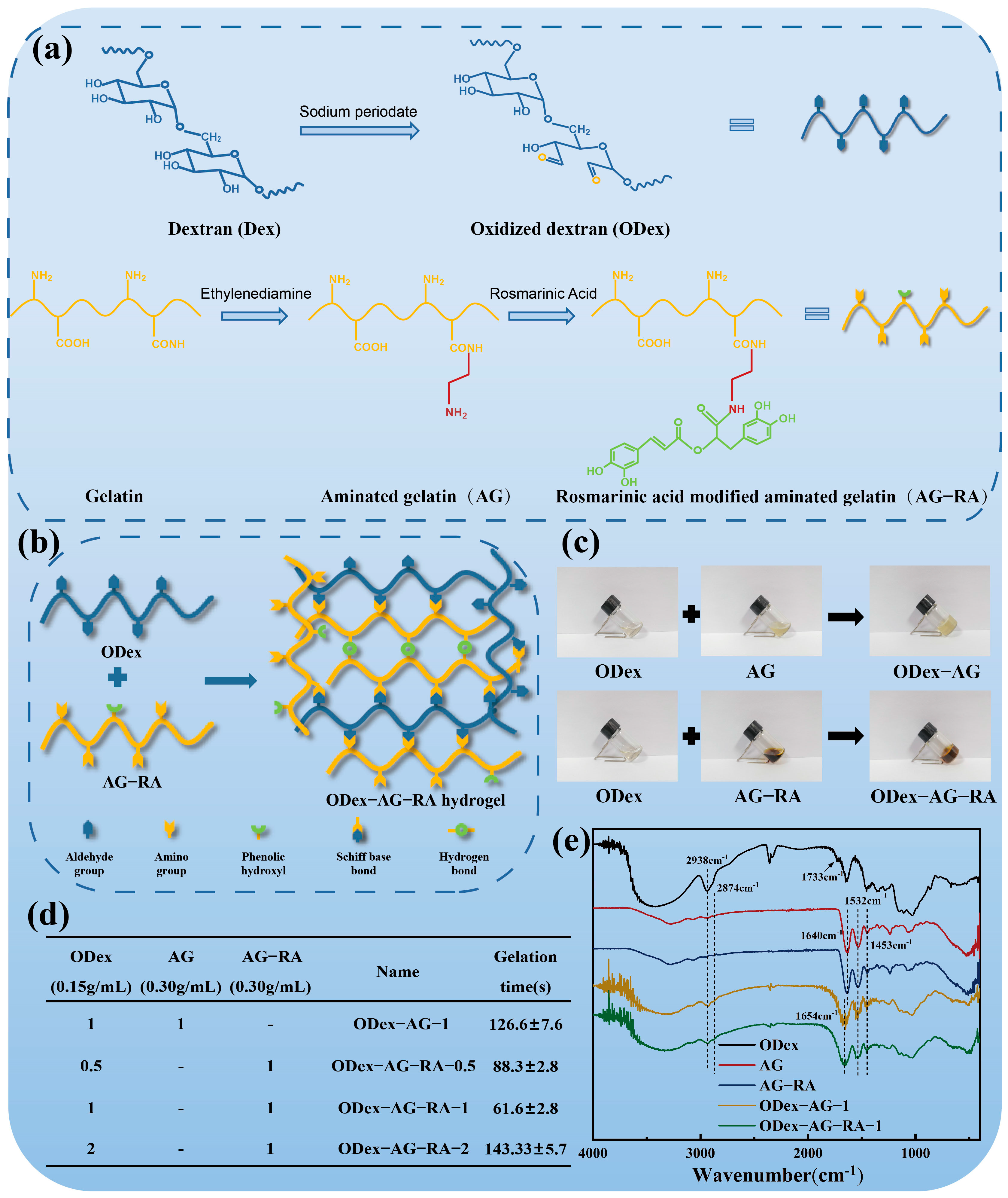
2.1. Design and Structural Characterization of the Hydrogels
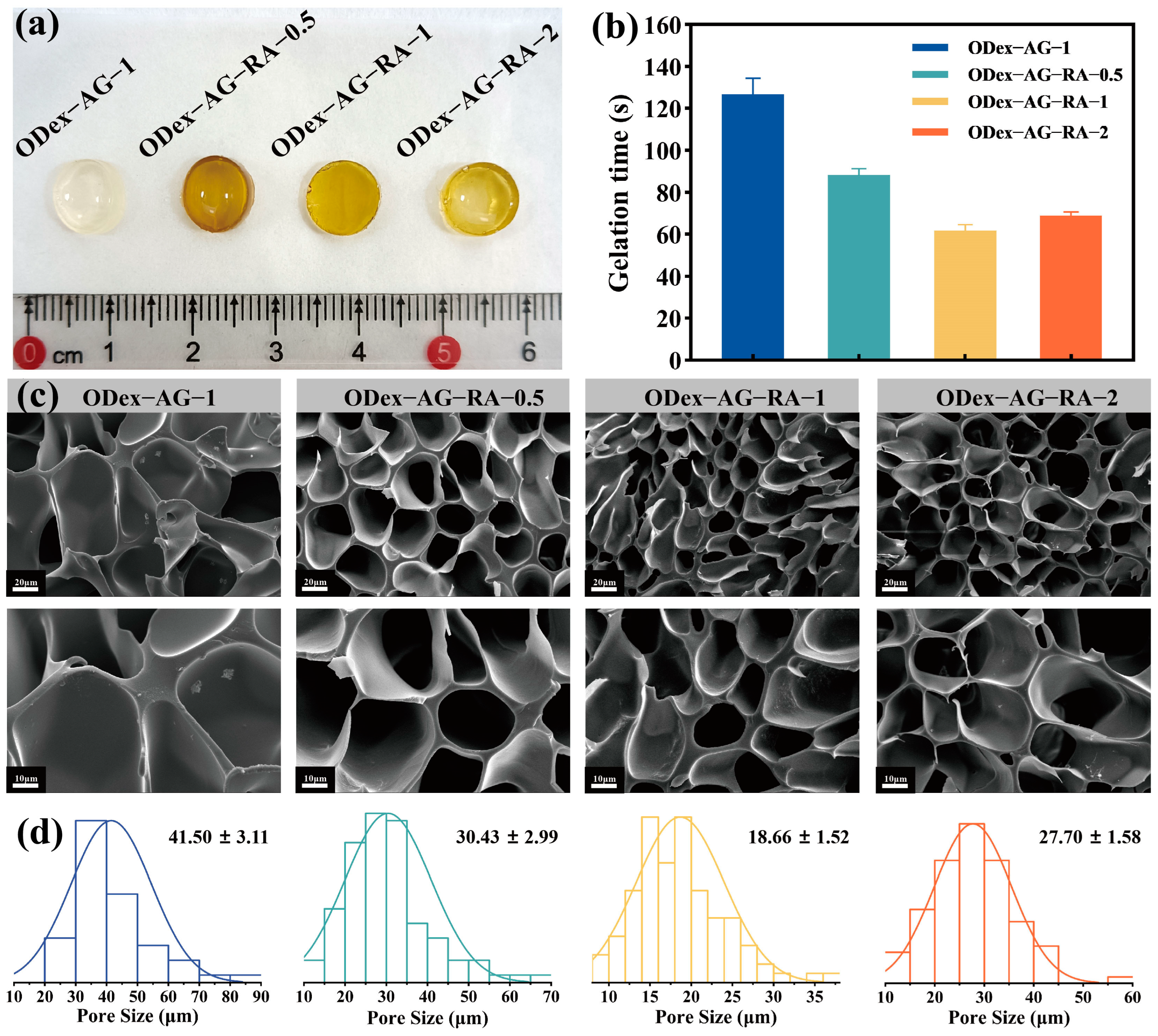
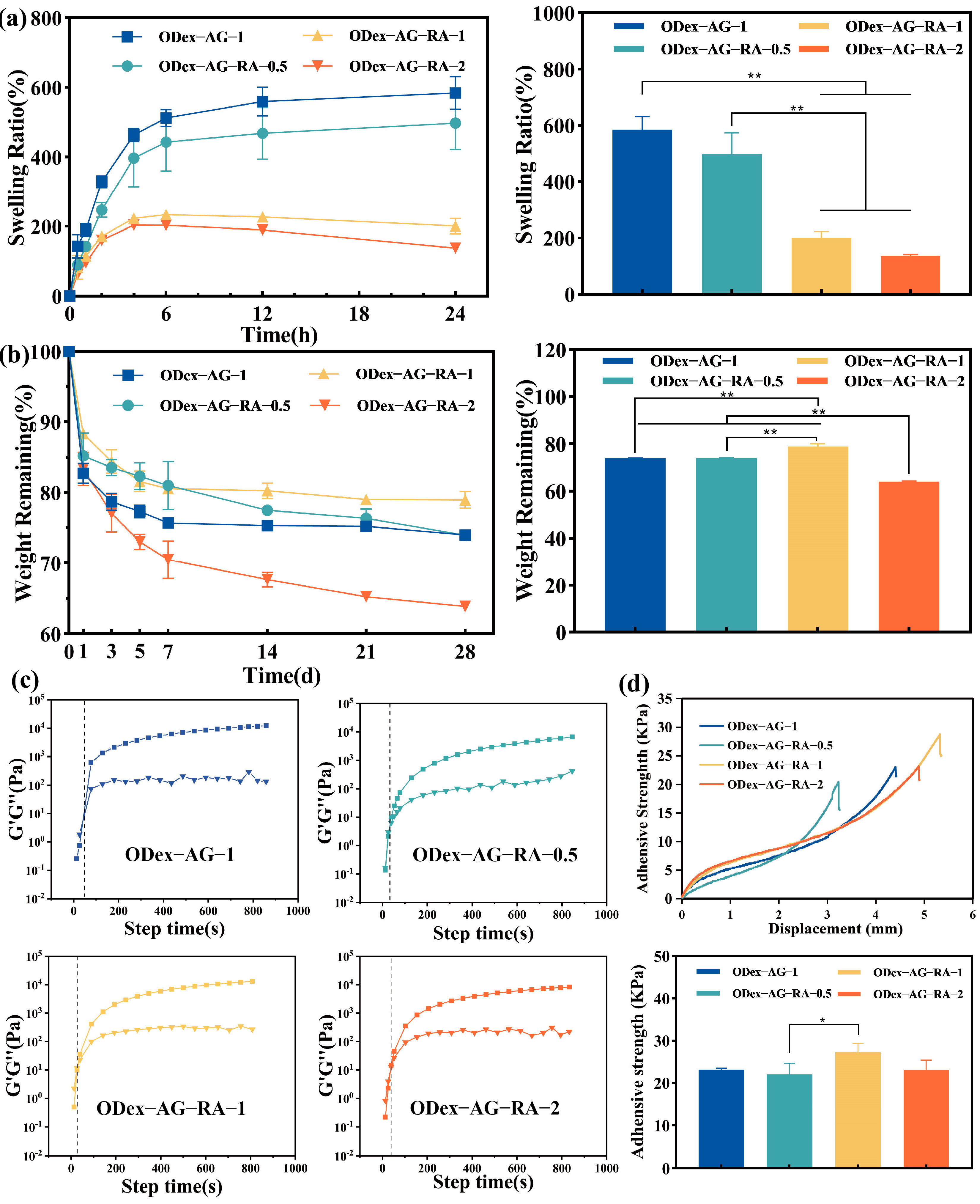
2.2. Physical Properties of the Hydrogels
2.3. Rheological Properties
2.4. Adhesive Property
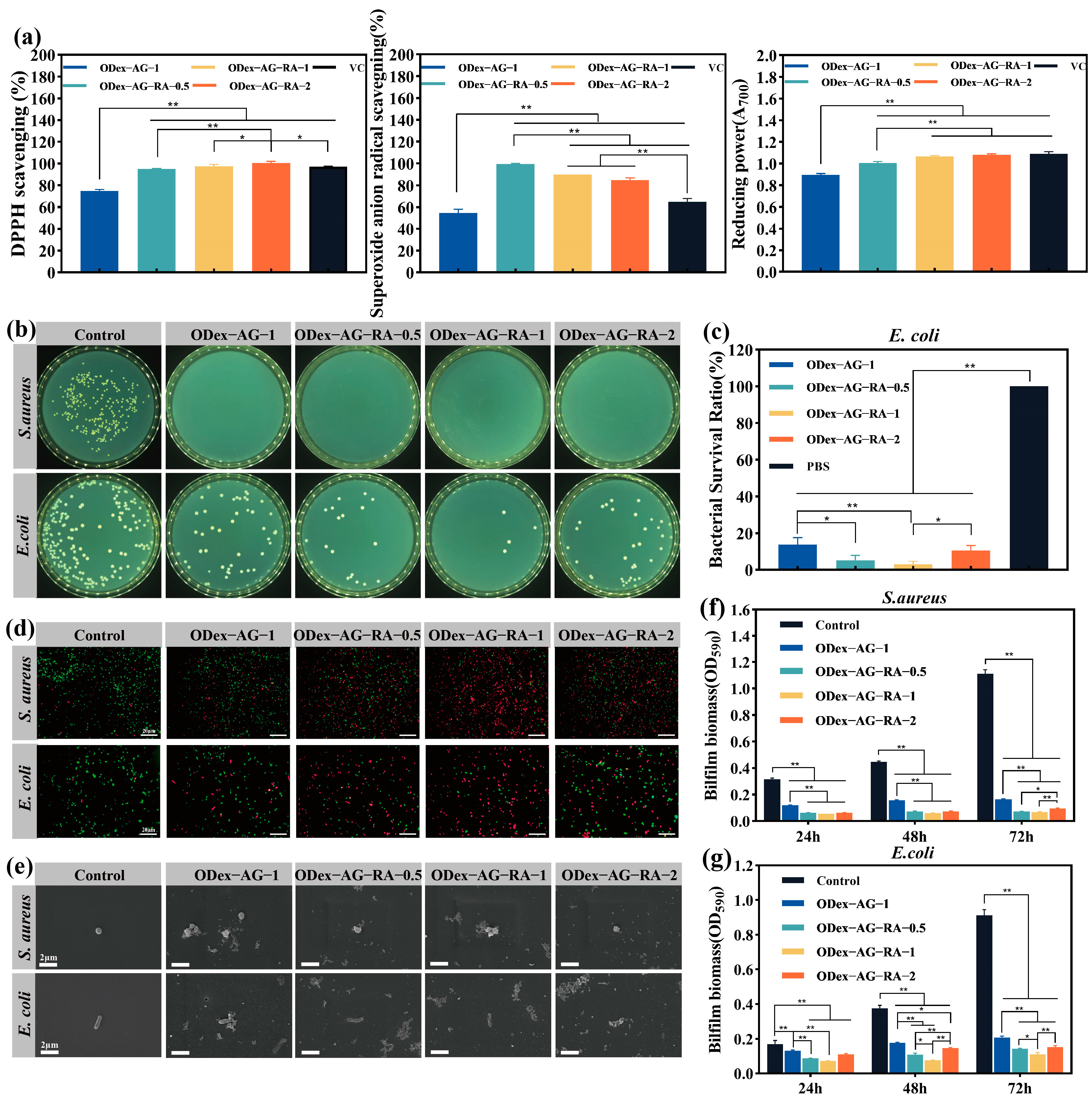
2.5. In Vitro Antioxidant and Antibacterial Activities
2.6. Hemocompatibility
2.7. In Vitro Cell Compatibility
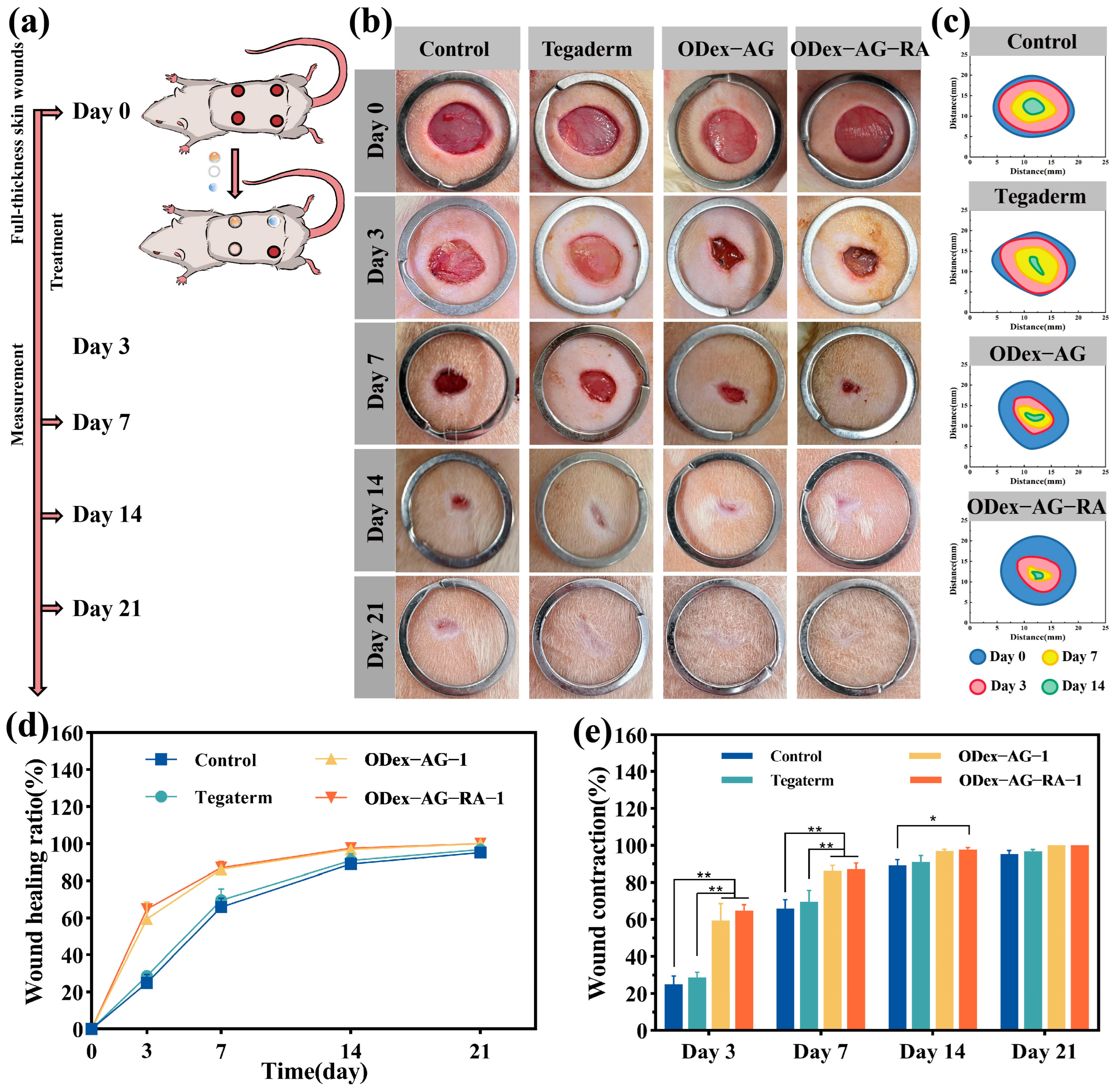
2.8. In Vivo Wound Healing and Histological Evaluation
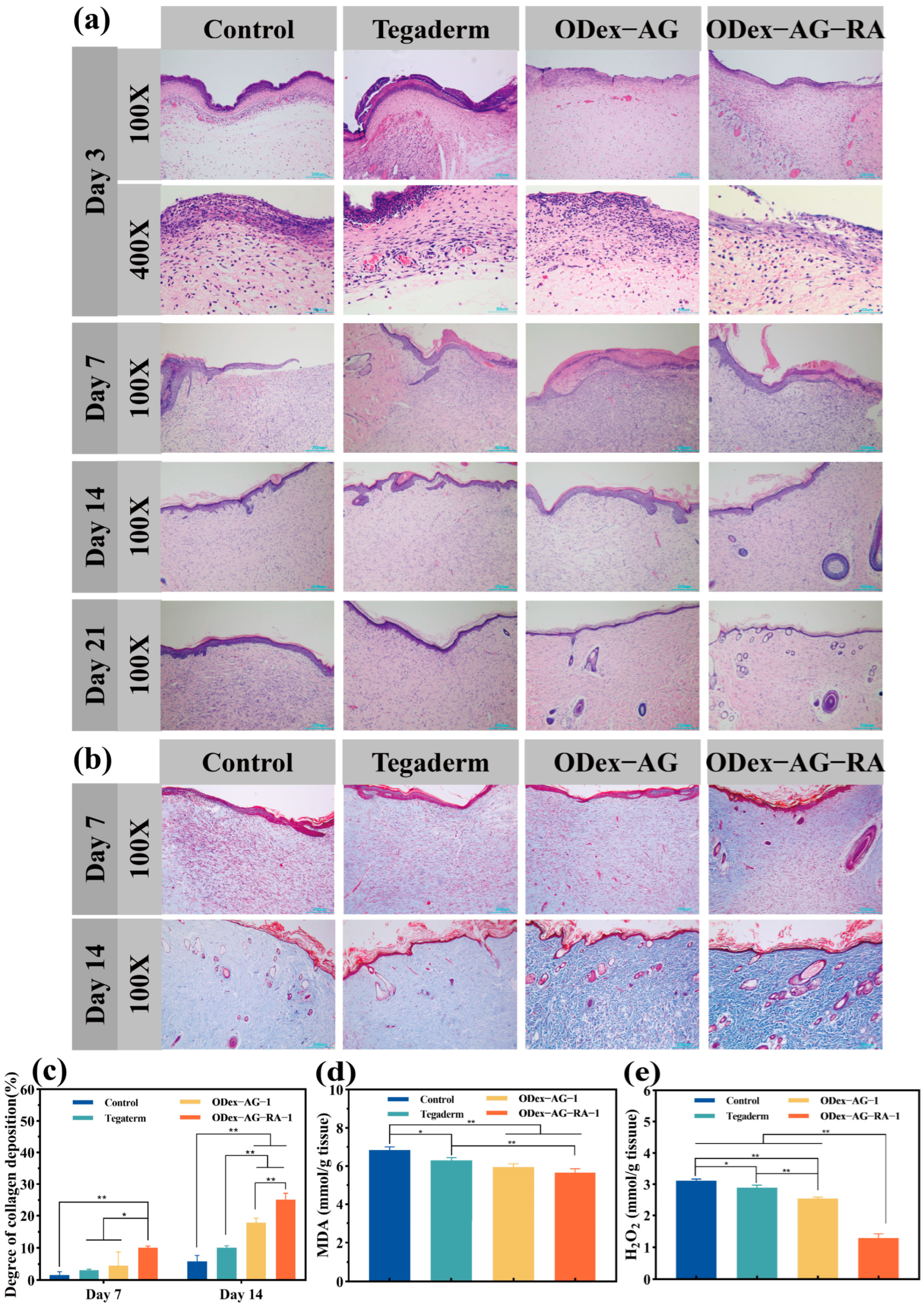
2.8.1. Macroscopic Observation and Histopathology
2.8.2. Content of Antioxidant Factor in Regenerated Skin Tissue
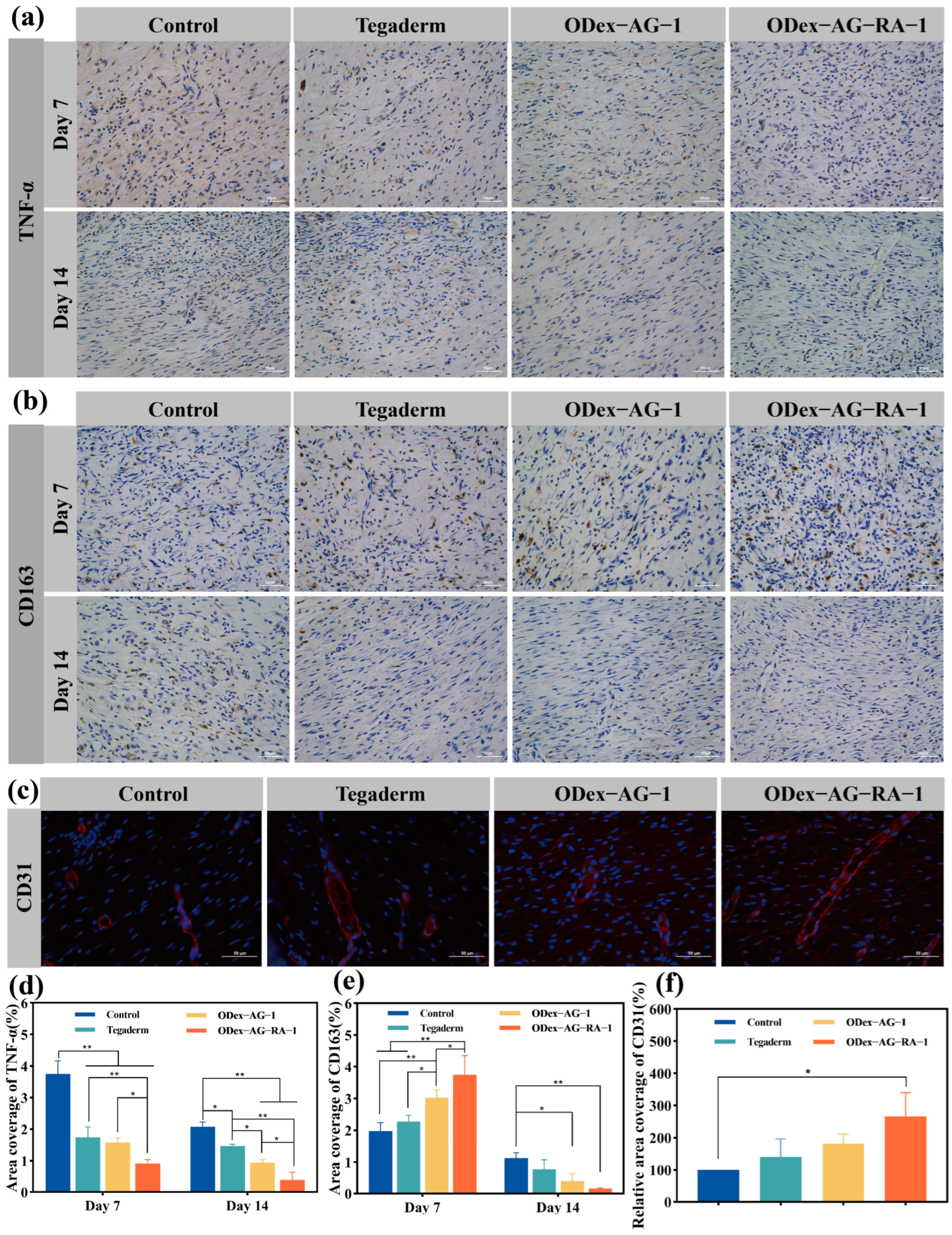
2.8.3. Expression of Inflammatory Factors
2.8.4. Neovascularization in Regenerative Skin Tissue
3. Materials and Methods
3.1. Materials
3.2. Preparation of ODex
3.3. Preparation of AG and AG−RA
3.4. Preparation of ODex−AG−RA Hydrogel
3.5. Characterization of Physical and Chemical Properties
3.5.1. Gelation Time
3.5.2. Morphology Analysis
3.5.3. Swelling and In Vitro Degradation Test
3.5.4. Rheological Property of Hydrogels
3.5.5. Adhesive Strength Test
3.6. Antioxidant and Antibacterial Activities of Hydrogels In Vitro
3.7. Biocompatibility of Hydrogels
3.7.1. Hemolytic Test
3.7.2. Cytocompatibility
3.8. In Vivo Wound Closure Evaluation
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Murphree, R.W. Impairments in Skin Integrity. Nurs. Clin. N. Am. 2017, 52, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.; Valdera, F.; Varon, D.; Kankuri, E.; Nuutila, K. The Immune and Regenerative Response to Burn Injury. Cells 2022, 11, 3073. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.H.; Mathieu, L.; Maibach, H.I. Acute chemical skin injuries in the United States: A review. Crit. Rev. Toxicol. 2018, 48, 540–554. [Google Scholar] [CrossRef]
- Luo, H.; Yin, X.Q.; Tan, P.F.; Gu, Z.P.; Liu, Z.M.; Tan, L. Polymeric antibacterial materials: Design, platforms and applications. J. Mater. Chem. B 2021, 9, 2802–2815. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Kang, Y.; Yao, C.; Li, X.; Li, L. Conjugated Molecule-Assisted Supramolecular Hydrogel with Enhanced Antibacterial and Antibiofouling Properties. ACS Appl. Bio. Mater. 2022, 5, 3107–3114. [Google Scholar] [CrossRef]
- Zhang, Z.; Bu, J.; Li, B.; Xuan, H.; Jin, Y.; Yuan, H. Dynamic Double Cross-Linked Self-Healing Polysaccharide Hydrogel Wound Dressing Based on Schiff Base and Thiol-Alkynone Reactions. Int. J. Mol. Sci. 2022, 23, 13817. [Google Scholar] [CrossRef]
- Sisso, A.M.; Boit, M.O.; DeForest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. A 2020, 108, 1112–1121. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical applications. J. Mater. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef] [PubMed]
- Gulfam, M.; Jo, S.H.; Vu, T.T.; Ali, I.; Rizwan, A.; Joo, S.B.; Park, S.H.; Lim, K.T. NIR-degradable and biocompatible hydrogels derived from hyaluronic acid and coumarin for drug delivery and bio-imaging. Carbohydr. Polym. 2023, 303, 120457. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef] [PubMed]
- Luanda, A.; Badalamoole, V. Past, present and future of biomedical applications of dextran-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 228, 794–807. [Google Scholar] [CrossRef]
- Kang, J.I.; Park, K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2021, 8, 341–354. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, X.; Lu, J.; Liu, X.; Chen, J.; Chen, Y. Mussel-Inspired Adhesive, Antibacterial, and Stretchable Composite Hydrogel for Wound Dressing. Macromol. Biosci. 2023, 23, e2200370. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Kang, J.I.; Park, K.M.; Park, K.D. In situ forming and reactive oxygen species-scavenging gelatin hydrogels for enhancing wound healing efficacy. Acta Biomater. 2020, 103, 142–152. [Google Scholar] [CrossRef]
- Moeini, S.; Karimi, E.; Oskoueian, E. Antiproliferation effects of nanophytosome-loaded phenolic compounds from fruit of Juniperus polycarpos against breast cancer in mice model: Synthesis, characterization and therapeutic effects. Cancer Nanotechnol. 2022, 13, 20. [Google Scholar] [CrossRef]
- Poorbagher, M.R.M.; Karimi, E.; Oskoueian, E. Hepatoprotective effect of nanoniosome loaded Myristica fragrans phenolic compounds in mice-induced hepatotoxicity. J. Cell. Mol. Med. 2022, 26, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.K.; Bolvari, N.E.; Pour, S.M.; Moghadam, S.K.; Shafaei, N.; Karimi, E.; Oskoueian, E. Encapsulated phenolic compounds from Ferula gummosa leaf: A potential phytobiotic against Campylobacter jejuni infection. J. Food Process. Preserv. 2022, 48, e16802. [Google Scholar]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules. 2022, 27, 3292. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Effects of rosmarinic acid on nervous system disorders: An updated review. Naunyn-Schmiedebergs Arch. Pharmacol. 2020, 393, 1779–1795. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, J.; Takano, H.; Xu, Z.; Nishiwaki, H.; Yonekura, L.; Yang, R.; Tamura, H. Rosmarinic acid and its ester derivatives for enhancing antibacterial, α-glucosidase inhibitory, and lipid accumulation suppression activities. J Food Biochem. 2019, 43, e12719. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Di Renzo, L.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. Rosmarinic Acid as Potential Anti-Inflammatory Agent. Rev. Recent Clin. Trials 2018, 13, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Jin, Y.; Lin, A.; Wang, P.; Wang, Y.; Zhu, H. Rosmarinic acid relieves cisplatin-induced ovary toxicity in female mice via suppression of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22839. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ji, M.; Wu, L.; Lv, L.; Liang, Q.; Deng, R.; Deng, Z.; Liu, X.; Ren, L.; Feng, X.; et al. Rosmarinic Acid Prevents Cisplatin-Induced Liver and Kidney Injury by Inhibiting Inflammatory Responses and Enhancing Total Antioxidant Capacity. Thereby Activating the Nrf2 Signaling Pathway. Molecules 2022, 27, 7815. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Zhang, M.; Bai, B.; Yang, Y.; Fan, S. The antibacterial mechanism of perilla rosmarinic acid. Biotechnol. Appl. Biochem. 2022, 69, 1757–1764. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Kello, M.; Čoma, M.; Slobodníková, L.; Drobná, E.; Holková, I.; Garajová, M.; Mrva, M.; Zachar, V.; Lukáč, M. Derivatization of Rosmarinic Acid Enhances its in vitro Antitumor. Antimicrobial and Antiprotozoal Properties. Molecules 2019, 24, 1078. [Google Scholar] [CrossRef]
- Cao, Z.; Luo, Y.; Li, Z.; Tan, L.; Liu, X.; Li, C.; Zheng, Y.; Cui, Z.; Yeung, K.W.K.; Liang, Y.; et al. Antibacterial Hybrid Hydrogels. Macromol. Biosci. 2021, 21, e2000252. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Jin, D.; Xin, Y.; Tian, L.; Wang, T.; Zhao, D.; Wang, Z.; Wang, J. Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules 2022, 12, 1410. [Google Scholar] [CrossRef]
- Wu, L.H.; He, Y.Y.; Mao, H.L.; Gu, Z.W. Bioactive hydrogels based on polysaccharides and peptides for soft tissue wound management. J. Mater. Chem. B 2022, 10, 7148–7160. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Yang, T.P.; Peng, H.H.; Chen, J.W. Preparation and characterization of polygalacturonic acid/rosmarinic acid membrane crosslinked by short chain hyaluronate for preventing postoperative abdominal adhesion. Carbohydr. Polym. 2012, 87, 1749–1755. [Google Scholar] [CrossRef]
- Miguel, H.M.; Javier, C.L.; Eva, E.C.; María, R.A.; Blanca, V.L. Chitosan-Rosmarinic acid conjugates with antioxidant, anti-inflammatory and photoprotective properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar]
- Zhang, W.W.; Zhang, Y.N.; Yang, L.R. Study of the Antioxidant Capacities of Four Natural Substances. Food Res. Dev. 2022, 43, 43–50. [Google Scholar]
- Yuan, Y.; Shen, S.; Fan, D. A physicochemical double cross-linked multifunctional hydrogel for dynamic burn wound healing: Shape adaptability, injectable self-healing property and enhanced adhesion. Biomaterials 2021, 276, 120838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Strong Collagen Hydrogels by Oxidized Dextran Modification. ACS Sustain. Chem. Eng. 2014, 2, 1318–1324. [Google Scholar] [CrossRef]
- Pan, J.F.; Yuan, L.; Guo, C.A.; Geng, X.H.; Fei, T.; Fan, W.S.; Li, S.; Yuan, H.F.; Yan, Z.Q.; Mo, X.M. Fabrication of modified dextran-gelatin in situ forming hydrogel and application in cartilage tissue engineering. J. Mater. Chem. B 2014, 2, 8346–8360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Luo, L.; Ding, Q.; Tang, S. Bio-orthogonally crosslinked catechol-chitosan hydrogel for effective hemostasis and wound healing. Carbohydr. Polym. 2022, 281, 119039. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004, 57, 453–455. [Google Scholar]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, T.; Tang, Y.; Luo, G.; Liang, G.; He, W. Epigenetic regulation of macrophage polarization in wound healing. Burns Trauma 2023, 11, tkac057. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gao, R.; Yang, C.; Feng, Z.; Ou-Yang, W.; Pan, X.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. ECM-mimetic immunomodulatory hydrogel for methicillin-resistant Staphylococcus aureus-infected chronic skin wound healing. Sci. Adv. 2022, 8, eabn7006. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, G.; Lei, M.; Lei, J.; Li, D.; Zheng, H. A pH-sensitive oxidized-dextran based double drug-loaded hydrogel with high antibacterial properties. Int. J. Biol. Macromol. 2021, 182, 385–393. [Google Scholar] [CrossRef]
- Li, D.D.; Mo, X.M. Oxidized dextran/aminated carboxymethyl chitosan two-component hydrogel adhesive based on Schiff base reaction. Chin. J. Tissue Eng. Res. 2018, 22, 3527–3532. [Google Scholar]
- Yin, Y.; Li, D.; Xu, Q.; Zhang, J.; Zhao, J. Synthesis and characterization of a novel rosmarinic acid modified gelatin with antioxidant function. J. Shenyang Pharm. Univ. 2023. accepted. [Google Scholar]
- Mondal, P.; Chatterjee, K. Injectable and self-healing double network polysaccharide hydrogel as a minimally-invasive delivery platform. Carbohydr. Polym. 2022, 291, 119585. [Google Scholar] [CrossRef]
- Dang, R.; Chen, L.; Sefat, F.; Li, X.; Liu, S.; Yuan, X.; Ning, X.; Zhang, Y.S.; Ji, P.; Zhang, X. A Natural Hydrogel with Prohealing Properties Enhances Tendon Regeneration. Small 2022, 18, e2105255. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Wang, Y.; Li, J.; Bao, J.; Xu, X.; Zhang, C.; Li, Y.; Wu, H.; Gu, Z. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr. Polym. 2021, 257, 117598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M. Construction and Application of Biomimetic Smart Matrices for Preventing Fibrotic Scarring of Skin Wound. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2020. [Google Scholar]
- Yin, G.L.; Zhao, L.; Zou, C.M. Ultrasound-assisted Extraction of Polyphenols from Ilex latifolia Thunb. and Its Antioxidant and Hypoglycemic Activities. Food Res. Dev. 2020, 41, 48–55. [Google Scholar]
- Wei, Z.H. Construction of Nano-Silver/Amphiphilic Composite Membrane and Its Inhibition on Bacterial Biofilm Formation. Master’s Thesis, Henan University, Kaifeng, China, 2020. [Google Scholar]
- Li, Z.; Li, B.; Li, X.; Lin, Z.; Chen, L.; Chen, H.; Jin, Y.; Zhang, T.; Xia, H.; Lu, Y.; et al. Ultrafast in-situ forming halloysite nanotube-doped chitosan/oxidized dextran hydrogels for hemostasis and wound repair. Carbohydr. Polym. 2021, 267, 118155. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Xu, Q.; Wei, X.; Ma, Q.; Li, D.; Zhao, J. Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory. Molecules 2023, 28, 4034. https://doi.org/10.3390/molecules28104034
Yin Y, Xu Q, Wei X, Ma Q, Li D, Zhao J. Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory. Molecules. 2023; 28(10):4034. https://doi.org/10.3390/molecules28104034
Chicago/Turabian StyleYin, Yi, Qianqian Xu, Xin Wei, Qianyun Ma, Dongsheng Li, and Juanjuan Zhao. 2023. "Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory" Molecules 28, no. 10: 4034. https://doi.org/10.3390/molecules28104034




