Influence of Irradiance and Wavelength on the Antioxidant Activity and Carotenoids Accumulation in Muriellopsis sp. Isolated from the Antofagasta Coastal Desert
Abstract
:1. Introduction
2. Results and Discussion
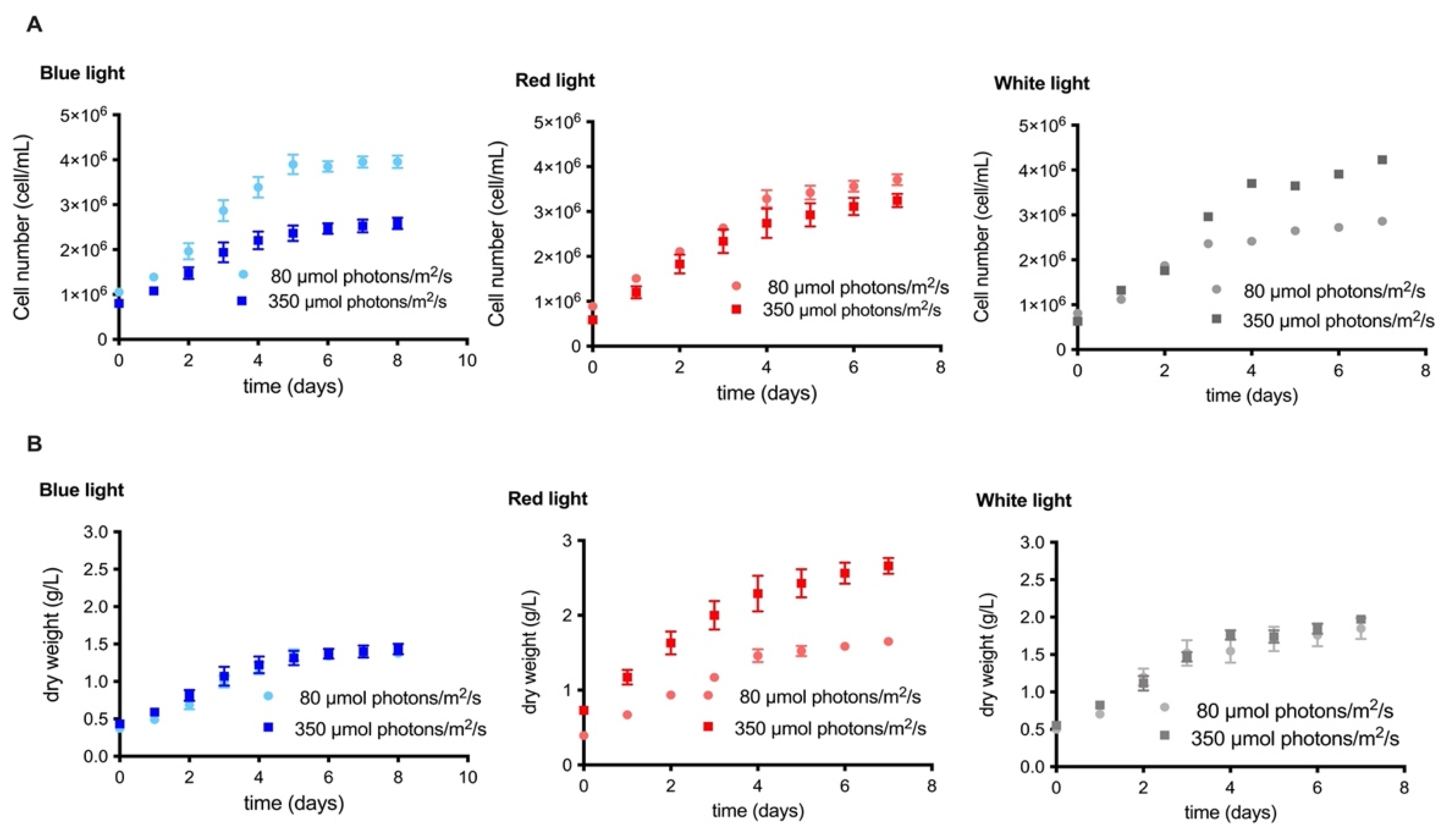
2.1. Comparison of the Effect of Irradiance and Wavelength on the Growth Kinetics and Biomass Production of the MCH-35 Microalgae
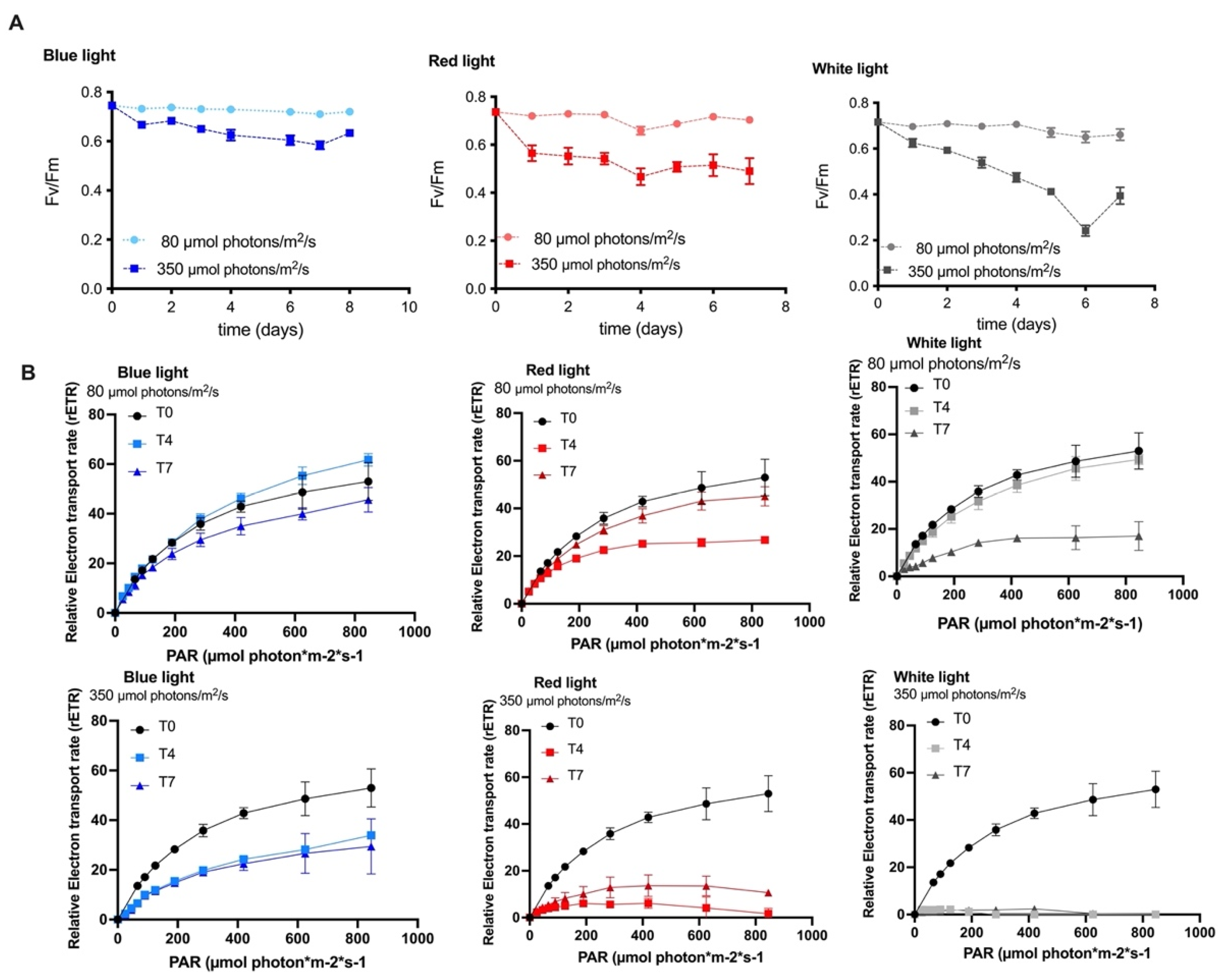
2.2. The Effect of Varying Wavelengths and Light Intensities on the Photosynthetic Activity of the MCH-35 Microalgae
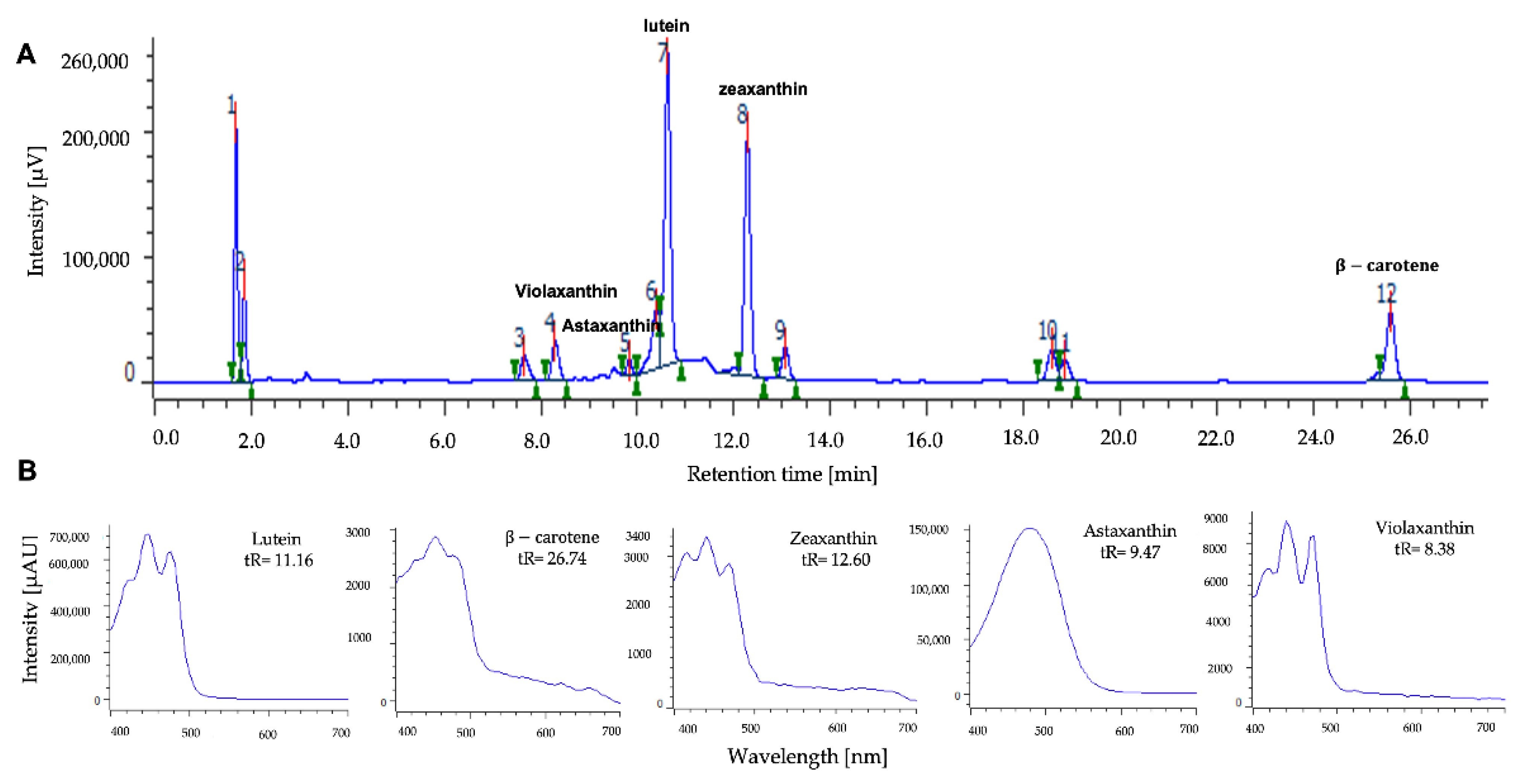
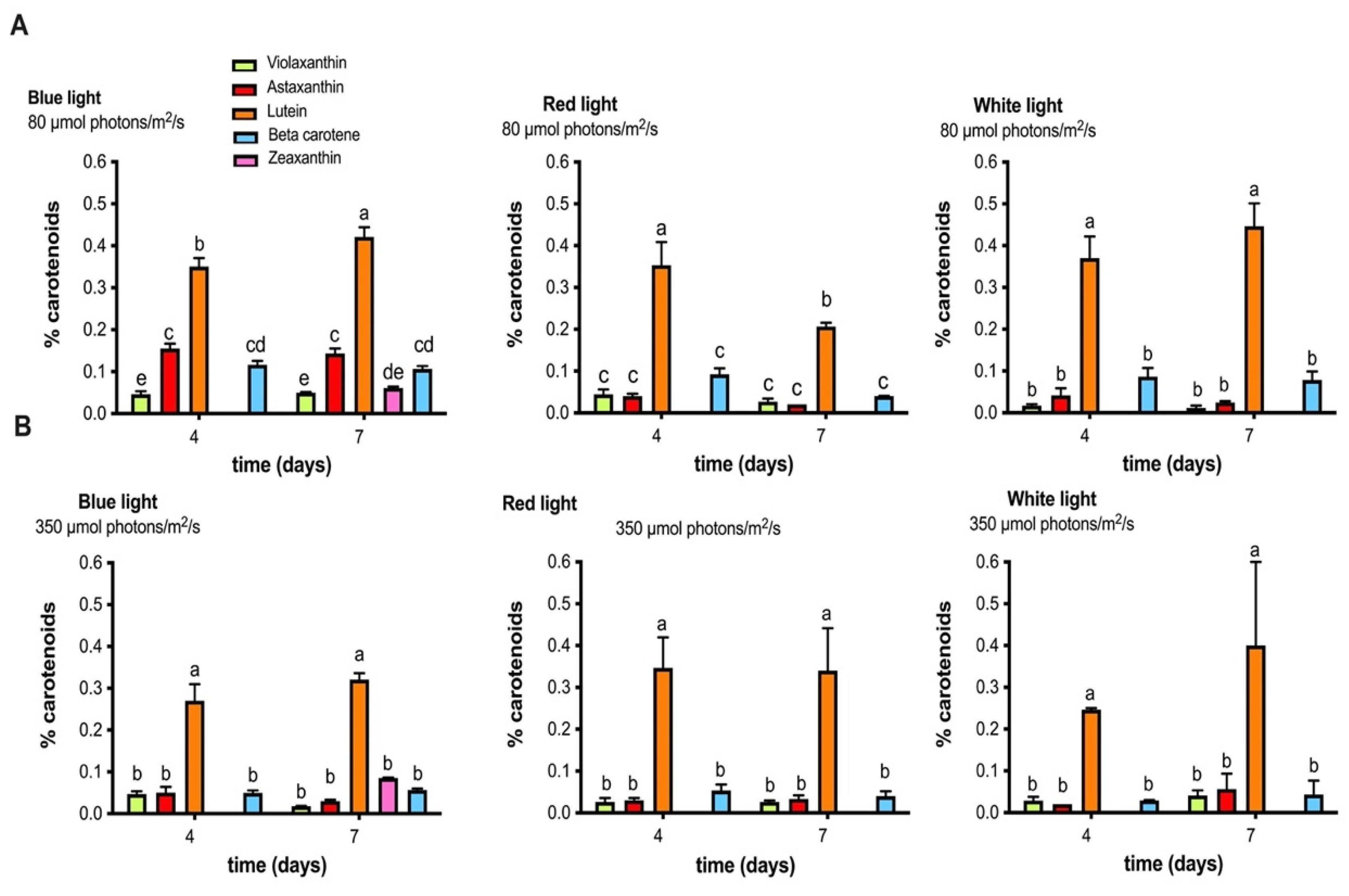
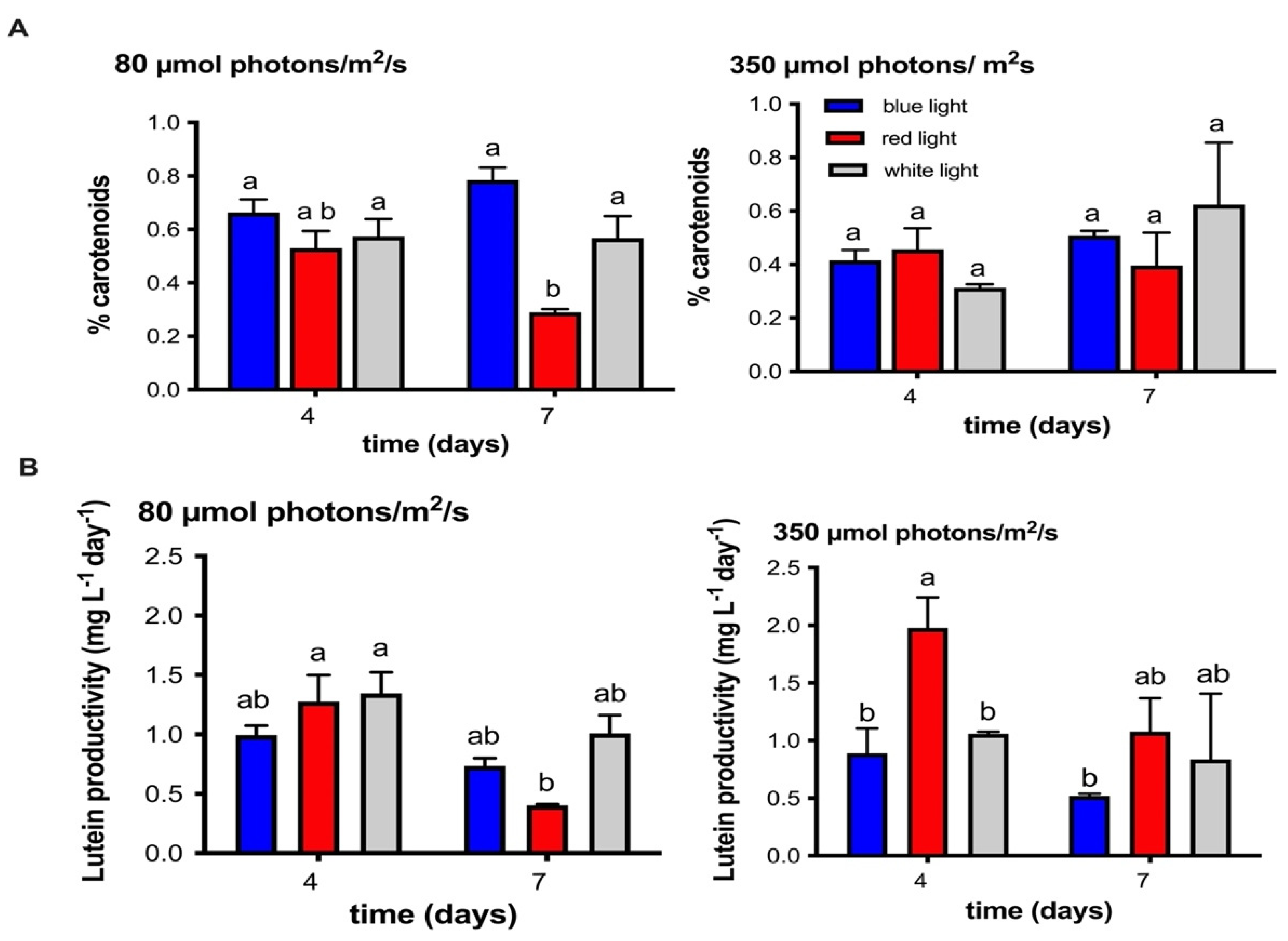
2.3. Identification and Quantification of Carotenoid Pigments Produced by the MCH-35 Microalgae Exposed to Varying Light Intensities and Wavelengths
2.4. Influence of Different Wavelengths and Irradiances on the Lipid Content
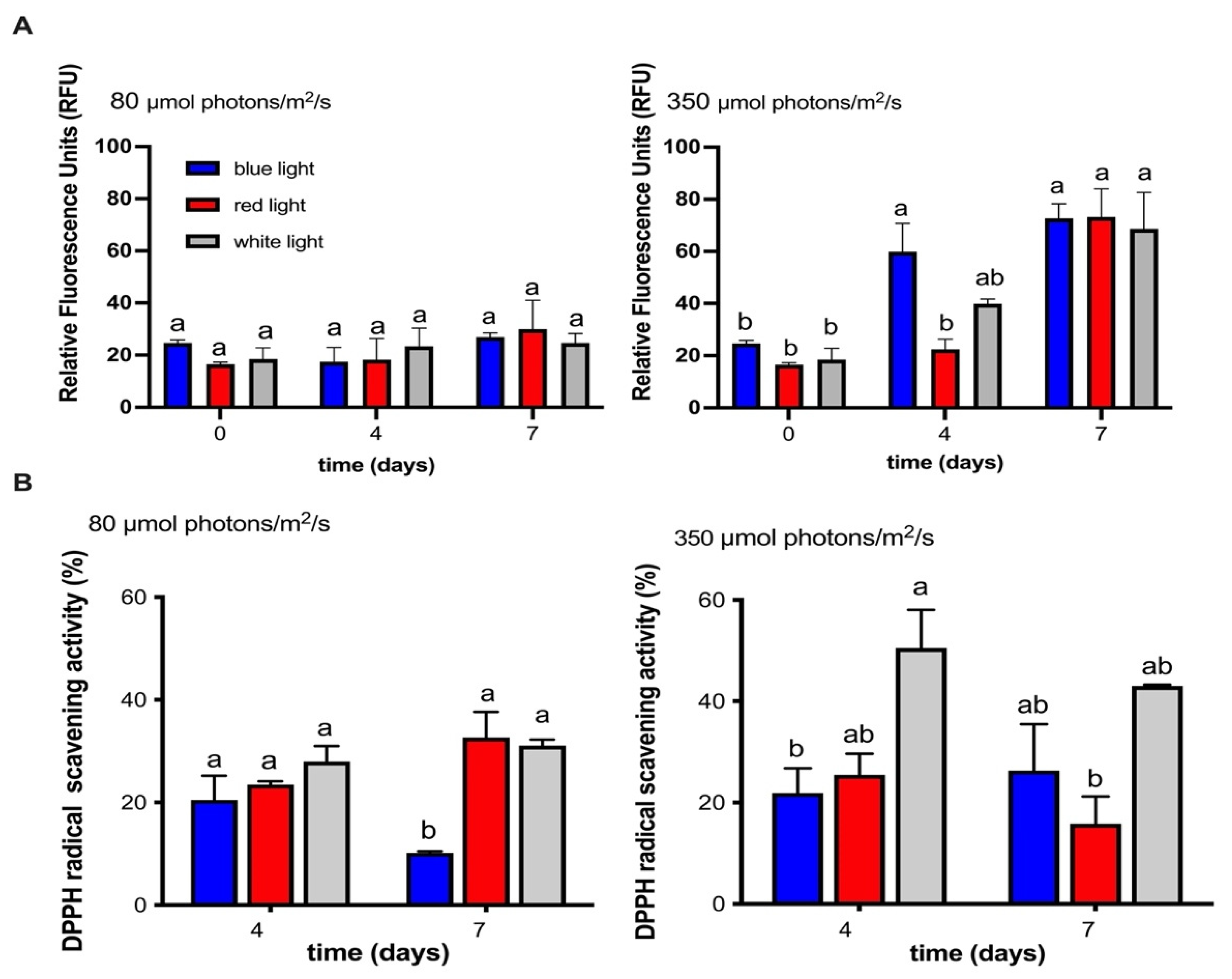
2.5. Influence of Different Wavelengths and Irradiances on the Antioxidant Activity of the MCH-35 Microalgae
3. Materials and Methods
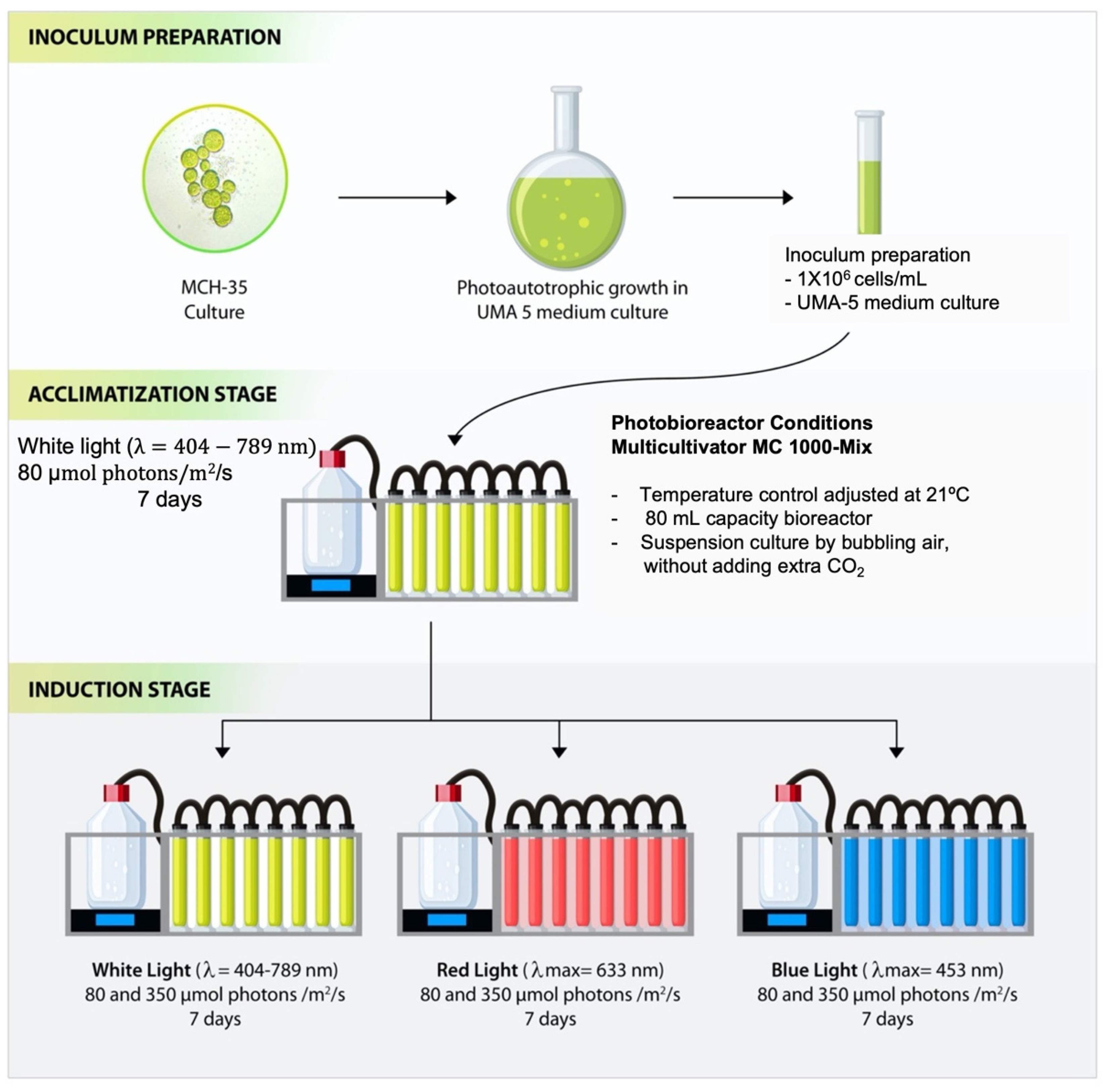
3.1. Cell Cultures and Treatment Conditions
3.2. Determination of Cell Growth and Dry Weight of the MCH-35 Microalgae
3.3. Influence of Light Color and Irradiance on the Photosynthetic State of the MCH-35 Microalgae
3.4. Organic Carotenoids Extraction from the Microalgae MCH-35
3.5. Determination of the Carotenoids Content in the Microalgae MCH-35
3.6. Determination of Total Lipid Production by MCH-35
3.7. Determination of the Antioxidant Capacity of the Microalgae MCH-35 with 2,2-Diphenyl-picrylhydrazyl (DPPH) Radical Scavenging Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sun, Z.; Li, T.; Zhou, Z.G.; Jiang, Y. Microalgae as a Source of lutein: Chemistry, Biosynthesis, and Carotenogenesis. Adv. Biochem. Eng. Biotechnol. 2015, 153, 37–58. [Google Scholar] [CrossRef]
- Da Silva Vaz, B.; Moreira, J.; Morais, M.; Costa, J. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Malsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Kou, Y.; Liu, M.; Sun, P.; Dong, Z.; Liu, J. High light boosts salinity stress-induced biosynthesis of astraxanthin and lipids in the green alga Chromochloris zofingiensis. Algal Res. 2020, 50, 101976. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Premaratne, R.G.M.M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Astaxanthin accumulation in the green microalga Haematococcus pluvialis: Effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnol. Rep. 2020, 28, e0053. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hogdson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [Green Version]
- Kroumov, A.D.; Módenes, A.N.; Trigueros, D.E.G.; Espinoza-Quiñones, F.R.; Borba, C.E.; Scheufele, F.B.; Hinterholz, C.L. A systems approach for CO2 fixation from flue gas by microalgae—Theory review. Process Biochem. 2016, 51, 1817–1832. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as source of carotenoids. Mar. Drugs 2011, 9, 625. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haemaetococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Mallick, N.; Helmuth, M.F. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A. The Global Market for Carotenoids; FOD025F; BBC Publishing: London, UK, 2018; Available online: https://www.bccresearch.com/market-research/food-and-beverage/the-global-market-for-carotenoids.htm (accessed on 19 January 2022).
- Fernandez-Sevilla, J.; Acién, F.G.; Molina, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Arunkumar, R.; Calvo, C.M.; Conrady, C.D.; Bernstein, P.S. What do we know about the macular pigment in AMD: The past, the present and the future? Eye 2018, 32, 992–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Campo, J.A.; Garcia-Gonzalez, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-J.; Tseng, Y.-F.; Chen, Y.-C.; Hsiao, Y.; Lee, P.-C.; Chen, T.-J.; Chen, C.-Y.; Kao, C.-Y.; Chang, J.-S.; Chen, J.-C.; et al. Transcriptome and physiological analysis of a lutein-producing alga Desmodesmus sp. reveals the molecular mechanisms for high lutein productivity. Algal Res. 2017, 21, 103–119. [Google Scholar] [CrossRef]
- Sunda, W.; Huntsman, S. Relationships among photoperiod, carbon fixation, growth, chlorophyll a, and cellular iron and zinc in a costal diatom. Limnol. Oceanogr. 2004, 45, 1742–1753. [Google Scholar] [CrossRef]
- Vaquero, I.; Mogedas, B.; Ruiz-Dominguez, M.C.; Vega, J.; Vilchez, C. Light-mediated lutein enrichment of an acid environment microalga. Algal Res. 2014, 6, 70–77. [Google Scholar] [CrossRef]
- McGee, D.; Archer, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Influence of spectral intensity and quality of LED lighting on photoacclimation, carbon allocation and high-value pigments in microalgae. Photosynth. Res. 2020, 143, 63–80. [Google Scholar] [CrossRef]
- Baer, S.; Heining, M.; Schwerna, P.; Buchholz, R.; Hübner, H. Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor. Algal Res. 2016, 14, 109–115. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Wang, Y.; Xi, Y.; Jiang, Z.; Zhang, X.; Wang, X.; Zhang, X. Light Emitting Diode Power Conversion capability and CO2 Fixation Rate of Microalgae Biofilm Cultured under Different Light Spectra. Energies 2020, 13, 1536. [Google Scholar] [CrossRef] [Green Version]
- Marticorena, P.; Gonzalez, L.; Riquelme, C.; Silva, F. Effects of beneficial bacteria on biomass, photosynthetic parameters and cell composition on the microalga Muriellopsis sp. adapted to grow in seawater. Aquac. Res. 2020, 51, 3609–3622. [Google Scholar] [CrossRef]
- Cruz-Balladares, V.; Marticorena, P.; Riquelme, C. Effect on growth and productivity of lutein from the chlorophyta microalga, strain MCH of Muriellospsis sp., when grown in sea water and outdoor conditions at the Atacama Desert. Electron. J. Biotechnol. 2021, 54, 77–85. [Google Scholar] [CrossRef]
- Teo, C.L.; Atta, M.; Bukhari, A.; Taisir, M.; Yusuf, A.M.; Idris, A. Enhancing growth and lipid production of marine microalgae for diesel production via the use of different LED wavelengths. Bioresour. Technol. 2014, 162, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ho, S.-H.; Chen, C.-N.; Chen, C.-Y.; Ng, I.-S.; Jing, K.-J.; Chang, J.-S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. For lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef]
- Duarte, J.H.; Costa, J.A.V. Blue light emitting diodes (LEDs) as an energy source in Chlorella fusca and Synechococcus nidulans cultures. Bioresour. Technol. 2017, 247, 1242–1245. [Google Scholar] [CrossRef]
- Fu, W.; Guomundsson, Ó.; Paglia, G.; Herjólfsson, G.; Andrésson, O. Enhancement of carotenoid biosynthesis in the green microalga Dunaniella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of and indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Chen, H.B.; Wu, J.Y.; Wang, C.F.; Fu, C.C.; Shieh, C.J.; Chen, C.I.; Wang, C.Y.; Liu, Y.C. Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem. Eng. J. 2010, 53, 52–53. [Google Scholar] [CrossRef]
- Das, P.; Lei, W.; Aziz, S.S.; Obbard, J.P. Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour. Technol. 2011, 102, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Amaro, H.; Pagels, F.; Azevedo, I.; Azevedo, J.; Pinto, I.S.; Malcata, F.; Guedes, A. Light-emitting diodes-a plus on microalgae biomass and high-value metabolite production. J. Appl. Phycol. 2020, 32, 3605–3618. [Google Scholar] [CrossRef]
- Schulze, P.S.; Barreira, L.A.; Pereira, H.G.; Perales, J.A.; Varela, J.C. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, D.; Sommerfeld, M.; Hu, Q. Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour. Technol. 2011, 102, 123–129. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Moheimani, N.R.; Cosgrove, J.J.; Parlevliet, D.; Bahri, P.A. Effects of different light spectra on the growth. Productivity and photosynthesis of two acclimated strains of Nannochloropsis sp. J. Appl. Phycol. 2017, 29, 1765–1774. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Moheimani, N.R.; Kosterink, N.R.; Cosgrove, J.J.; Parlevliet, D.; Gonzalez-Garcia, C.; Lubián, L.M. Photosynthetic performance of two Nannochloropsis spp. under different filtered light spectra. Algal Res. 2016, 19, 168–177. [Google Scholar] [CrossRef]
- Schagerl, M.; Pichler, C.; Donabaum, K. Patterns of major photosynthetic pigments in freshwater algae. 2. Dinophyta, Ruglenophyta, Chlorophyceae and Charales. Ann. Limnol. 2003, 39, 49–62. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Liu, X.; Ho, S.-H.; Xie, Y.; Chen, J. Strategies related to light quality and temperature to improve lutein production of marine microalga Chlamydomonas sp. Bioprocess Biosyst. Eng. 2019, 42, 435–443. [Google Scholar] [CrossRef]
- Pereira, S.; Otero, A. Haematococcus pluvialis bioprocess optimization: Effect of light quality, temperature and irradiance on growth, pigment content and photosynthetic response. Algal Res. 2020, 51, 102027. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P. Carotenoid production by Dunaliella salina under red light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Dini-Cohen, S.; Cohen, Z.; Merzlyak, M.N. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J. Appl. Phycol. 2008, 20, 245–251. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.; Harvey, P. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella Salina (Chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016, 106, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Ahn, J.; Jeon, H.; Jin, E. Development of a Dunaliella tertiolecta strain with increased zeaxanthin content using randon mutagenesis. Mar. Drugs 2017, 15, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Jin, P.; Cheng, J.J. A comprehensive comparable study of the physiological properties of four microalgal species under different light wavelength conditions. Planta 2018, 248, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.-H.; Chen, J. Enhancing lutein productivity of Chlamydomonas sp. Via high-intensity light exposure with corresponding carotenogenic genes expression profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Kim, D.G.; Roh, S.W.; Choi, J.-S.; Choi, Y.-E. Enhancement of astaxanthin production using Haematococcus pluvialis with novel LED wavelength shift strategy. Appl. Microbiol. Biotechnol. 2016, 100, 6231–6238. [Google Scholar] [CrossRef]
- Han, S.I.; Kim, S.; Lee, C.; Choi, Y.E. Blue-Red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J. Microbiol. 2019, 57, 101–106. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, Y.; Han, S.-H.; Hwang, S.-J. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour. Technol. 2013, 130, 75–80. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Katsoulas, N.; Karapanagiotidis, I.T.; Papapolymerou, G. Effect of nitrogen concentration two-stage and prolonged cultivation on growth rate, lipid and protein content of Chlorella vulgaris. J. Chem. Technol. 2019, 94, 1466–1473. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, F.; Jin, P. Effects of Light-emitting Diodes (LEDs) on the accumulation of lipid content in microalgae. Bioresour. Technol. 2017, 212, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Vadiveloo, A.; Moheimani, N.R.; Cosgrove, J.J.; Parisa, A.B.; Parlevliet, D. Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res. 2015, 8, 121–127. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2020, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yuan, C.; Hu, G.; Li, F. Effects of light intensity in the growth and lipid accumulation of microalga Scenedesmus sp. 11–1 under nitrogen limitation. Appl. Biochem. Biotechnol. 2012, 166, 2127–2137. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Chow, K.P.; Yung, K.K. Effect of different light sources on algal biomass and lipid production in internal LEDs-illuminated photobioreactor. J. Mar. Biol. Aquac. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Riveros, K.; Sepulveda, C.; Bazaes, J.; Marticorena, P.; Riquelme, C.; Acién, G. Overall development of a bioprocess for the outdoor production of Nannochloropsis gaditana for aquaculture. Aquac. Res. 2018, 49, 165–176. [Google Scholar] [CrossRef]
- Cerón-García, M.C.; González-López, C.V.; Camacho-Rodriguez, J.; López-Rosales, L.; Garcia-Camacho, F.; Molina-Grima, E. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC). Food Chem. 2018, 257, 316–324. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-MacAdoo, D.; Mata, M.T.; Riquelme, C. Influence of Irradiance and Wavelength on the Antioxidant Activity and Carotenoids Accumulation in Muriellopsis sp. Isolated from the Antofagasta Coastal Desert. Molecules 2022, 27, 2412. https://doi.org/10.3390/molecules27082412
Diaz-MacAdoo D, Mata MT, Riquelme C. Influence of Irradiance and Wavelength on the Antioxidant Activity and Carotenoids Accumulation in Muriellopsis sp. Isolated from the Antofagasta Coastal Desert. Molecules. 2022; 27(8):2412. https://doi.org/10.3390/molecules27082412
Chicago/Turabian StyleDiaz-MacAdoo, Daniela, Maria Teresa Mata, and Carlos Riquelme. 2022. "Influence of Irradiance and Wavelength on the Antioxidant Activity and Carotenoids Accumulation in Muriellopsis sp. Isolated from the Antofagasta Coastal Desert" Molecules 27, no. 8: 2412. https://doi.org/10.3390/molecules27082412





