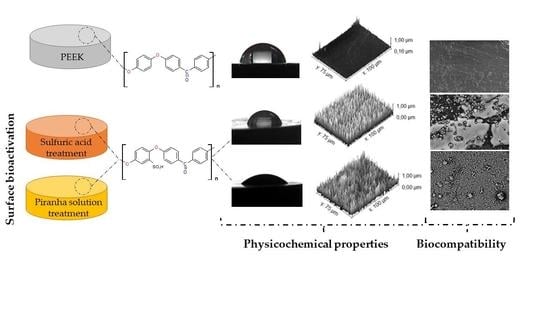
Use of Piranha Solution as An Alternative Route to Promote Bioactivation of PEEK Surface with Low Functionalization Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
2.2.1. Infrared Spectroscopy by Fourier Transform (FTIR)
2.2.2. Surface Wettability
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Surface Roughness Measurement
2.2.5. In Vitro Bioactivity
2.2.6. Statistical Analysis
3. Results and Discussion
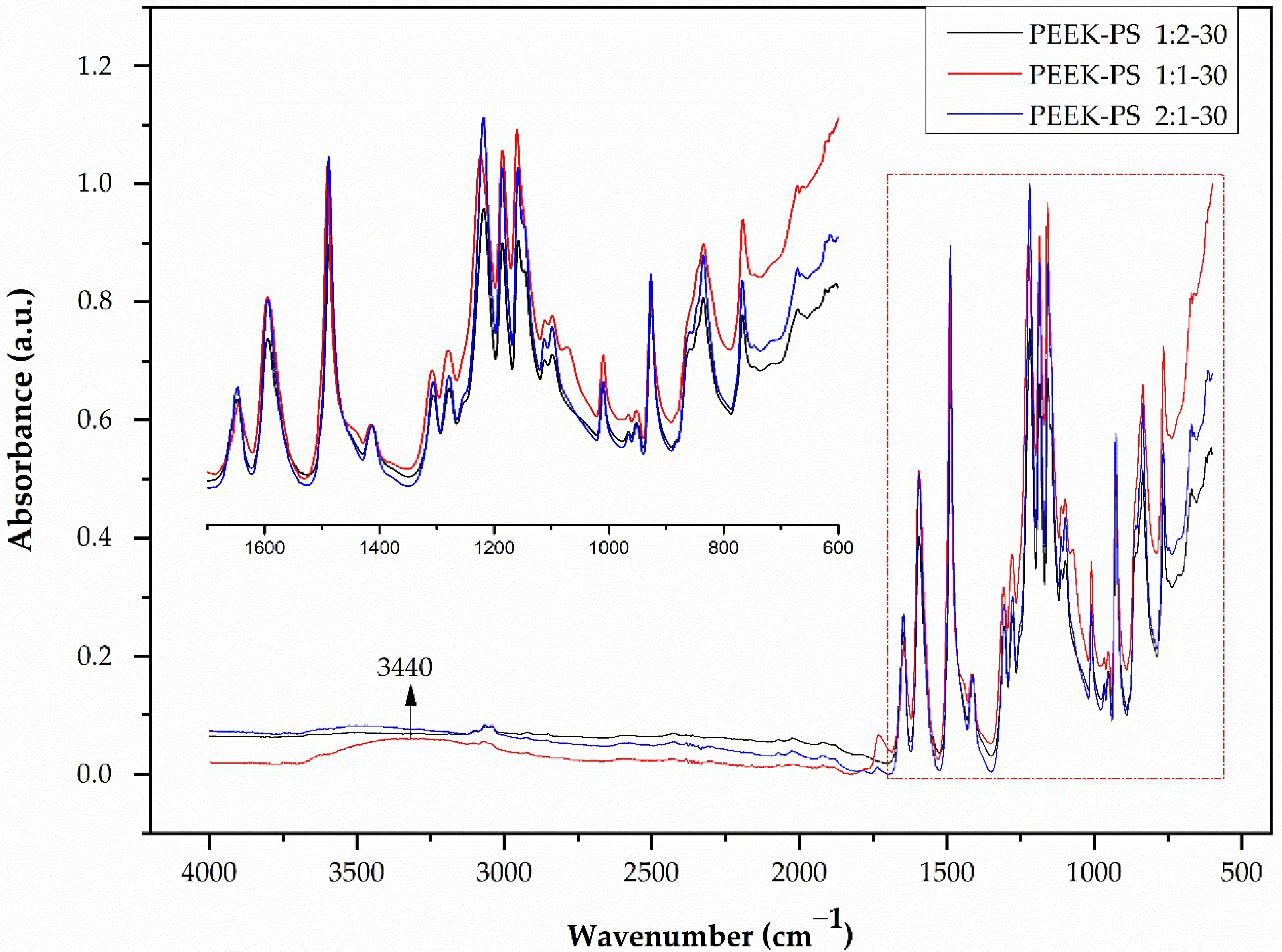
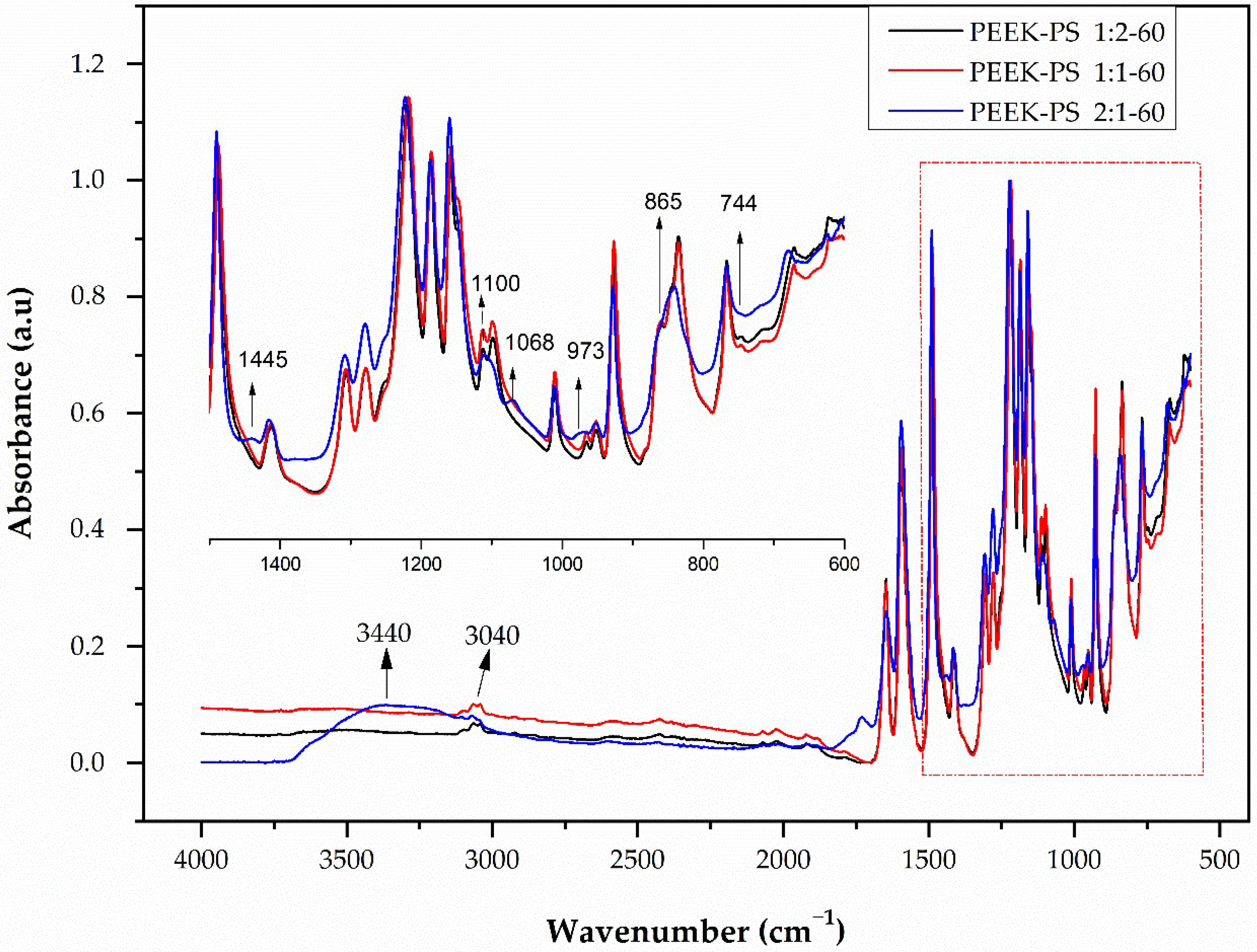
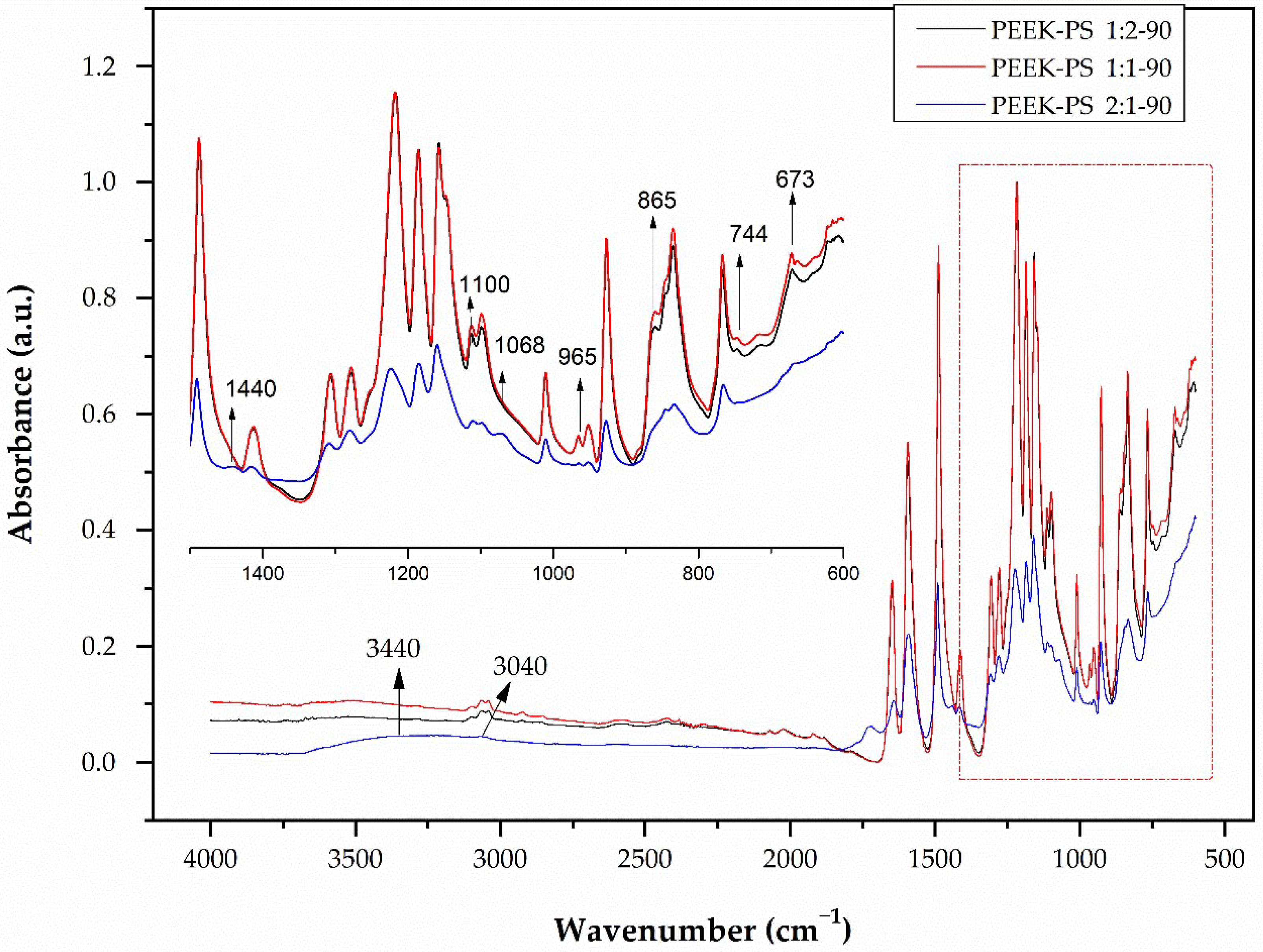
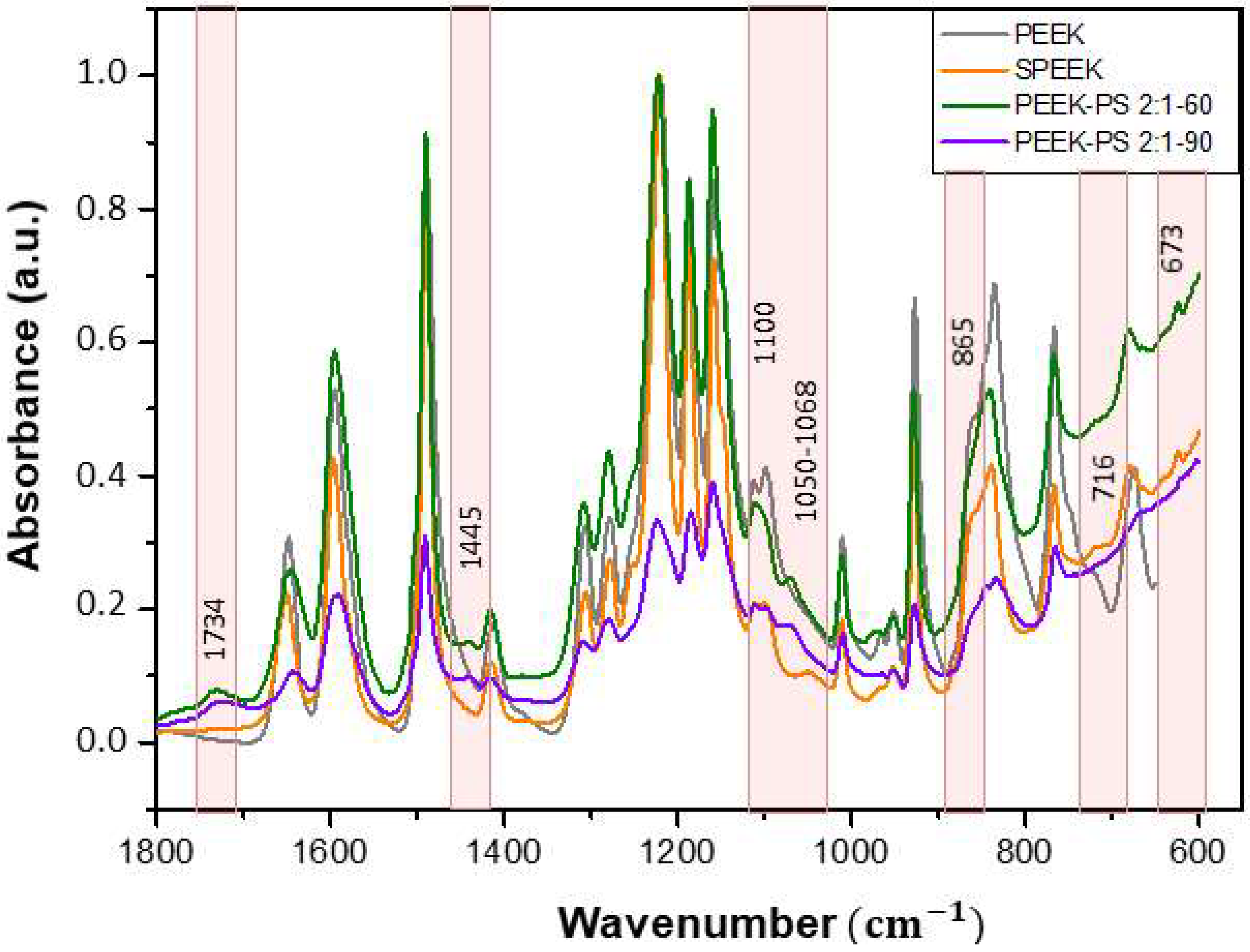
3.1. Infrared Spectroscopy by Fourier Transform (FTIR)
3.2. Student’s T-Test Applied to FTIR
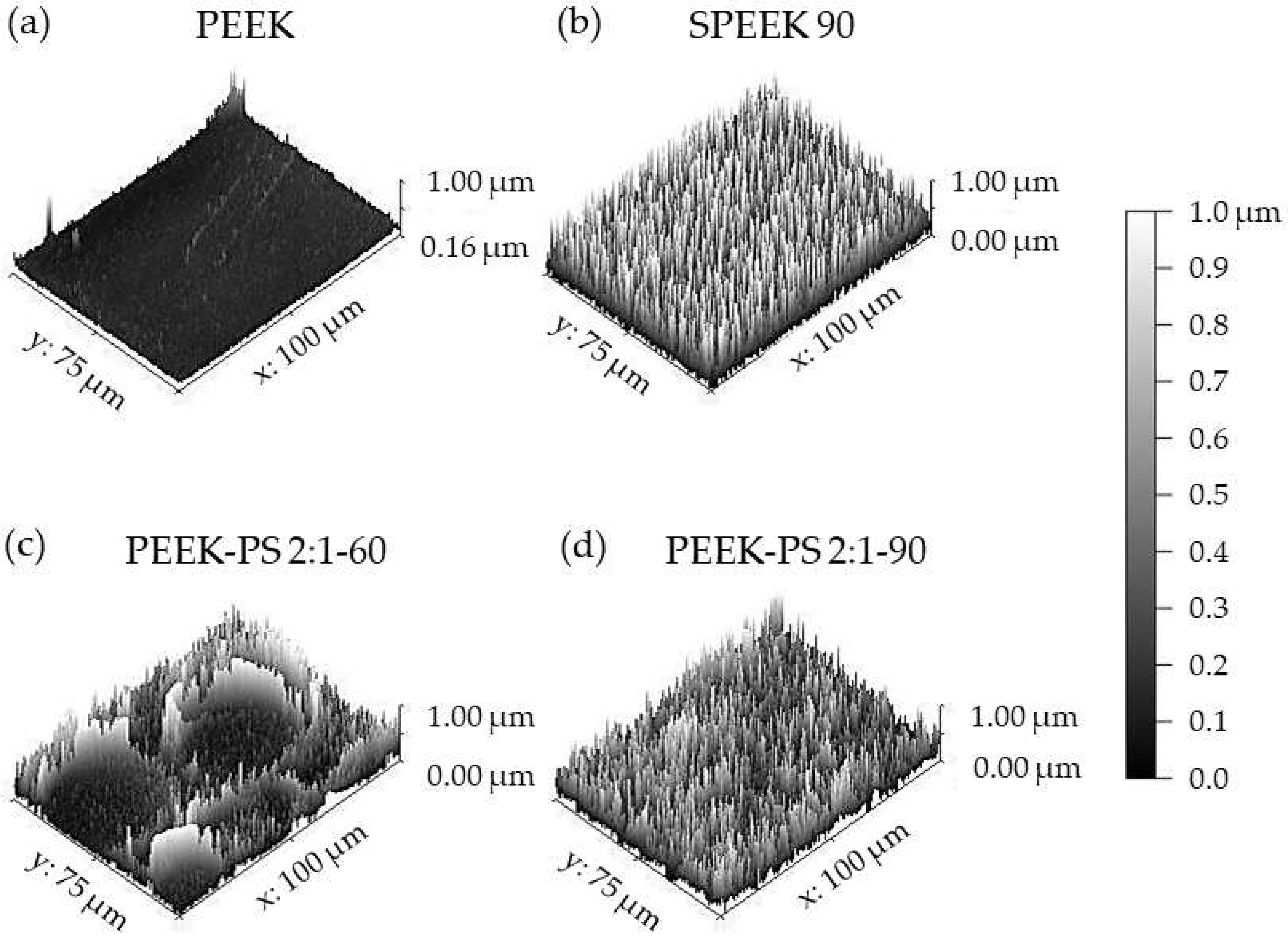
3.3. Scanning Electron Microscopy (SEM)
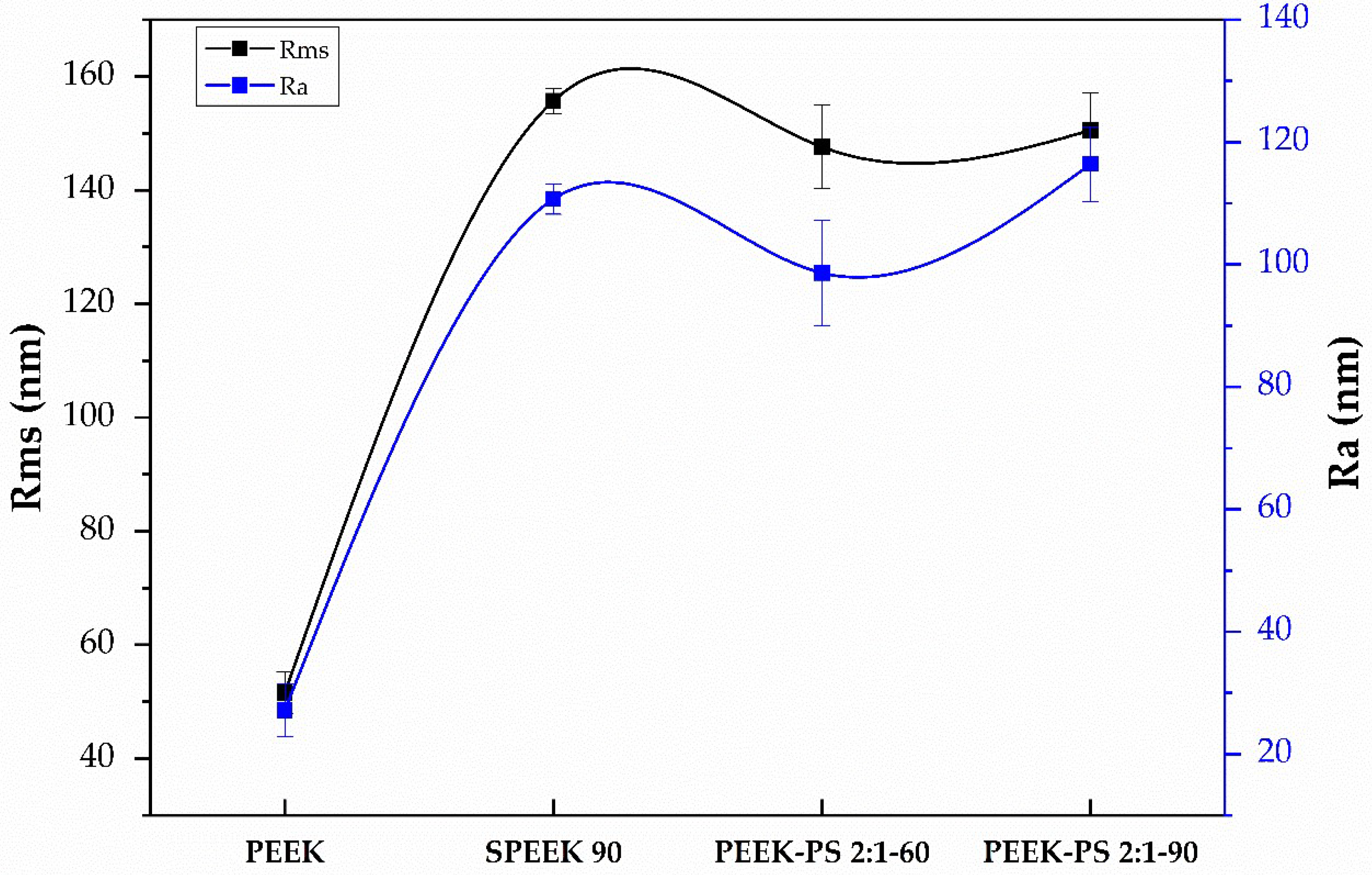
3.4. Surface Roughness Measurement
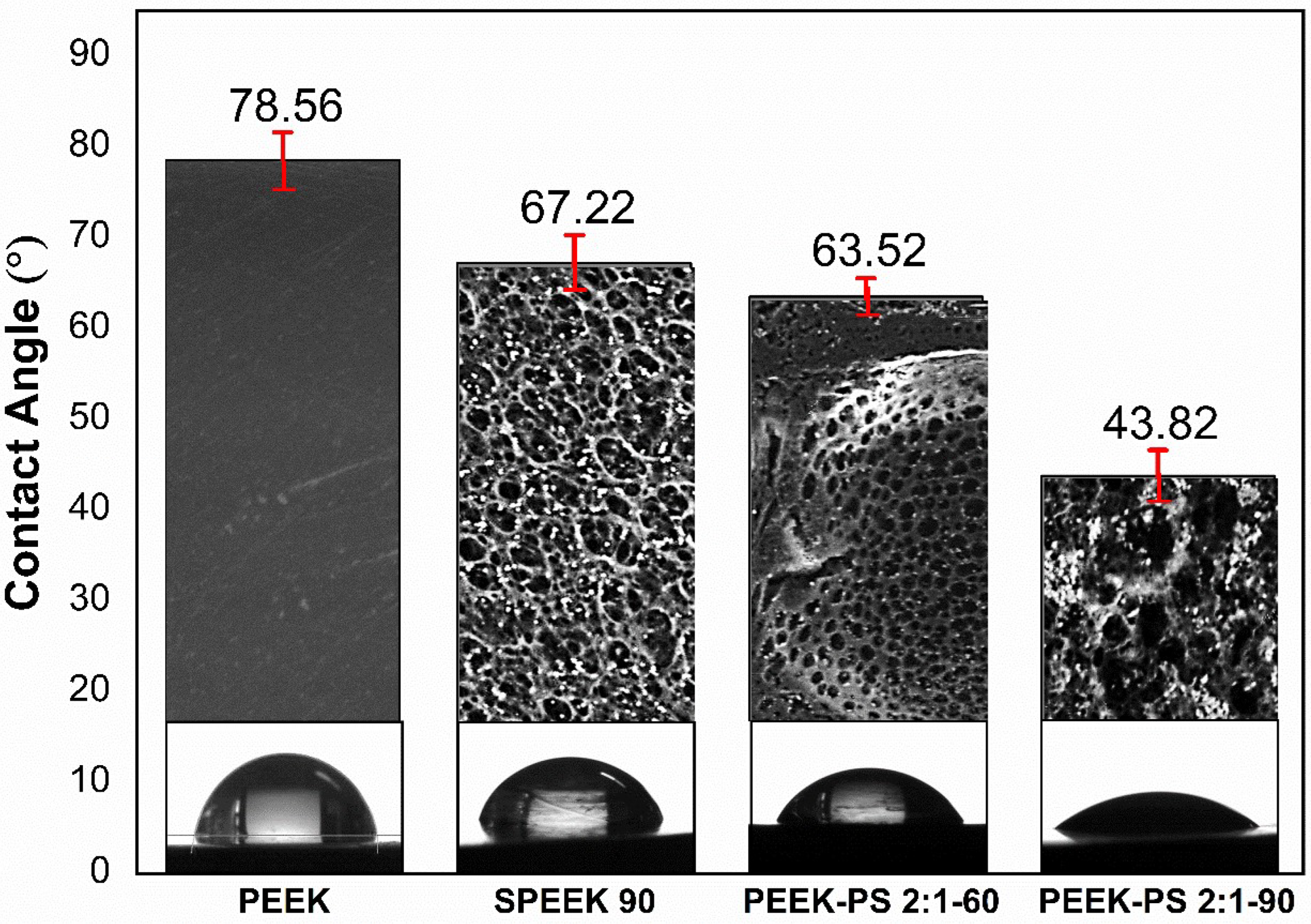
3.5. Contact Angle
3.6. In Vitro Bioactivity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kurtz, S.M. An overview of PEEK biomaterials. In PEEK Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–7. [Google Scholar]
- Liu, X.; Gan, K.; Liu, H.; Song, X.; Chen, T.; Liu, C. Antibacterial properties of nano-silver coated PEEK prepared through magnetron sputtering. Dent. Mater. 2017, 33, e348–e360. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Deng, Y.; Xie, K. AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf. B Biointerfaces 2017, 160, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, L.; Mehl, A.; Sereno, N.; Hämmerle, C.H. The improvement of adhesive properties of PEEK through different pre-treatments. Appl. Surf. Sci. 2012, 258, 7213–7218. [Google Scholar] [CrossRef]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.S.; Boothby, J.M.; Whittingslow, D.C. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015, 13, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham III, J.W.; Rabiei, A. Deposition, heat treatment and characterization of two layer bioactive coatings on cylindrical PEEK. Surf. Coat. Technol. 2016, 301, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Effect of low-temperature plasma on chitosan-coated PEEK polymer characteristics. Eur. Polym. J. 2016, 78, 1–13. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef]
- Sundriyal, P.; Sahu, M.; Prakash, O.; Bhattacharya, S. Long-term surface modification of PEEK polymer using plasma and PEG silane treatment. Surf. Interfaces 2021, 25, 101253. [Google Scholar] [CrossRef]
- Kyzioł, A.; Kyzioł, K. Surface functionalization with biopolymers via plasma-assisted surface grafting and plasma-induced graft polymerization—materials for biomedical applications. In Biopolymer Grafting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–151. [Google Scholar]
- Jurak, M.; Wiącek, A.E.; Terpiłowski, K. Properties of PEEK-supported films of biological substances prepared by the Langmuir-Blodgett technique. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 263–274. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Bishop, M.T.; Ellis, T.S.; Karasz, F.E. A sulphonated poly (aryl ether ketone). Br. Polym. J. 1985, 17, 4–10. [Google Scholar] [CrossRef]
- Bailly, C.; Williams, D.J.; Karasz, F.E.; MacKnight, W.J. The sodium salts of sulphonated poly (aryl-ether-ether-ketone)(PEEK): Preparation and characterization. Polymer 1987, 28, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.J. Polymer sulfonation-A versatile route to prepare proton-conducting membrane material for advanced technologies. Arab. J. Sci. Eng. 2003, 28, 183–194. [Google Scholar]
- Almasi, D.; Izman, S.; Assadian, M.; Ghanbari, M.; Kadir, M.A. Crystalline HA coating on PEEK via chemical deposition. Appl. Surf. Sci. 2014, 314, 1034–1040. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef] [Green Version]
- Montero, J.F.; Tajiri, H.A.; Barra, G.M.; Fredel, M.C.; Benfatti, C.A.; Magini, R.S.; Pimenta, A.L.; Souza, J.C. Biofilm behavior on sulfonated poly (ether-ether-ketone) (sPEEK). Mater. Sci. Eng. C 2017, 70, 456–460. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J. Prosthet. Dent. 2014, 112, 1278–1288. [Google Scholar] [CrossRef] [Green Version]
- Uhrenbacher, J.; Schmidlin, P.R.; Keul, C.; Eichberger, M.; Roos, M.; Gernet, W.; Stawarczyk, B. The effect of surface modification on the retention strength of polyetheretherketone crowns adhesively bonded to dentin abutments. J. Prosthet. Dent. 2014, 112, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Zandparsa, R.; Talua, N.A.; Finkelman, M.D.; Schaus, S.E. An in vitro comparison of shear bond strength of zirconia to enamel using different surface treatments. J. Prosthodont. 2014, 23, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.; Souza, R.; Dursun, E.; Monteiro, E.; Valandro, L.; Bottino, M. Surface treatments of zirconia to enhance bonding durability. Oper. Dent. 2015, 40, 636–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallmann, L.; Ulmer, P.; Lehmann, F.; Wille, S.; Polonskyi, O.; Johannes, M.; Köbel, S.; Trottenberg, T.; Bornholdt, S.; Haase, F. Effect of surface modifications on the bond strength of zirconia ceramic with resin cement resin. Dent. Mater. 2016, 32, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Eulálio, H.Y.C.; Vieira, M.; Fideles, T.B.; Tomás, H.; Silva, S.M.; Peniche, C.A.; Fook, M.V.L. Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing. Materials 2020, 13, 5005. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, T.; Noguchi, T.; Imagawa, K. Photodegradation of PEEK sheets under tensile stress. Polym. Degrad. Stab. 2006, 91, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Doyle, C.S.; James, B.J.; Hyland, M.M. Valence band XPS and FT-IR evaluation of thermal degradation of HVAF thermally sprayed PEEK coatings. Polym. Degrad. Stab. 2010, 95, 792–797. [Google Scholar] [CrossRef]
- Doğan, H.; Inan, T.Y.; Koral, M.; Kaya, M. Organo-montmorillonites and sulfonated PEEK nanocomposite membranes for fuel cell applications. Appl. Clay Sci. 2011, 52, 285–294. [Google Scholar] [CrossRef]
- Dutra Filho, J.C.; Santos, T.R.d.; Gomes, A.d.S. Nanostructured Polyelectrolytes Based on SPEEK/TiO2 for Direct Ethanol Fuel Cells (DEFCs). Polímeros 2014, 24, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Silthampitag, P.; Chaijareenont, P.; Tattakorn, K.; Banjongprasert, C.; Takahashi, H.; Arksornnukit, M. Effect of surface pretreatments on resin composite bonding to PEEK. Dent. Mater. J. 2016, 35, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Stawarczyk, B.; Wieland, M.; Attin, T.; Hämmerle, C.H.; Fischer, J. Effect of different surface pre-treatments and luting materials on shear bond strength to PEEK. Dent. Mater. 2010, 26, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, M.M.; Chou, J.-H. Fluid-kinetics enhanced selective etching process of NiPt film by piranha chemistry in silicide formation for complementary metal oxide semiconductor fabrication. Thin Solid Film. 2012, 520, 5482–5488. [Google Scholar] [CrossRef]
- Al-Gharabli, S.; Kujawa, J.; Mavukkandy, M.O.; Arafat, H.A. Functional groups docking on PVDF membranes: Novel Piranha approach. Eur. Polym. J. 2017, 96, 414–428. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhao, Y.; Jin, G.; Lu, T.; Li, J.; Qiao, Y.; Ning, C.; Zhang, X.; Chu, P.K.; Liu, X. Influence of sulfur content on bone formation and antibacterial ability of sulfonated PEEK. Biomaterials 2016, 83, 115–126. [Google Scholar] [CrossRef]
- Lohbauer, U.; Zipperle, M.; Rischka, K.; Petschelt, A.; Müller, F.A. Hydroxylation of dental zirconia surfaces: Characterization and bonding potential. J. Biomed. Mater. Res. Part B: Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 87, 461–467. [Google Scholar] [CrossRef]
- Siddiq, A.R.; Kennedy, A.R. Porous poly-ether ether ketone (PEEK) manufactured by a novel powder route using near-spherical salt bead porogens: Characterisation and mechanical properties. Mater. Sci. Eng. C 2015, 47, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, D.; Machigashira, M.; Miyamoto, M.; Takeuchi, H.; Noguchi, K.; Izumi, Y.; Ban, S. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent. Mater. J. 2009, 28, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, K.; Liu, H.; Jiang, L.; Liu, X.; Song, X.; Niu, D.; Chen, T.; Liu, C. Bioactivity and antibacterial effect of nitrogen plasma immersion ion implantation on polyetheretherketone. Dent. Mater. 2016, 32, e263–e274. [Google Scholar] [CrossRef]
- Han, X.; Sharma, N.; Spintzyk, S.; Zhou, Y.; Xu, Z.; Thieringer, F.M.; Rupp, F. Tailoring the biologic responses of 3D printed PEEK medical implants by plasma functionalization. Dent. Mater. 2022, 38, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What affects the biocompatibility of polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, Z.; Peng, Y.; Hu, L.; Guney, T.; Tang, B. Facile Surface Modification Method for Synergistically Enhancing the Biocompatibility and Bioactivity of Poly(ether ether ketone) That Induced Osteodifferentiation. ACS Appl. Mater. Interfaces 2019, 11, 27503–27511. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [Green Version]
- Hieda, A.; Uemura, N.; Hashimoto, Y.; Toda, I.; Baba, S. In vivo bioactivity of porous polyetheretherketone with a foamed surface. Dent. Mater. J. 2017, 36, 2016–2277. [Google Scholar] [CrossRef] [Green Version]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 39. [Google Scholar] [CrossRef]
- Fook, M.V.L. Desenvolvimento de técnica de deposição de hidroxiapatita pelo método biomimético na superfície polietileno de ultra-alto peso molecular para aplicação como biomaterial. Tese, Doutorado em Química, Instituto de Química, Universidade Estadual Paulista, Araraquara, Brazil, 2005. [Google Scholar]
- Ren, Y.; Sikder, P.; Lin, B.; Bhaduri, S.B. Microwave assisted coating of bioactive amorphous magnesium phosphate (AMP) on polyetheretherketone (PEEK). Mater. Sci. Eng. C 2018, 85, 107–113. [Google Scholar] [CrossRef]
- Hong, W.; Guo, F.; Chen, J.; Wang, X.; Zhao, X.; Xiao, P. Bioactive glass–chitosan composite coatings on PEEK: Effects of surface wettability and roughness on the interfacial fracture resistance and in vitro cell response. Appl. Surf. Sci. 2018, 440, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Tulyaganov, D.U.; Makhkamov, M.E.; Urazbaev, A.; Goel, A.; Ferreira, J.M. Synthesis, processing and characterization of a bioactive glass composition for bone regeneration. Ceram. Int. 2013, 39, 2519–2526. [Google Scholar] [CrossRef]
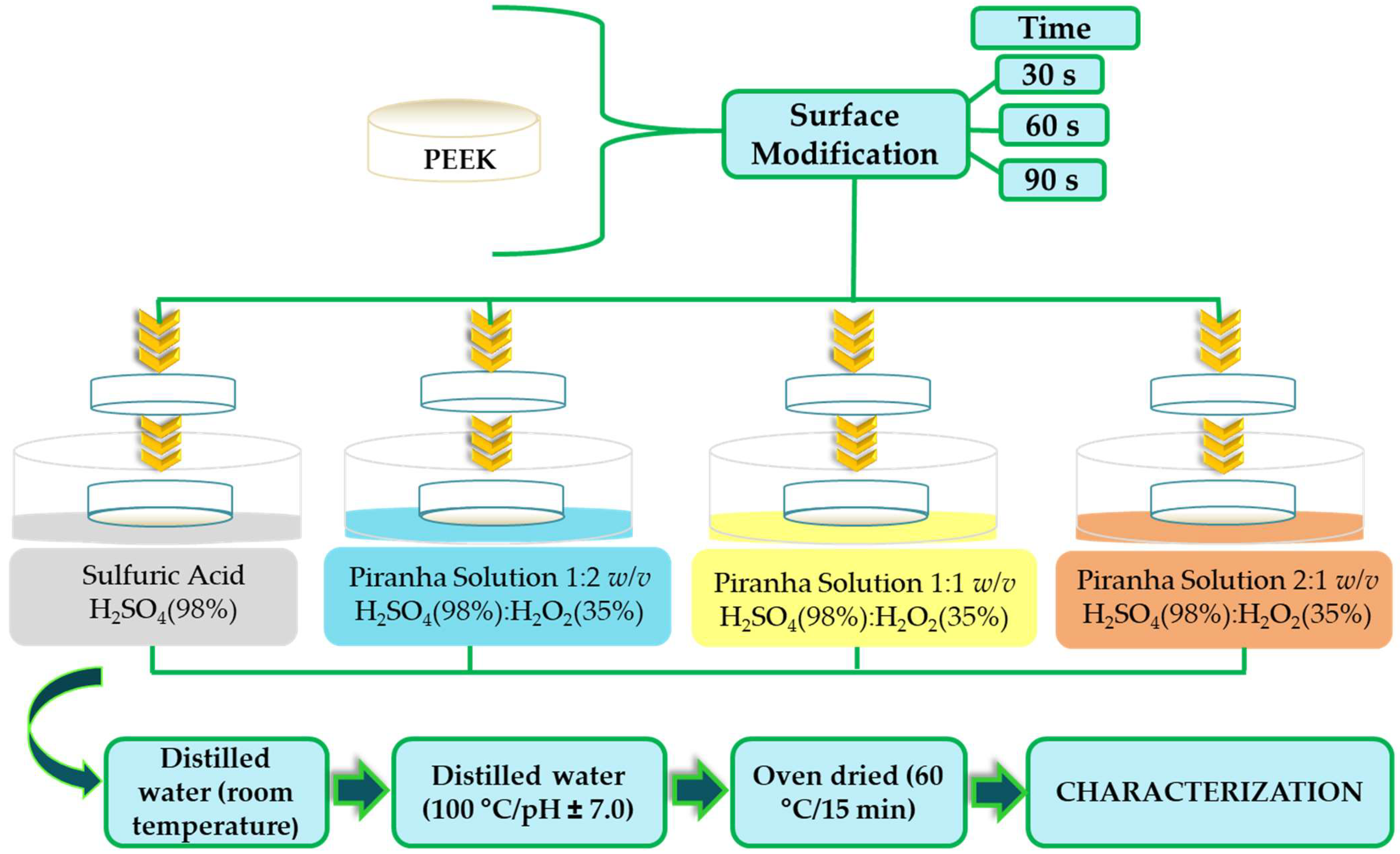





| Treatment | Immersion Time (s) | Codification |
|---|---|---|
| Sulfuric Acid H2SO4 (98%) | 30 60 90 | SPEEK 30 SPEEK 60 SPEEK 90 |
| Piranha Solution 1:2 v/v H2SO4 (98%):H2O2 (35%) | 30 60 90 | PEEK-PS 1:2-30 PEEK-PS 1:2-60 PEEK-PS 1:2-90 |
| Piranha Solution 1:1 v/v H2SO4 (98%):H2O2 (35%) | 30 60 90 | PEEK-PS 1:1-30 PEEK-PS 1:1-60 PEEK-PS 1:1-90 |
| Piranha Solution 2:1 v/v H2SO4 (98%):H2O2 (35%) | 30 60 90 | PEEK-PS 2:1-30 PEEK-PS 2:1-60 PEEK-PS 2:1-90 |
| Samples | Vibration Bands (O–H) p-Value | Vibration Bands (S=O) p-Value |
|---|---|---|
| (SPEEK 90 and PEEK-PS 2:1-60) | 0.002768897 | 0.00074459 |
| (SPEEK 90 and PEEK-PS 2:1-90) | 0.04471751 | 0.002609884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.S.F.d.; Rodrigues, J.F.B.; da Silva, M.C.; Barreto, M.E.V.; da Silva, H.N.; de Lima Silva, S.M.; Fook, M.V.L. Use of Piranha Solution as An Alternative Route to Promote Bioactivation of PEEK Surface with Low Functionalization Times. Molecules 2023, 28, 74. https://doi.org/10.3390/molecules28010074
Santos FSFd, Rodrigues JFB, da Silva MC, Barreto MEV, da Silva HN, de Lima Silva SM, Fook MVL. Use of Piranha Solution as An Alternative Route to Promote Bioactivation of PEEK Surface with Low Functionalization Times. Molecules. 2023; 28(1):74. https://doi.org/10.3390/molecules28010074
Chicago/Turabian StyleSantos, Flavia Suzany Ferreira dos, José Filipe Bacalhau Rodrigues, Milena Costa da Silva, Maria Eduarda Vasconcelos Barreto, Henrique Nunes da Silva, Suédina Maria de Lima Silva, and Marcus Vinicius Lia Fook. 2023. "Use of Piranha Solution as An Alternative Route to Promote Bioactivation of PEEK Surface with Low Functionalization Times" Molecules 28, no. 1: 74. https://doi.org/10.3390/molecules28010074