Fraxinol Stimulates Melanogenesis in B16F10 Mouse Melanoma Cells through CREB/MITF Signaling
Abstract
:1. Introduction
2. Results
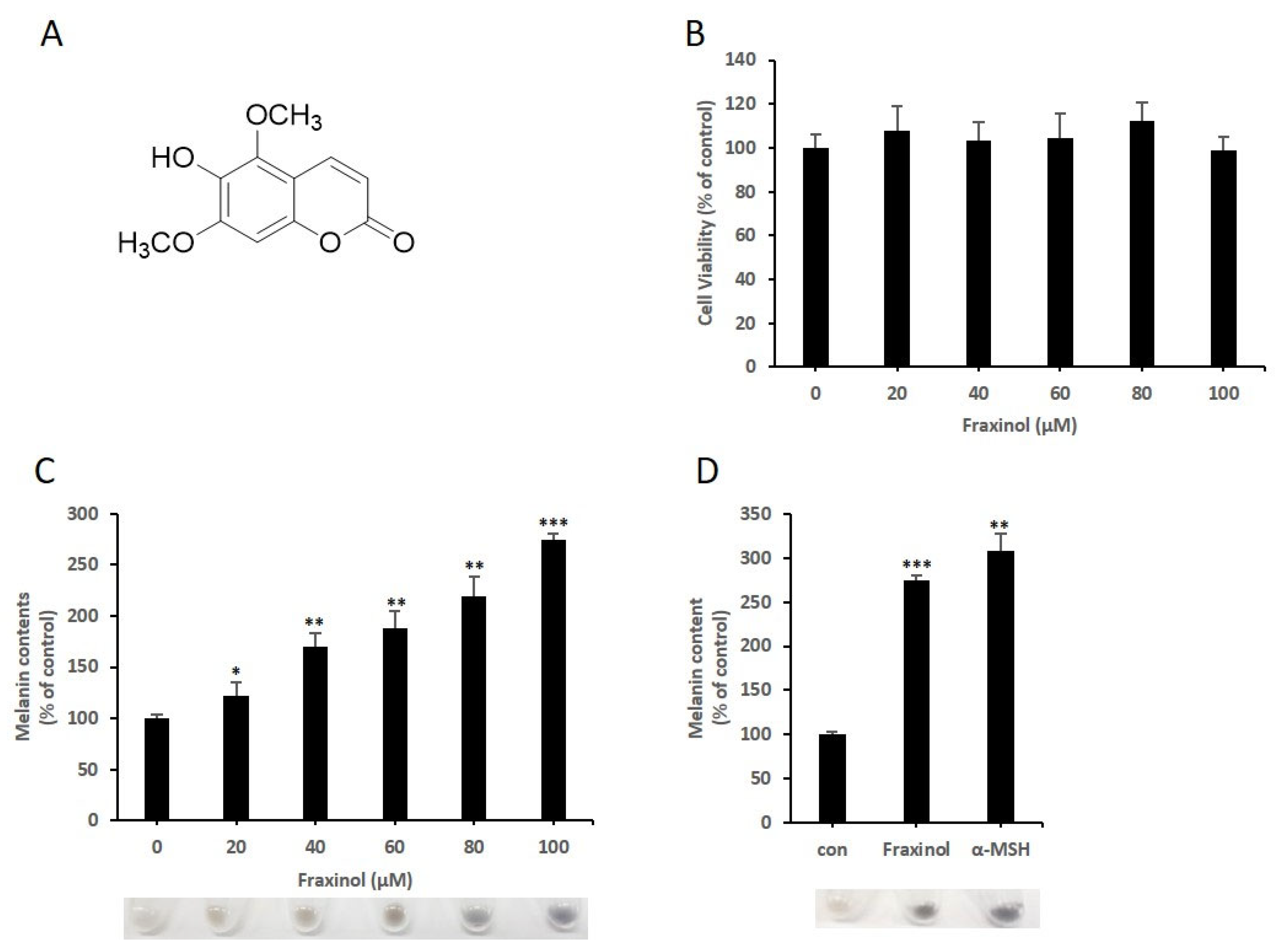
2.1. Fraxinol Induces the Production of Cellular Melanin in B16-F10 Melanoma Cells without Cytotoxicity
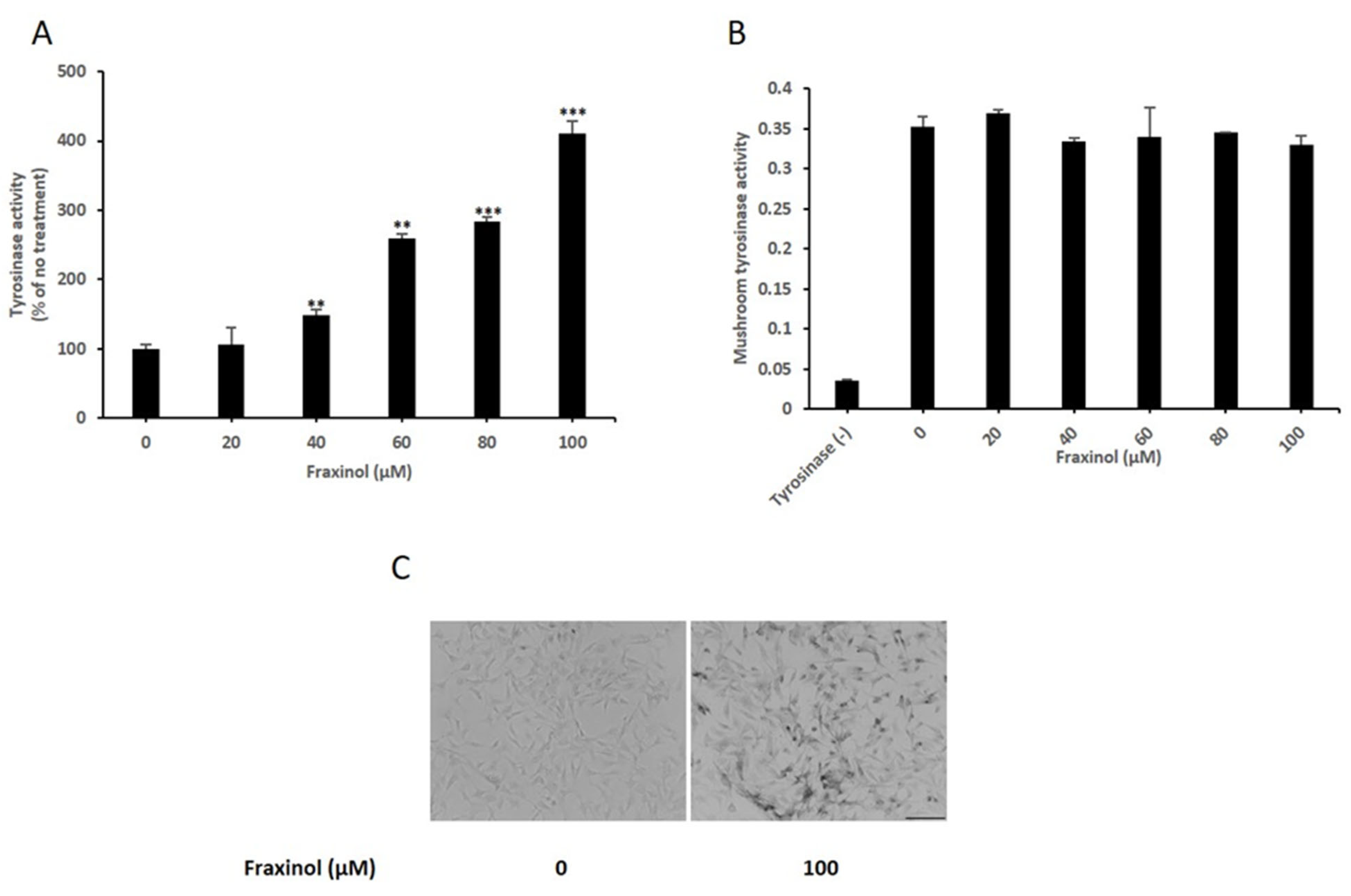
2.2. Fraxinol Increases Intracellular TYR Activity in B16-F10 Cells
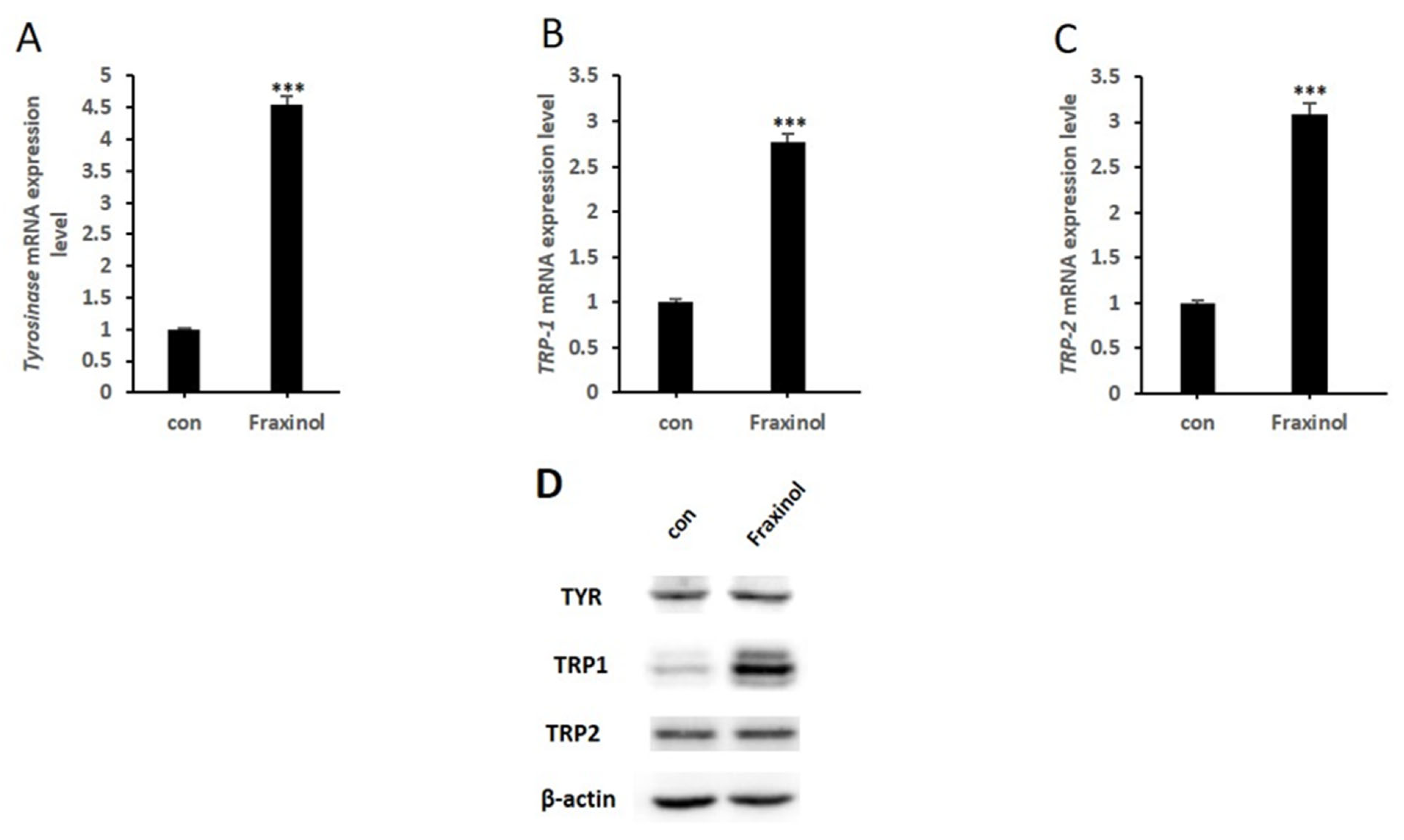
2.3. Fraxinol Enhances the Expression Level of Melanogenic Enzymes
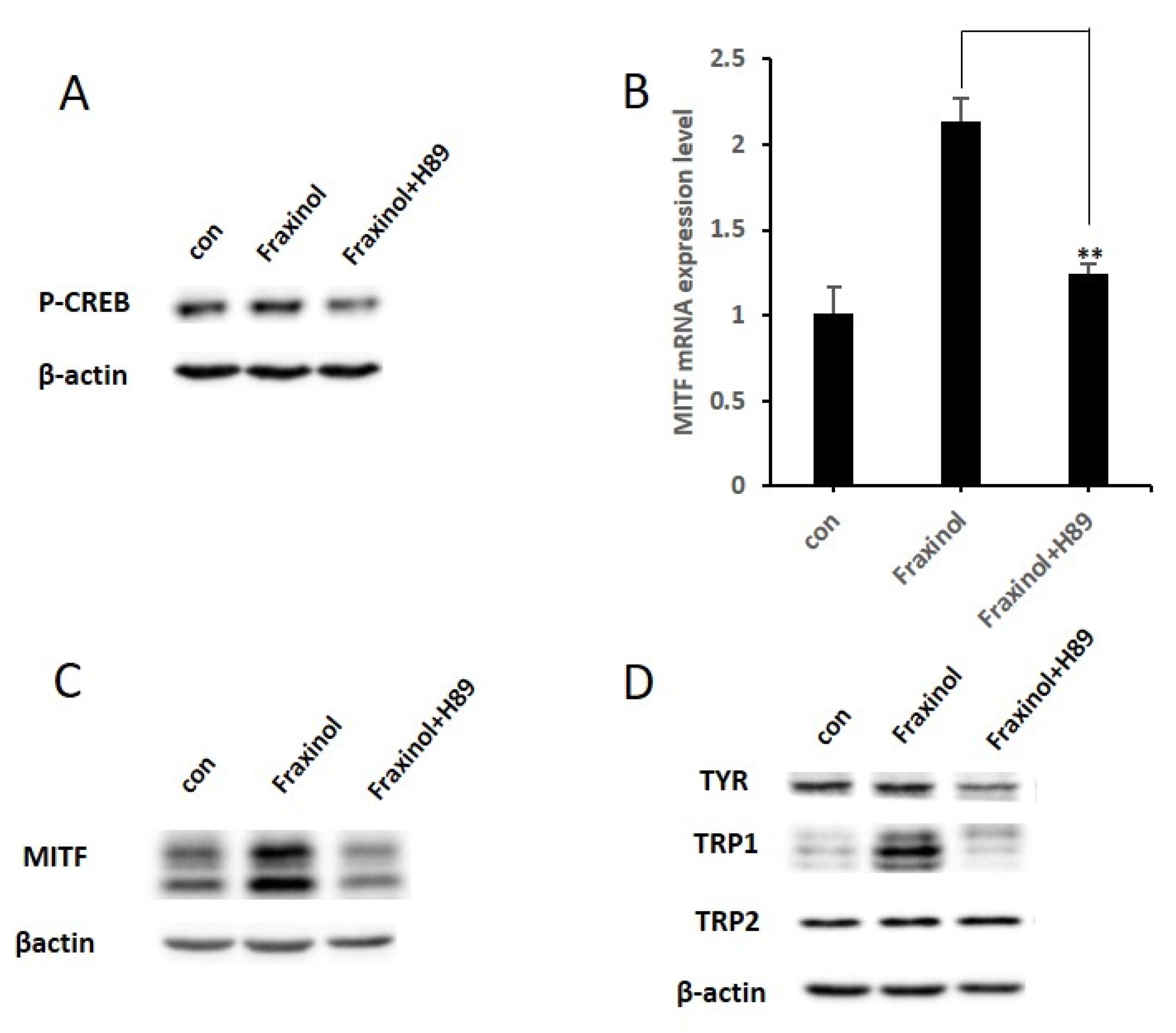
2.4. Fraxinol Upregulates the Phosphorylation of CREB and Expression Level of MITF
2.5. PKA Inhibitor Attenuates CREB Phosphorylation and MITF Expression Induced by Fraxinol
2.6. PKA Inhibitor Reduces Melanin Production and the Increased Tyrosinase Activity by Fraxinol Treatment
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Measurement of Melanin Content
4.5. Intracellular Tyrosinase Activity and Cell-Free Mushroom Tyrosinase Activity
4.6. L-DOPA Staining
4.7. RNA Isolation and qRT-PCR Analysis
4.8. Western Blot Assay
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ohbayashi, N.; Fukuda, M. Recent advances in understanding the molecular basis of melanogenesis in melanocytes. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Kondo, T.; Hearing, V.J. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev. Dermatol. 2011, 6, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, M.W.; Maddukuri, S.; Karanfilian, K.M.; Elias, M.L.; Lambert, W.C. The physiology of melanin deposition in health and disease. Clin. Dermatol. 2019, 37, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef]
- Speeckaert, R.; van Geel, N. Vitiligo: An update on pathophysiology and treatment options. Am. J. Clin. Dermatol. 2017, 18, 733–744. [Google Scholar] [CrossRef]
- Boo, Y.C. Up- or downregulation of melanin synthesis using amino acids, peptides, and their analogs. Biomedicines 2020, 8, 322. [Google Scholar] [CrossRef]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Bae-Harboe, Y.S.; Park, H.Y. Tyrosinase: A central regulatory protein for cutaneous pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jimenez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; Garcia-Borron, J.C.; Hearing, V.J. Tyrosinase related protein 1 (trp1) functions as a dhica oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, trp-2, is a melanogenic enzyme termed dopachrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of melanogenesis cascade for identifying pathophysiology and therapeutic approach of pigmentary disorders and melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.Y.; Noh, M. The regulation of epidermal melanogenesis via camp and/or pkc signaling pathways: Insights for the development of hypopigmenting agents. Arch. Pharmacal Res. 2013, 36, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J. The medical treatment of vitiligo: An historical review. Dermatol. Clin. 2017, 35, 107–116. [Google Scholar] [CrossRef]
- Gianfaldoni, S.; Wollina, U.; Tirant, M.; Tchernev, G.; Lotti, J.; Satolli, F.; Rovesti, M.; Franca, K.; Lotti, T. Herbal compounds for the treatment of vitiligo: A review. Open Access Maced. J. Med. Sci. 2018, 6, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, J.; Lu, J.; Ding, Y.; Jiang, L.; Hu, S.; Chen, J.; Zeng, Q. The role and mechanism of asian medicinal plants in treating skin pigmentary disorders. J. Ethnopharmacol. 2019, 245, 112173. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Pang, G.X.; Li, G.; Dou, J.; Nie, L.F.; Himit, H.; Kabas, M.; Aisa, H.A. Synthesis and biological evaluation of furocoumarin derivatives on melanin synthesis in murine b16 cells for the treatment of vitiligo. Bioorg. Med. Chem. 2016, 24, 5960–5968. [Google Scholar] [CrossRef]
- Pang, G.X.; Niu, C.; Mamat, N.; Aisa, H.A. Synthesis and in vitro biological evaluation of novel coumarin derivatives containing isoxazole moieties on melanin synthesis in b16 cells and inhibition on bacteria. Bioorg. Med. Chem. Lett. 2017, 27, 2674–2677. [Google Scholar] [CrossRef]
- Vandal, J.; Abou-Zaid, M.M.; Ferroni, G.; Leduc, L.G. Antimicrobial activity of natural products from the flora of northern ontario, canada. Pharm. Biol. 2015, 53, 800–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfraz, I.; Rasul, A.; Jabeen, F.; Younis, T.; Zahoor, M.K.; Arshad, M.; Ali, M. Fraxinus: A plant with versatile pharmacological and biological activities. Evid. Based Complement. Altern. Med. 2017, 2017, 4269868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostova, I.; Iossifova, T. Chemical components of fraxinus species. Fitoterapia 2007, 78, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Akter, K.M.; Kim, H.J.; Park, W.S.; Khalil AA, K.; Ahn, M.J. Anti-helicobacter pylori activity of compounds isolated from fraxinus mandshurica bark. Nat. Prod. Sci. 2020, 26, 158–164. [Google Scholar]
- Akter, K.M.; Park, W.S.; Kim, H.J.; Khalil, A.A.K.; Ahn, M.J. Comparative studies of fraxinus species from korea using microscopic characterization, phytochemical analysis, and anti-lipase enzyme activity. Plants 2020, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xue, G.; Liu, F.; Gong, X. Immunosuppressive effect of extracts from leaves of fraxinus mandshurica rupr. Bioengineered 2017, 8, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and function of human tyrosinase and tyrosinase-related proteins. Chemistry 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Shibahara, S.; Takeda, K.; Yasumoto, K.; Udono, T.; Watanabe, K.; Saito, H.; Takahashi, K. Microphthalmia-associated transcription factor (mitf): Multiplicity in structure, function, and regulation. J. Investig. Dermatol. Symp. Proc. 2001, 6, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrzesniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against uv damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Tien Guan Thng, S.; Kumar Verma, N.; Gautam, H.K. Melanogenesis inhibitors. Acta Derm.-Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, S.S.; Lombardo, D.A.; Abou-Zaid, M.M. A metabolomic and hplc-ms/ms analysis of the foliar phenolics, flavonoids and coumarins of the fraxinus species resistant and susceptible to emerald ash borer. Molecules 2018, 23, 2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, C.I.; Setaluri, V. Cyclic amp (camp) signaling in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Solca, F.F.; Salomon, Y.; Eberle, A.N. Heterogeneity of the msh receptor among b16 murine melanoma subclones. J. Recept. Res. 1991, 11, 379–390. [Google Scholar] [CrossRef]
- Siegrist, W.; Eberle, A.N. In situ melanin assay for msh using mouse b16 melanoma cells in culture. Anal. Biochem. 1986, 159, 191–197. [Google Scholar] [CrossRef]
- Chung, S.; Lim, G.J.; Lee, J.Y. Quantitative analysis of melanin content in a three-dimensional melanoma cell culture. Sci. Rep. 2019, 9, 780. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, B.X.; Zhang, C.; Shen, N.; Zhang, Y.Y.; Wang, A.X.; Tu, C.X. Ginsenosides rb1 and rg1 stimulate melanogenesis in human epidermal melanocytes via pka/creb/mitf signaling. Evid. Based Complement. Altern. Med. 2014, 2014, 892073. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Park, E.; Kim, J.; Hong, S.; Park, J.; Park, S.; Lee, S.; et al. Glycyrrhizin induces melanogenesis by elevating a camp level in b16 melanoma cells. J. Investig. Dermatol. 2005, 124, 405–411. [Google Scholar] [CrossRef] [Green Version]
- del Marmol, V.; Ito, S.; Jackson, I.; Vachtenheim, J.; Berr, P.; Ghanem, G.; Morandini, R.; Wakamatsu, K.; Huez, G. Trp-1 expression correlates with eumelanogenesis in human pigment cells in culture. FEBS Lett. 1993, 327, 307–310. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, P.; Hu, Y.; He, L. Regulation of melanocyte pivotal transcription factor mitf by some other transcription factors. Mol. Cell. Biochem. 2011, 354, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, V.; Atkar, R.; Batchelor, J.; McDonald, B.; Novakovic, L.; Patel, J.V.; Ravenscroft, J.; Rush, E.; Shah, D.; Shah, R.; et al. British association of dermatologists guidelines for the management of people with vitiligo 2021. Br. J. Dermatol. 2021, 186, 18–29. [Google Scholar] [CrossRef]


| Target | Sequence (5′ to 3′) | Accession No. | |
|---|---|---|---|
| MITF | Forward | GAAACCTTGCTATGCTGGAAATG | BC108976.2 |
| Reverse | GAGCTTGCTGTATGTGGTACTT | ||
| TYR | Forward | CCTCATGGACAAAGACGACTAC | BC079678.1 |
| Reverse | CCTTTCAGTCCCACTCTGTTT | ||
| TRP1 | Forward | GCTCCAGACAATCTGGGATATG | BC076598.1 |
| Reverse | AGTAACAACGCAGCCACTAC | ||
| TRP2 | Forward | CCTGTCTCTCCAGAAGTTTGAC | BC082330.1 |
| Reverse | CCAGTGTTCCGTCTGCTTTA | ||
| β-actin | Forward | GAGGTATCCTGACCCTGAAGTA | NM_007393 |
| Reverse | CACACGCAGCTCATTGTAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.Y.; Akter, K.-M.; Ahn, M.-J.; Kim, K.D.; Yoo, J.; Lee, J.-H.; Lee, J.-H.; Hwangbo, C. Fraxinol Stimulates Melanogenesis in B16F10 Mouse Melanoma Cells through CREB/MITF Signaling. Molecules 2022, 27, 1549. https://doi.org/10.3390/molecules27051549
Moon SY, Akter K-M, Ahn M-J, Kim KD, Yoo J, Lee J-H, Lee J-H, Hwangbo C. Fraxinol Stimulates Melanogenesis in B16F10 Mouse Melanoma Cells through CREB/MITF Signaling. Molecules. 2022; 27(5):1549. https://doi.org/10.3390/molecules27051549
Chicago/Turabian StyleMoon, Sun Young, Kazi-Marjahan Akter, Mi-Jeong Ahn, Kwang Dong Kim, Jiyun Yoo, Joon-Hee Lee, Jeong-Hyung Lee, and Cheol Hwangbo. 2022. "Fraxinol Stimulates Melanogenesis in B16F10 Mouse Melanoma Cells through CREB/MITF Signaling" Molecules 27, no. 5: 1549. https://doi.org/10.3390/molecules27051549






