The Influence of High-Intensity Ultrasonication on Properties of Cellulose Produced from the Hop Stems, the Byproduct of the Hop Cones Production
Abstract
:1. Introduction
2. Results
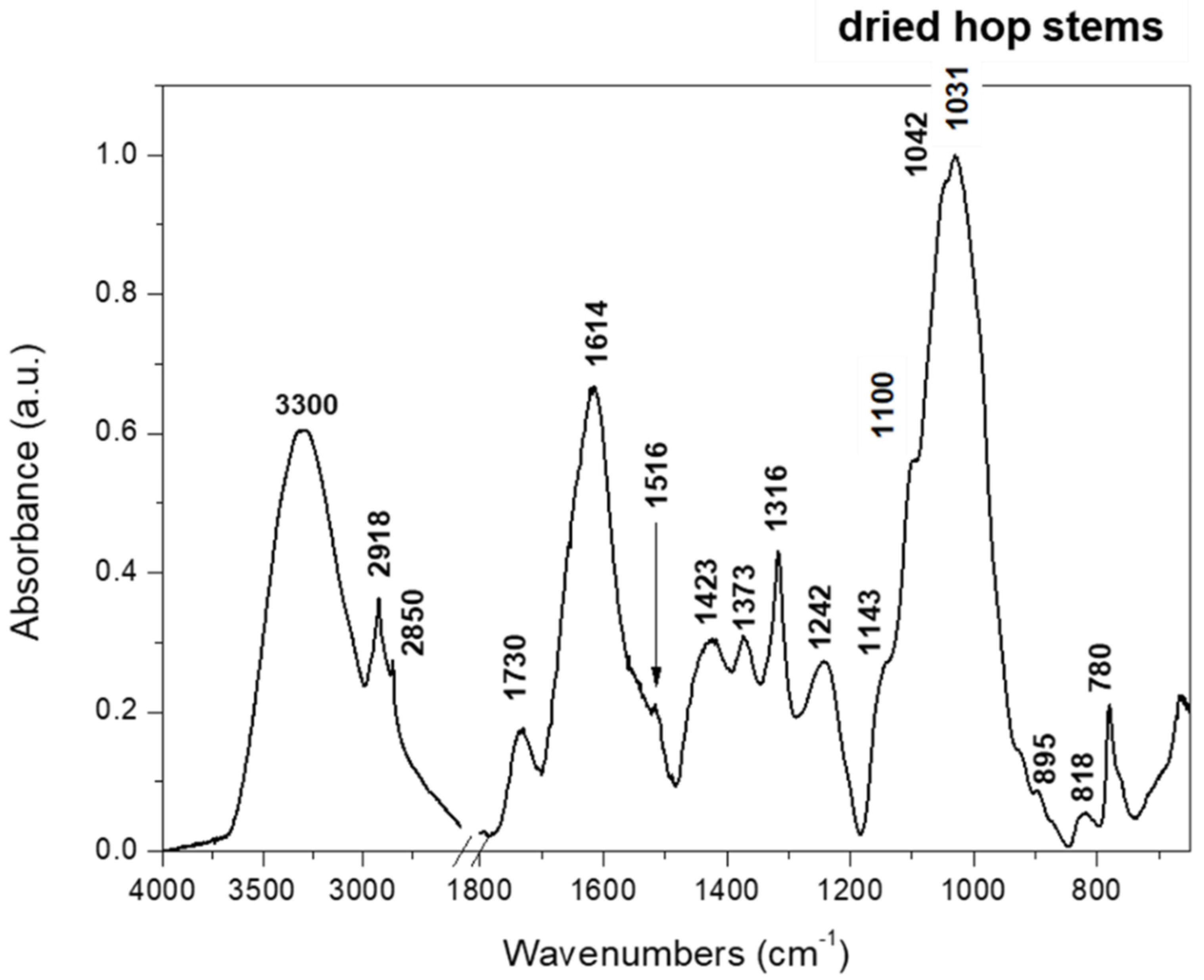
2.1. Characterization of Hop Stems
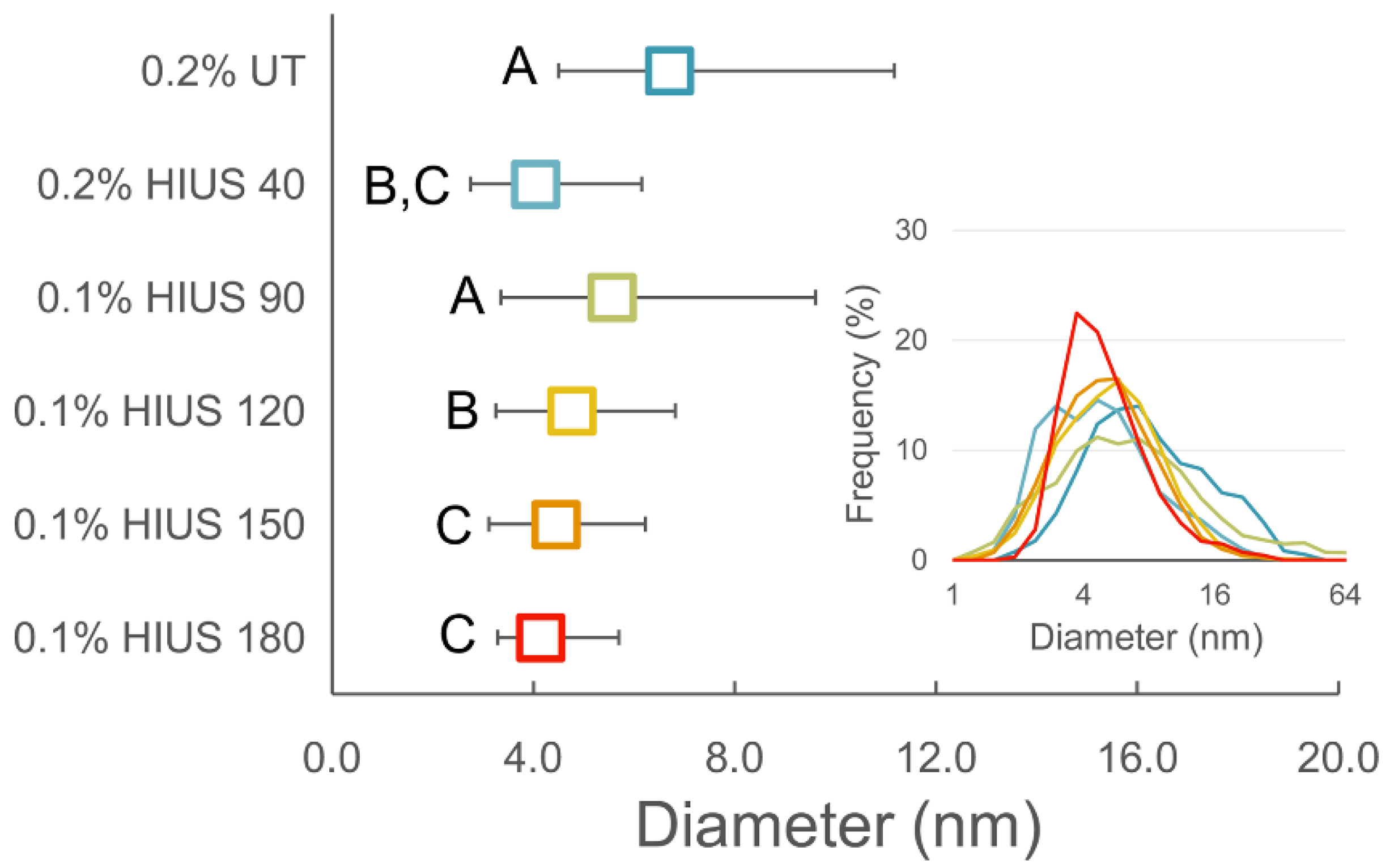
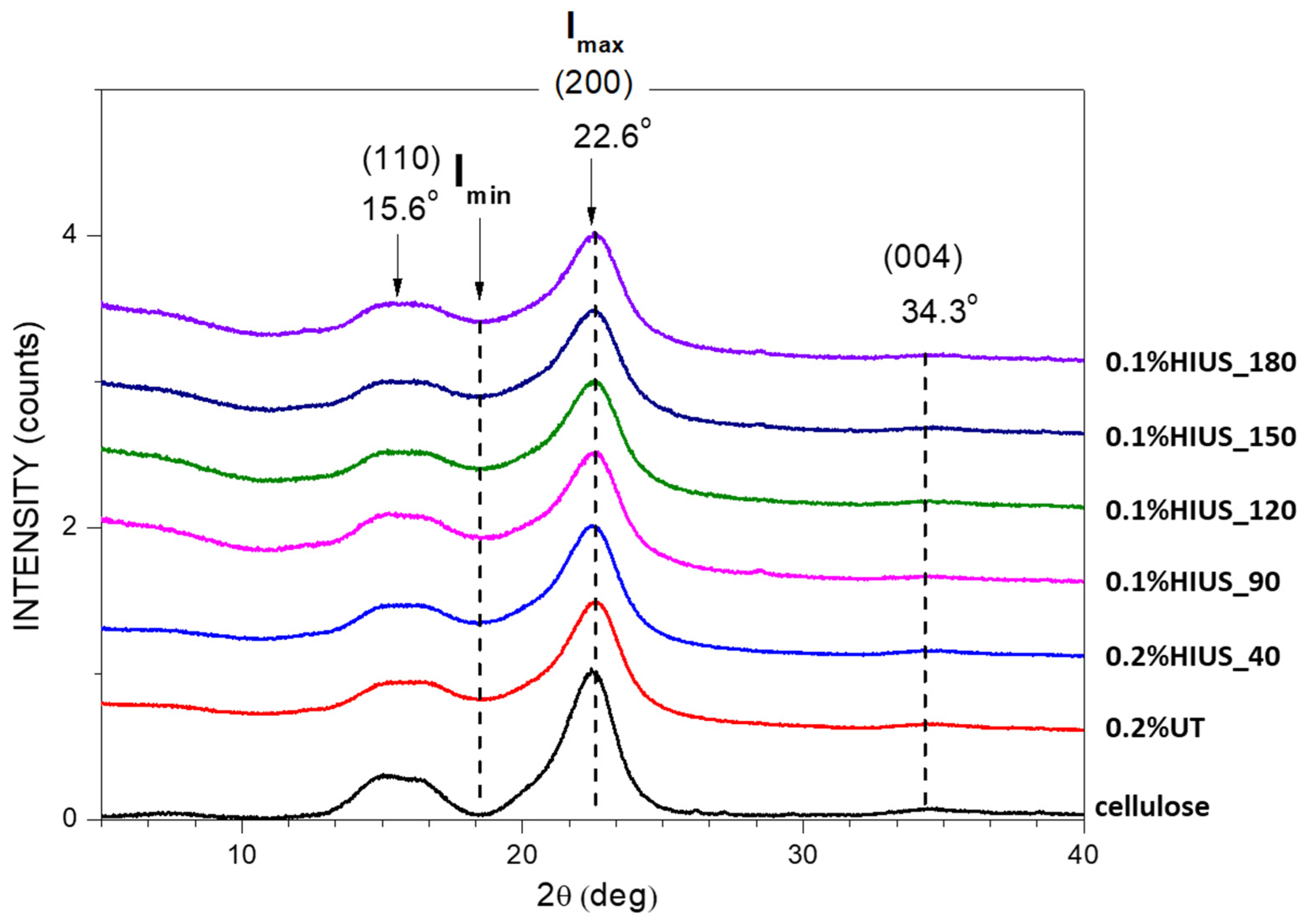
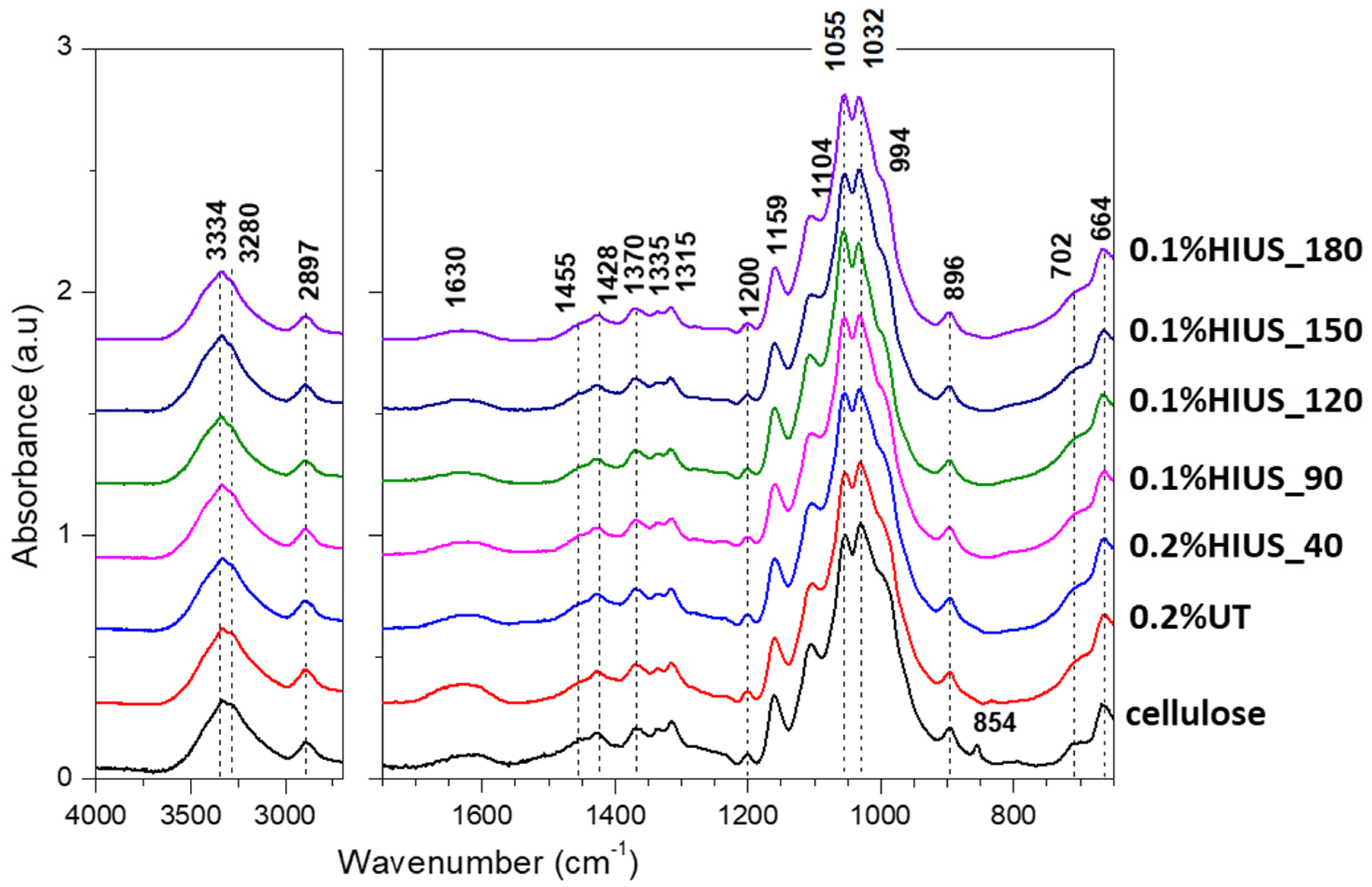
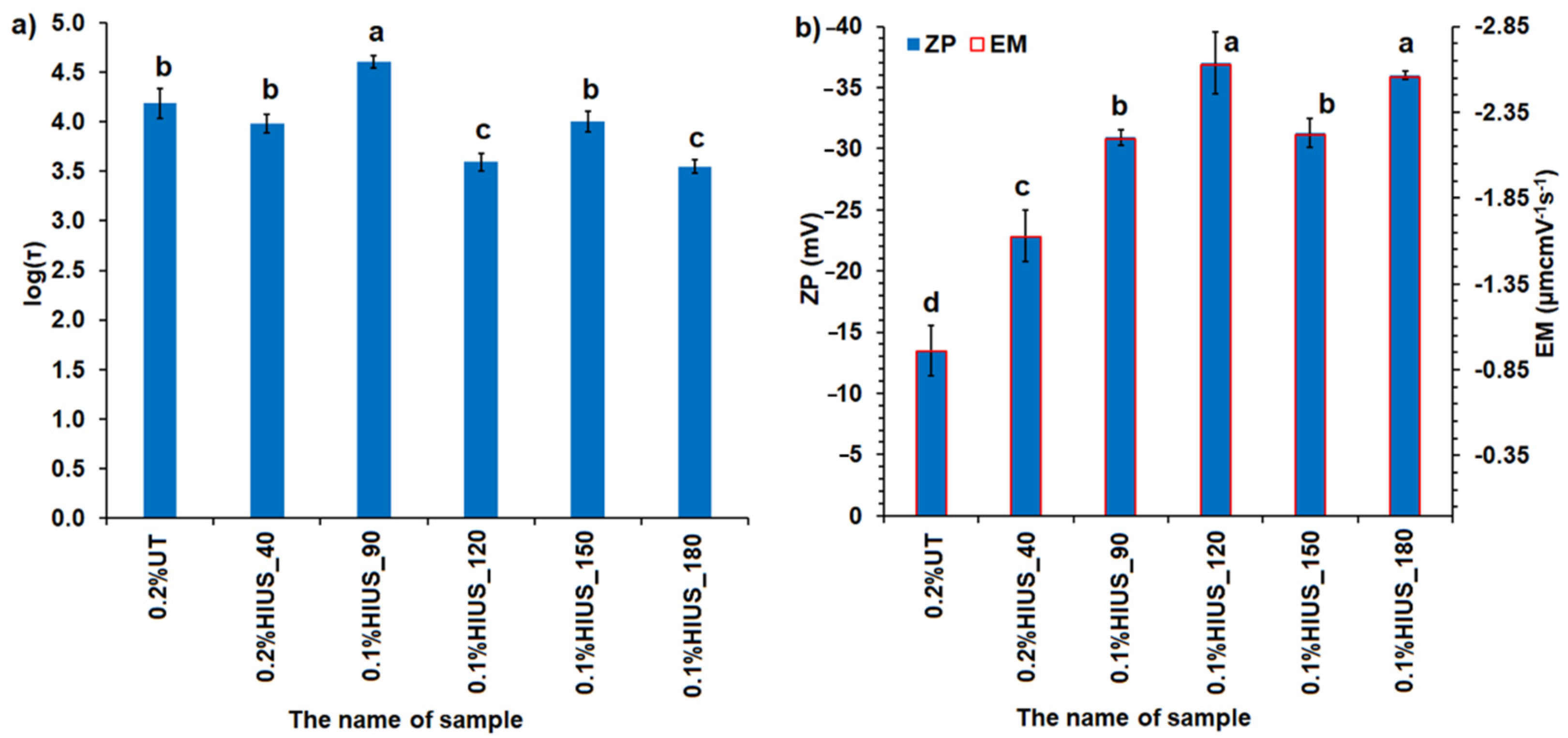
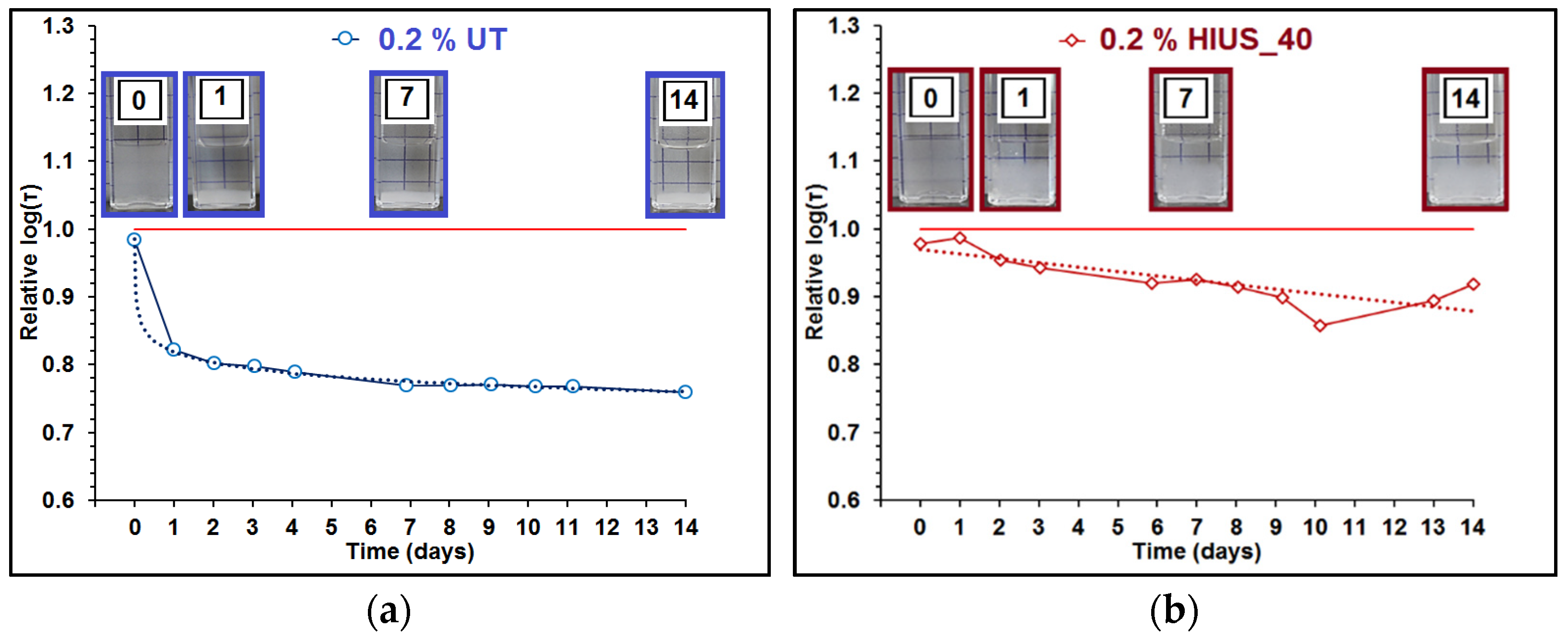
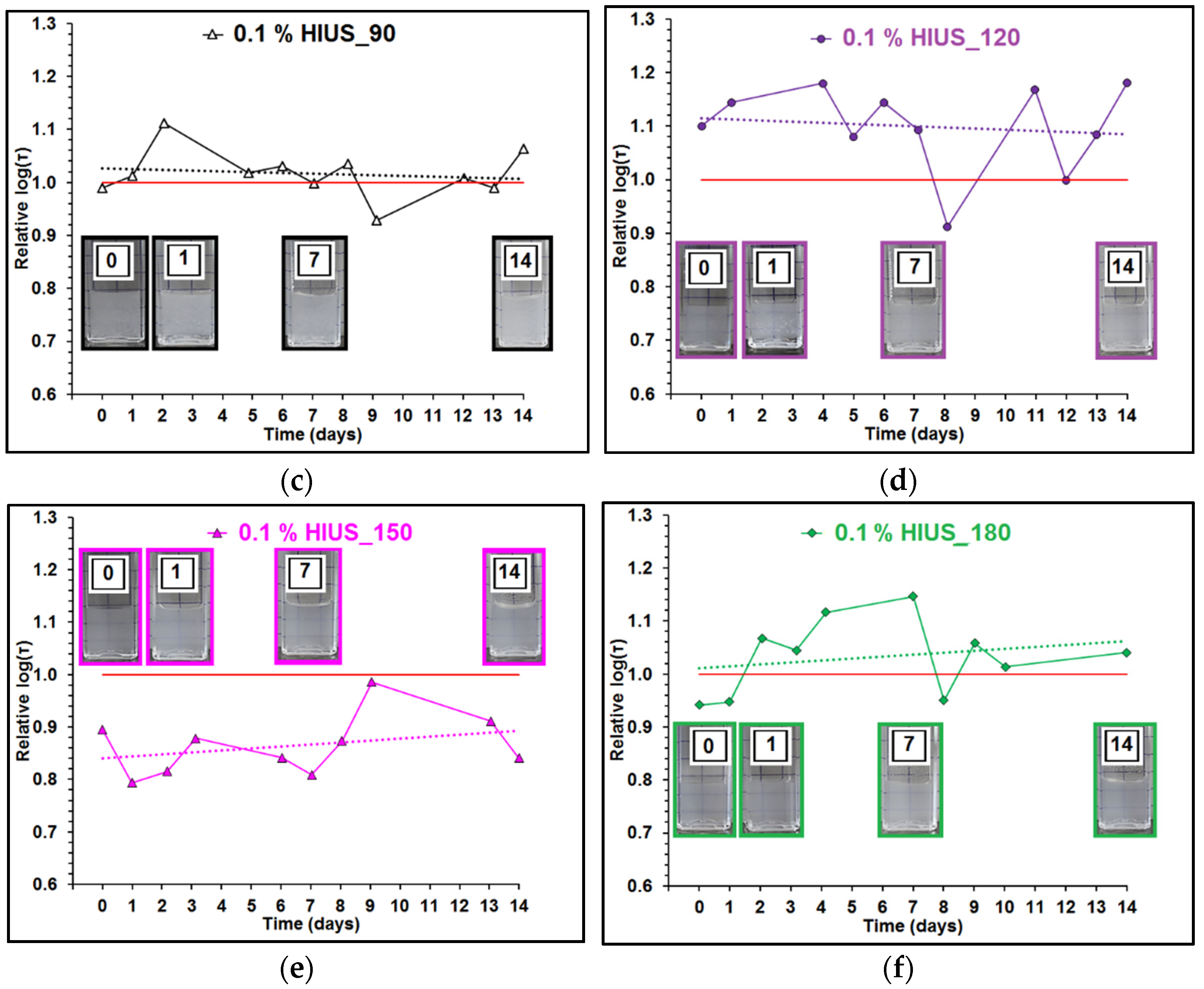
2.2. Characterization of Cellulose and Nanocellulose Obtained from Hop Stems
3. Materials and Methods
3.1. Materials
3.2. Characterization of Hop Stems
3.3. Isolation of Cellulose and Preparation of Nanocellulose
3.4. Atomic Force Microscopy (AFM)
3.5. X-ray Diffraction
3.6. Thermal Properties—Differential Scanning Calorimetry (DSC)
3.7. Fourier Transform Infrared Spectroscopy
3.8. Zeta Potential and Sedimentation of Cellulose and Nanocellulose Dispersions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Reddy, N.; Yang, Y. Properties of natural cellulose fibers from hop stems. Carbohydr. Polym. 2009, 77, 898–902. [Google Scholar] [CrossRef]
- Hop Report for the Harvest Year 2018. 19 May 2019. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/hops/hops-reports_en (accessed on 15 October 2020).
- Dufresne, A. (Ed.) Nanocellulose: From Nature to High Performance Tailored Materials; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Brett, C.; Waldron, K.; Black, M.; Chapman, J. (Eds.) Physiology and Biochemistry of Plant Cell Wall in Topics in Plant Physiology; Unwin Hyman Ltd.: London, UK, 1990; pp. 4–57. [Google Scholar]
- Bras, J.; Viet, D.; Bruzzese, C.; Dufresne, A. Correlation between stiffness of sheets prepared from cellulose whiskers and nanoparticles dimensions. Carbohydr. Polym. 2011, 84, 211–215. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Zdunek, A. Tailored nanocellulose structure depending on the origin. Example of apple parenchyma and carrot root celluloses. Carbohydr. Polym. 2019, 210, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J.; Jarvis, M.C. Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant Sci. 2012, 3, 204. [Google Scholar] [CrossRef] [Green Version]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Alonso-Simon, A.; Garcia-Angulo, P.; Melida, H.; Encina, A.; Alvarez, J.M.; Acebes, J.L. The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors. Plant Signal. Behav. 2011, 6, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Do Nascimento, D.M.; Almeida, J.S.; Vale, M.S.; Leitão, R.C.; Muniz, C.R.; de Figueirêdo, M.C.B.; Morais, J.P.S.; de Freitas Rosa, M. A comprehensive approach for obtaining cellulose nanocrystal from coconut fiber. Part I: Proposition of technological pathways. Ind. Crops Prod. 2016, 93, 66–75. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Zdunek, A. Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Largo-Gosens, A.; Hernández-Altamirano, M.; García-Calvo, L.; Alonso-Simón, A.; Álvarez, J.; Acebes, J.L. Fourier transform mid infrared spectroscopy applications for monitoring the structural plasticity of plant cell walls. Front. Plant Sci. 2014, 5, 303. [Google Scholar] [CrossRef] [Green Version]
- Szymańska-Chargot, M.; Chylińska, M.; Kruk, B.; Zdunek, A. Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydr. Polym. 2015, 115, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Flandez, J.; González, I.; Resplandis, J.B.; El Mansouri, N.-E.; Vilaseca, F.; Mutjé, P. Management of corn stalk waste as reinforcement for polypropylene injection moulded composites. Bioresources 2012, 2, 1836–1849. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, D.; Chen, M.; Weiming, Y.; Wang, L. Characteristics of corn stover components pyrolysis at low temperature based on detergent fibers. Front. Bioeng. Biotechnol. 2019, 7, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfiligoj Smole, M.; Hribernik, S.; Stana Kleinschek, K.; Kreže, T. Plant fibres for textile and technical applications. In Advances in Agrophysical Research; Grundas, S., Stepniewski, A., Eds.; IntechOpen: London, UK, 2013; Chapter 15; pp. 369–398. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Li, Q.; Renneckar, S. Supramolecular structure characterization of molecularly thin cellulose I nanoparticle. Biomacromolecules 2011, 12, 650–659. [Google Scholar] [CrossRef]
- Yeng, L.C.; Wahit, M.U.; Othman, N. Thermal and flexural properties of regenerated cellulose(rc)/poly(3-hydroxybutyrate)(phb)biocomposites. J. Teknol. 2015, 75, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Dai, D.; Huang, B.; Salih, S. (Eds.) Fourier transform infrared spectroscopy for natural fibres. In Fourier Transform—Materials Analysis; InTech Open: London, UK, 2012; pp. 45–68. [Google Scholar] [CrossRef] [Green Version]
- Kačurakova, M.; Smith, A.C.; Gidley, M.J.; Wilson, R.H. Molecular interactions in bacterial cellulose composites studied by 1D FT-IR and dynamic 2D FT-IR spectroscopy. Carbohydr. Res. 2002, 337, 1145–1153. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterization of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, F.; Wang, Q.; Zhang, Y.; Kong, L. Homogeneous isolation of nanocellulose from eucalyptus pulp by high pressure homogenization. Ind. Crops Prod. 2017, 104, 237–241. [Google Scholar] [CrossRef]
- Horne, D.S.; Hemar, Y.; Davidson, C.M. Diffusing wave spectroscopy studies of gel formation. In Food Colloids, Biopolymers and Materials; Dickinson, E., Van Vliet, T., Eds.; RSC Publishing: London, UK, 2003; pp. 17–25. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, K.; Yokota, S.; Kondo, T. Difference between bamboo- and wood-derived cellulose nanofibers prepared by the aqueous counter collision method. Nord. Pulp Pap. Res. J. 2014, 29, 69–76. [Google Scholar] [CrossRef]
- Raj, P.; Mayahi, A.; Lahtinen, P.; Varanasi, S.; Garnier, G.; Martin, D.; Batchelor, W. Gel point as a measure of cellulose nanofibre quality and feedstock development with mechanical energy. Cellulose 2016, 23, 3051–3064. [Google Scholar] [CrossRef]
- Mahmud, S.; Hasan, K.M.F.; Jahid, M.A.; Mohiuddin, K.; Zhang, R.; Zhu, J. Comprehensive review on plant fiber-reinforced polymeric biocomposites. J. Mater. Sci. 2021, 56, 7231–7264. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Walkiewicz, A.; Pertile, G.; Frąc, M.; Cieślak, K.J.; Zdunek, A. Evaluation of Nanocomposite Made of Polylactic Acid and Nanocellulose from Carrot Pomace Modified with Silver Nanoparticles. Polymers 2020, 12, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, K.M.F.; Horváth, P.G.; Bak, M.; Le, D.H.A.; Mucsi, Z.M.; Alpár, T. Rice straw and energy reed fibers reinforced phenol formaldehyde resin polymeric biocomposites. Cellulose 2021, 28, 7859–7875. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horváth, P.G.; Kóczán, Z.; Le, D.H.A.; Bak, M.; Bejó, L.; Alpár, T. Novel insulation panels development from multilayered coir short and long fiber reinforced phenol formaldehyde polymeric biocomposites. J. Polym. Res. 2021, 28, 467. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1990, 73, 491–497. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Mayinger, F. Optical Measurements Techniques and Applications; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Delgado, A.V.; Gonzales-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]

| NDS | NDF | H | C | L |
|---|---|---|---|---|
| g/100 g d.m. | g/100 g d.m. | g/100 g d.m. | g/100 g d.m. | g/100 g d.m. |
| 51.07 ± 0.51 | 48.93 ± 0.51 | 10.94 ± 0.46 | 29.06 ± 0.86 | 8.93 ± 0.40 |
| Xc% | dhkl | |
|---|---|---|
| (%) | (nm) | |
| cellulose | 67.2 | 3.71 |
| 0.2%UT | 68.4 | 3.20 |
| 0.2%HIUS_40 | 66.9 | 3.10 |
| 0.1%HIUS_90 | 58.7 | 3.01 |
| 0.1%HIUS_120 | 60.4 | 2.96 |
| 0.1%HIUS_150 | 61.3 | 2.91 |
| 0.1%HIUS_180 | 60.4 | 2.94 |
| SEMIMELTING | DECOMPOSITION | |||
|---|---|---|---|---|
| Tmax | Enthalpy | Tmax | Enthalpy | |
| (°C) | (J/g) | (°C) | (J/g) | |
| 0.2%UT | 255.60 | 20.354 | 339.86 | 85.841 |
| 0.2%HIUS_40 | 333.58 | 25.457 | 361.73 | 13.601 |
| 0.1%HIUS_90 | 323.88 | 37.785 | 358.94 | 14.028 |
| 0.1%HIUS_120 | 323.88 | 22.700 | 353.56 | 21.663 |
| 0.1%HIUS_150 | 331.83 | 21.747 | 364.56 | 11.422 |
| 0.1%HIUS_180 | 321.77 | 25.870 | 358.78 | 25.870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska-Chargot, M.; Cieśla, J.; Pękala, P.; Pieczywek, P.M.; Oleszek, W.; Żyła, M.; Szkopek, Z.; Zdunek, A. The Influence of High-Intensity Ultrasonication on Properties of Cellulose Produced from the Hop Stems, the Byproduct of the Hop Cones Production. Molecules 2022, 27, 2624. https://doi.org/10.3390/molecules27092624
Szymańska-Chargot M, Cieśla J, Pękala P, Pieczywek PM, Oleszek W, Żyła M, Szkopek Z, Zdunek A. The Influence of High-Intensity Ultrasonication on Properties of Cellulose Produced from the Hop Stems, the Byproduct of the Hop Cones Production. Molecules. 2022; 27(9):2624. https://doi.org/10.3390/molecules27092624
Chicago/Turabian StyleSzymańska-Chargot, Monika, Jolanta Cieśla, Patrycja Pękala, Piotr M. Pieczywek, Wiesław Oleszek, Marcin Żyła, Zbigniew Szkopek, and Artur Zdunek. 2022. "The Influence of High-Intensity Ultrasonication on Properties of Cellulose Produced from the Hop Stems, the Byproduct of the Hop Cones Production" Molecules 27, no. 9: 2624. https://doi.org/10.3390/molecules27092624







