Nanoinhibitory Impacts of Salicylic Acid, Glycyrrhizic Acid Ammonium Salt, and Boric Acid Nanoparticles against Phytoplasma Associated with Faba Bean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis of Nanomaterials
2.2. Characterization of Nanomaterials by Using Dynamic Light Scattering (DLS)
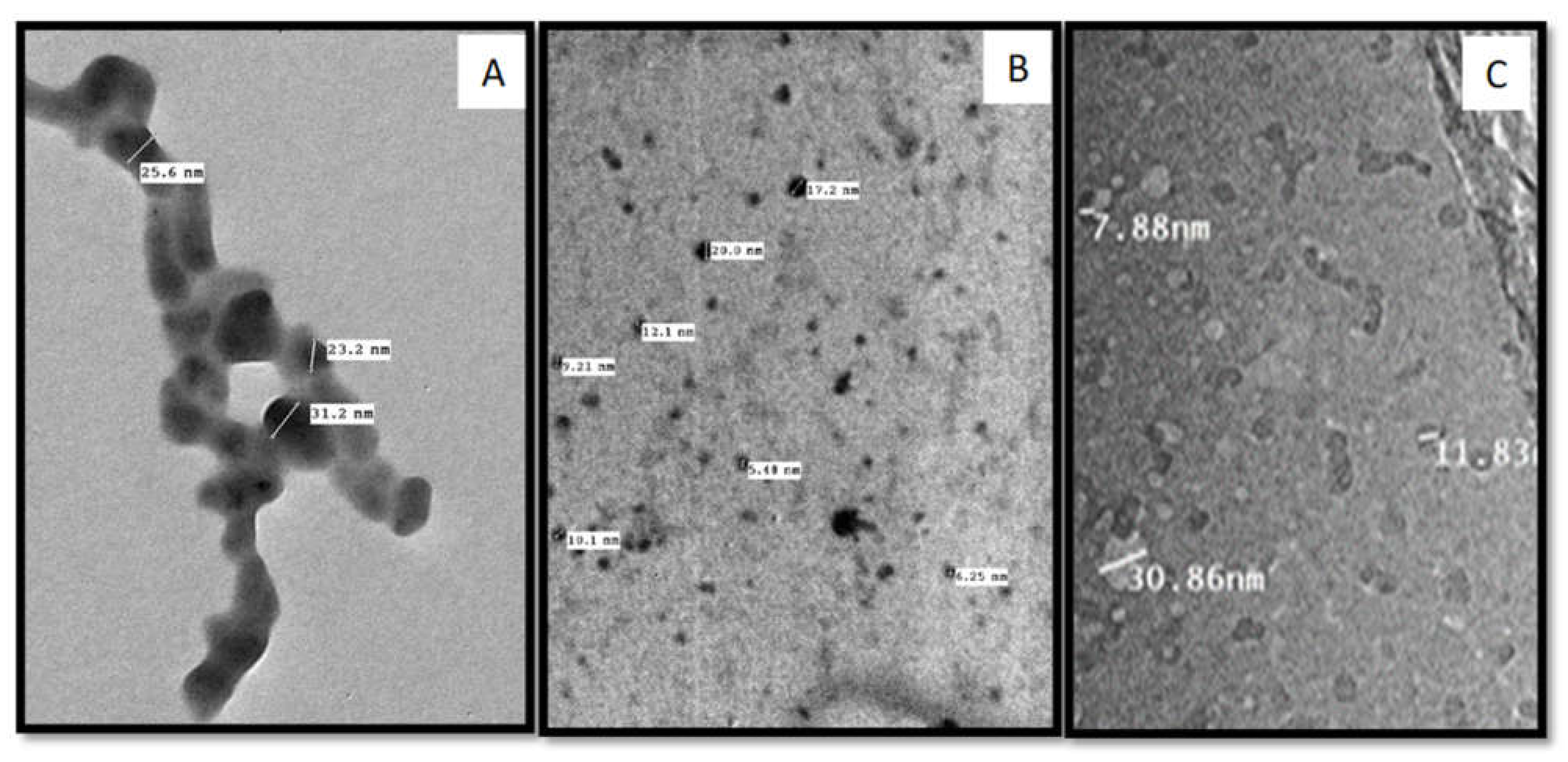
2.3. Transmission Electron Microscopy (TEM)
2.4. Experimental Design and Plant Material
2.5. Treatments Application
2.6. Nucleic Acid Extraction
2.7. Nested PCR for Phytoplasma Detection
2.8. Incidence and Severity of the Phytoplasma Disease
2.9. Photosynthetic Efficiency, Growth, and Green Pods Yield
2.10. Statistical Analysis
3. Results
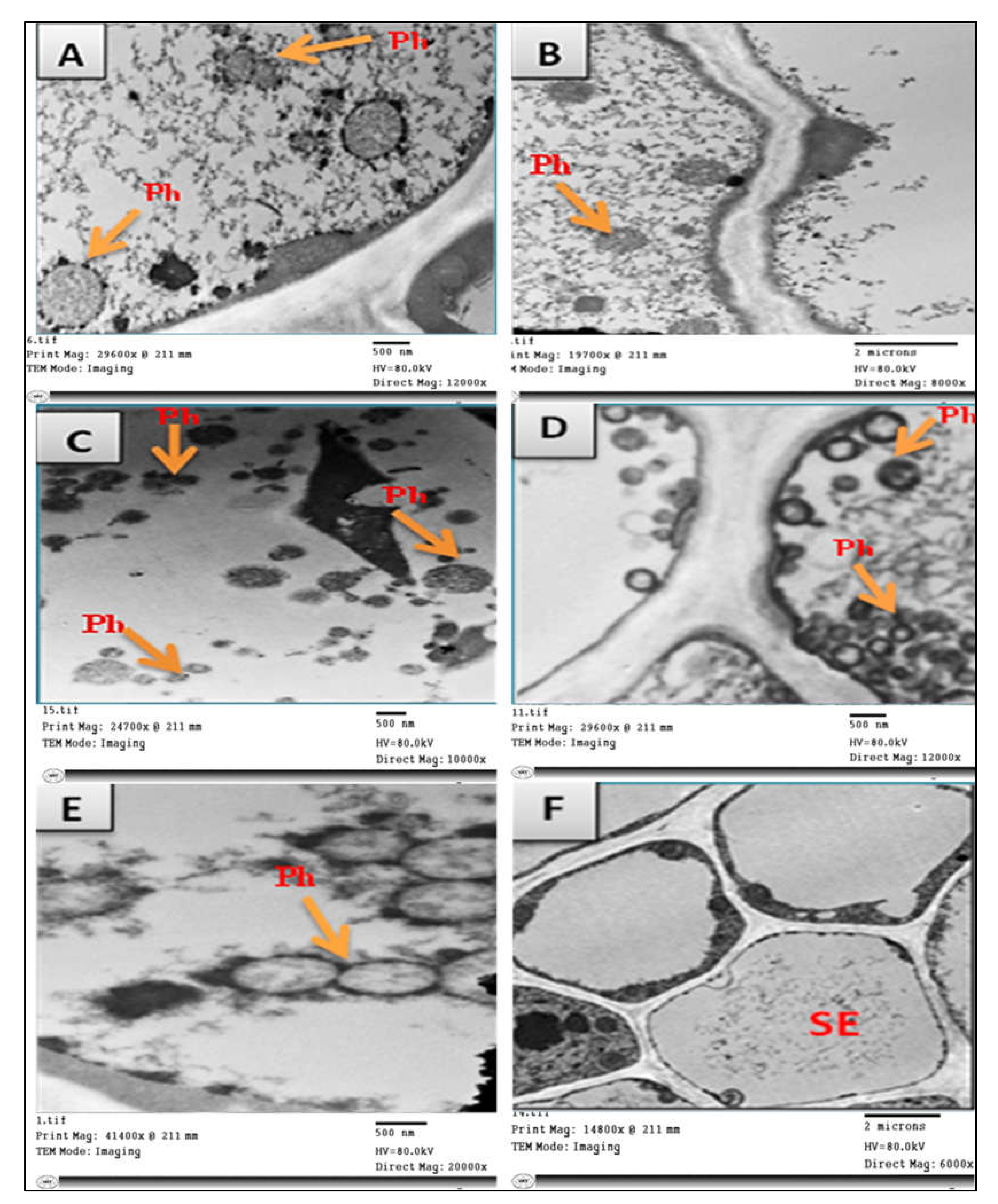
3.1. Symptomatology and Nested PCR for Phytoplasma Detection
3.2. Characterization of Nanomaterials
3.3. Transmission Electron Microscopy (TEM)
3.4. Effect of SA-NP, GAS-NP, and BA-NP Treatments on Phytoplasma-Infected Faba Bean Plants
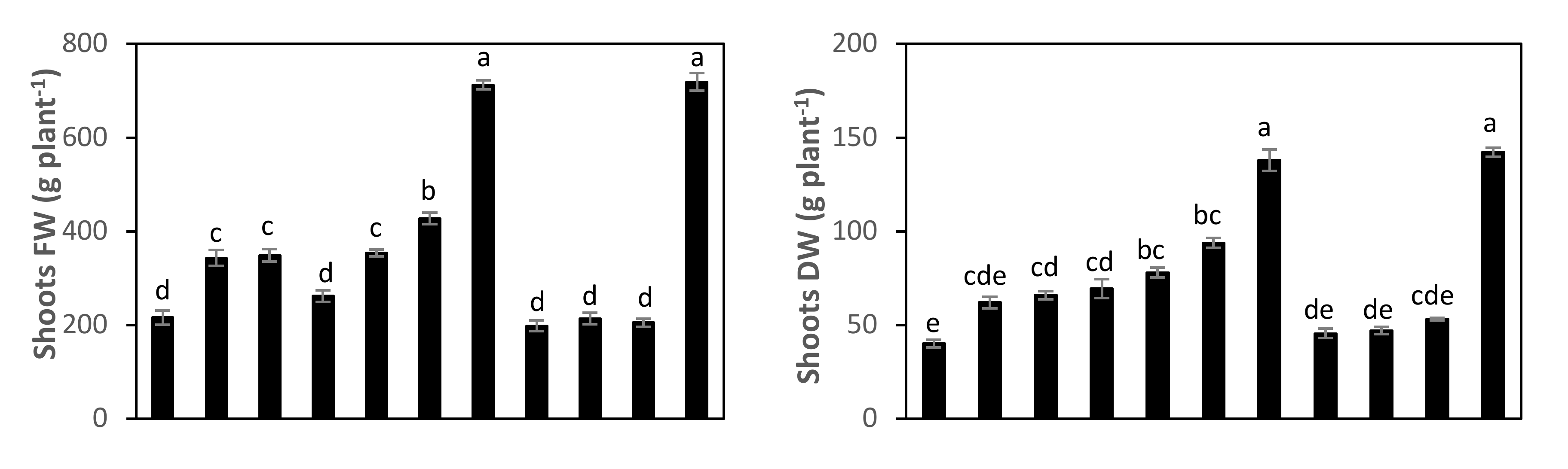
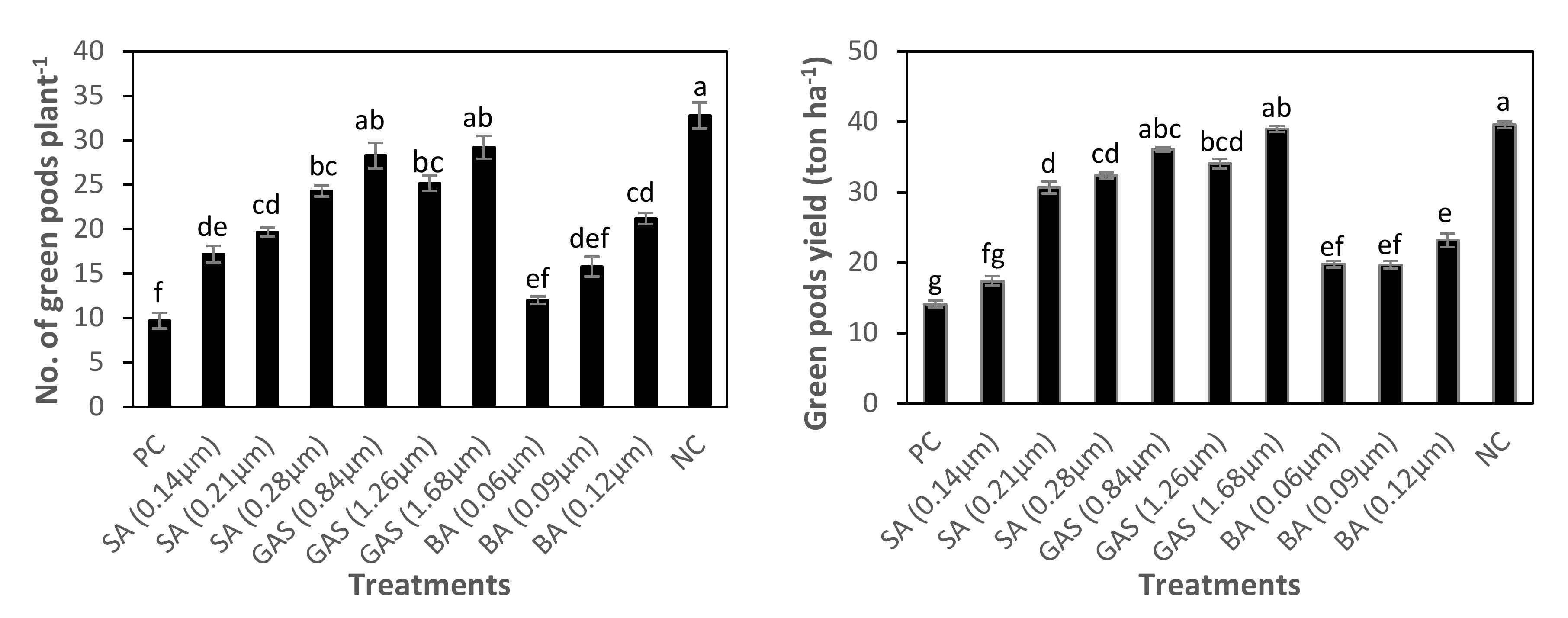
3.5. Effect of SA-NP, GAS-NP, and BA-NP Treatments on Photosynthetic Efficiency, Growth, and Green Pods Yield of Phytoplasma-Infected Faba Bean Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Crepon, K.; Marget, P.; Peyronnet, C.; Carrouee, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop. Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous Gibberellic Acid or Dilute Bee Honey Boosts Drought Stress Tolerance in Vicia faba by Rebalancing Osmoprotectants, Antioxidants, Nutrients, and Phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, H.; Vandenberg, A. Seed Mineral Composition and Protein Content of Faba Beans (Vicia faba L.) with Contrasting Tannin Contents. Agronomy 2020, 10, 511. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Nagendran, K.; Rai, A.B.; Singh, B.; Rao, G.P.; Bertaccini, A. Global status of phytoplasma diseases in vegetable crops. Front. Microbiol. 2019, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Hogenhout, S.A.; Oshima, K.; Ammar, E.-D.; Kakizawa, S.; Kingdom, H.N.; Namba, S. Phytoplasmas: Bacteria that manipulate plants and insects. Mol. Plant Pathol. 2008, 9, 403–423. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhatt, B.P. Occurrence of phytoplasma phyllody and witches’ broom disease of faba bean in Bihar. J. Environ. Biol. 2013, 34, 837–840. [Google Scholar]
- Al-Saleh, M.A.; Amer, M.A. Molecular characterization of the 16Sr II group of phytoplasma associated with faba bean (Vicia Faba L.) in Saudi Arabia. J. Anim. Plant Sci. 2014, 24, 221–228. [Google Scholar]
- Hamed, A.H.; El Attar, A.K.; El-Banna Om-Hashim, M. First record of a phytoplasma associated with Faba Bean (Vicia faba L.) Witches’-broom in Egypt. Int. J. Virol. 2014, 10, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Kumari, B.; Mallick, M.A.; Solanki, M.K.; Solanki, A.C.; Hora, A.; Guo, W. Plant Growth Promoting Rhizobacteria (PGPR): Modern Prospects for Sustainable Agriculture. In Plant Health under Biotic Stress; Ansari, R., Mahmood, I., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Torres Suarez, G.; Gamarra Gamarra, D.; Villar, C.M.; Llacza Munive, S.L.; Satta, E.; Carrasco Lozano, E.C.; Bertaccini, A. Detection and identification of a 16SrIII-J subgroup phytoplasma associated with faba bean in Peru. J. Phytopathol. 2021, 169, 203–208. [Google Scholar] [CrossRef]
- Bertaccini, A. Phytoplasmas: Diversity, taxonomy, and epidemiology. Front. Biosci. 2007, 12, 673–689. [Google Scholar] [CrossRef] [Green Version]
- Zamharir, G.M.; Taheri, M. Effect of new resistance inducers on grapevine phytoplasma disease. Arch. Phytopathol. Plant Prot. 2019, 52, 1207–1214. [Google Scholar] [CrossRef]
- Raskin, I.; Skubatz, H.; Tang, W.; Meeuse, B.J.D. Salicylic acid levels in thermogenic and non-thermogenic plants. Ann. Bot. 1990, 66, 369–373. [Google Scholar] [CrossRef]
- Malamy, J.; Hennig, J.; Klessig, D.F. Temperature-dependent induction of SA and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell 1992, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Pompei, R.; Flore, O.; Marccialis, M.A.; Pani, A.; Loddo, B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Oyama, K.; Kawada-Matsuo, M.; Oogai, Y.; Hayashi, T.; Nakamura, N.; Komatsuzawa, H. Antibacterial effects of glycyrrhetinic acid and its derivatives on Staphylococcus aureus. PLoS ONE 2016, 11, e0165831. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J. Tratado de Fitofármacos y Nutracéuticos; Corpus: Barcelona, Spain, 2004; pp. 905–911. [Google Scholar]
- Fu, Y.; Chen, J.; Li, Y.; Zheng, Y.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, J.; Kim, K.; Jeong, J. The Anti-Angiogenic Activities of Glycyrrhizic Acid in Tumor Progression. Phytother. Res. 2013, 27, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Amirghofran, Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran. J. Immunol. 2010, 7, 65. [Google Scholar] [PubMed]
- Krausse, R.; Bielenberg, J.; Blaschek, W.; Ullmann, U. In vitro anti-Helicobacter pylori activity of extractum liquiritiae, glycyrrhizin and its metabolites. J. Antimicrob. Chemother. 2004, 54, 243–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Seta, F.; Schmidt, M.; Vu, B.; Essmann, M.; Larsen, B. Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J. Antimicrob. Chemother. 2009, 63, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, P.L.; Robert, I.K.; William, C.K. Boric Acid and Inorganic Borate Pesticides. In Handbook of Pesticide Toxicology, 2nd ed.; Strong, P.L., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 1429–1437. ISBN 978-0-12-426260-7. [Google Scholar]
- See, A.; Salleh, A.S.; Abu Bakar, F.; Yusof, N.A.; Abdulamir, A.S.; Yook Heng, L. Risk and Health Effect of Boric Acid. Am. J. Appl. Sci. 2010, 7, 620–627. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against Bean Yellow Mosaic Virus and human pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef] [Green Version]
- Cordero, T.; Mohamed, M.A.; López-Moya, J.J.; Daròs, J.A. A recombinant potato virus y infectious clone tagged with the rosea1 visual marker (pvy-ros1) facilitates the analysis of viral infectivity and allows the production of large amounts of anthocyanins in plants. Front. Microbiol. 2017, 8, 611. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; López-Camarillo, C.; Pozos-Guillen, A.; Leyva-Porras, C.; Silva-Cázares, M.B. Nanomaterial Complexes Enriched With Natural Compounds Used in Cancer Therapies: A Perspective for Clinical Application. Front. Oncol. 2021, 11, 1032. [Google Scholar] [CrossRef]
- Doi, Y.; Teranaka, M.; Yora, K.; Asuyama, H. Mycoplasma or PLT grouplike microrganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’ broom, aster yellows or Pawlownia Witches’ Broom. Jpn. J. Phytopathol. 1967, 33, 259–266. [Google Scholar] [CrossRef]
- Sertkaya, G.; Martini, M.; Ermacora, P.; Musetti, R.; Osler, R. Detection and characterization of phytoplasmas in diseased stone fruits and pear by PCR-RFLP analysis in Turkey. Phytoparasitica 2005, 33, 380–390. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Shalaby, O.Y.; Dwidar, E.F.; Mokbel, S.A.; El-Attar, A.K. Ultrastructural changes in tomato plant induced by phytoplasma infection and attempts for its elimination using tissue culture techniques. Egypt. J. Virol. 2016, 13, 34–51. [Google Scholar]
- Gad, S.M.; Kheder, A.A.; Awad, M.A. Detection and molecular identification of phytoplasma associated with Gazania in Egypt. J. Virol. Sci. 2019, 6, 12–23. [Google Scholar]
- Pollini, P.C.; Bissanin, R.; Giunchedi, L. Occurrence of European Stone Fruit Yellows Phytoplasma (ESFYP) infection in peach orchards in northern-central Italy. J. Phytopathol. 2001, 149, 725–730. [Google Scholar] [CrossRef]
- Abou-Jawdah, Y.; Karakashian, A.; Sobh, H.; Martini, M.; Lee, L.M. An epidemic of almond witches’-broom in Lebanon: Classification and phylogenetic relationships of the associated phytoplasma. Plant Dis. 2002, 86, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Harrison, N.A. Detection and characterization of a lethal yellowing (16SrIV) group phytoplasma in Canary Island date palms affected by lethal decline in Texas. Plant Dis. 2002, 86, 676–681. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.A.; Shalaby, O.Y.; Dwidar, E.F.; Mokbel, S.A.; El-Attar, A.K. Occurrence, Etiology and Molecular Characterization of Phytoplasma Diseases on Solanum lycopersicum Crop in Egypt. Egypt. J. Virol. 2014, 11, 244–261. [Google Scholar]
- Amin, B.H.; El-Sharkawy, R.M. Bactericidal Activity of Silver Nanoparticles Produced by Fusarium solani against the Multidrug-Resistant Bacteria. Res. J. Pharma. Biol. Chem. Sci. 2019, 10, 203–211. [Google Scholar]
- EPPO. PM 7/133 (1) Generic detection of phytoplasmas. EPPO Bull. 2018, 48, 414–424. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, N.G. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. Nanoantibiotics’: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Shoala, T.; Al-Karmalawy, A.A.; Germoush, M.O.; ALshamrani, S.M.; Abdein, M.A.; Awad, N.S. Nanobiotechnological Approaches to Enhance Potato Resistance against Potato Leafroll Virus (PLRV) Using Glycyrrhizic Acid Ammonium Salt and Salicylic Acid Nanoparticles. Horticulturae 2021, 7, 402. [Google Scholar] [CrossRef]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H.; et al. Oxi1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Shoala, T. Positive Impacts of Nanoparticles in Plant Resistance against Different Stimuli. In Nanobiotechnology Applications in Plant Protection, 1st ed.; Abd-Elsalam, K.A., Prasad, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 267–279. [Google Scholar]






| No. of Treatments | Treatments | Concentration (µM) |
|---|---|---|
| 1 | PC | Un treated |
| 2 | PIP + SA-NP | 0.14 |
| 3 | PIP + SA-NP | 0.21 |
| 4 | PIP + SA-NP | 0.28 |
| 5 | PIP + GAS-NP | 0.84 |
| 6 | PIP + GAS-NP | 1.26 |
| 7 | PIP + GAS-NP | 1.68 |
| 8 | PIP + BA-NP | 0.06 |
| 9 | PIP + BA-NP | 0.09 |
| 10 | PIP + BA-NP | 0.124 |
| 11 | NC | Untreated |
| Treatments | Concentrations | Disease Incidence % | Disease Severity % |
|---|---|---|---|
| PC | --------- | 79.6 ± 1.5 a | 76.2 ± 1.5 a |
| PIP + SA-NP1 | 0.14 µM | 52.0 ± 2.2 c | 40.0 ± 1.0 d |
| PIP + SA-NP1.5 | 0.21 µM | 49.6 ± 0.9 c | 30.0 ± 1.0 f |
| PIP + SA-NP2 | 0.28 µM | 40.2 ± 1.1 d | 27.6 ± 1.2 f |
| PIP + GAS-NP1 | 0.84 µM | 39.8 ± 1.4 d | 18.2 ± 0.9 g |
| PIP + GAS-NP1.5 | 1.26 µM | 36.8 ± 2.0 d | 14.4 ± 0.5 h |
| PIP + GAS-NP2 | 1.68 µM | 20.0 ± 1.1 e | 12.0 ± 0.7 h |
| PIP + BA-NP1 | 0.06 µM | 64.8 ± 1.8 b | 66.0 ± 1.3 b |
| PIP + BA-NP1.5 | 0.09 µM | 63.8 ± 2.4 b | 57.4 ± 1.0 c |
| PIP + BA-NP2 | 0.124 µM | 63.8 ± 1.5 b | 35.0 ± 1.3 e |
| NC | -------- | 0.0 ± 00 f | 0.0 ± 0.0 i |
| Treatments | Fv/Fm | PI | SPAD Chlorophyll | Shoots Height (cm) | No. of Leaves Plant−1 | No. of Branches Plant−1 |
|---|---|---|---|---|---|---|
| PC | 0.74 ± 0.01 f | 3.17 ± 0.38 d | 39.2 ± 1.14 f | 57.3 ± 2.03 f | 66.2 ± 1.38 e | 13.7 ± 1.76 a |
| PIP+SA-NP1 | 0.81 ± 0.01 cde | 4.41 ± 0.26 bc | 47.8 ± 0.95 cde | 87.3 ± 1.31 e | 73.2 ± 3.05 de | 6.8 ± 0.48 bc |
| PIP+SA-NP1.5 | 0.82 ± 0.00 abc | 4.71 ± 0.17 bc | 48.2 ± 1.27 cde | 90.7 ± 1.75 de | 78.2 ± 2.80 cd | 6.0 ± 0.45 bc |
| PIP+SA-NP2 | 0.80 ± 0.01 cde | 4.99 ± 0.39 abc | 50.7 ± 0.62 bcd | 99.7 ± 6.14 cde | 90.3 ± 2.91 b | 6.3 ± 0.61 bc |
| PIP+GAS-NP1 | 0.81 ± 0.01 bcd | 5.00 ± 0.16 abc | 51.6 ± 1.18 abc | 102.8 ± 7.07 cd | 88.0 ± 1.57 bc | 6.7 ± 0.42 bc |
| PIP+GAS-NP1.5 | 0.82 ± 0.01 abc | 5.33 ± 0.40 ab | 52.3 ± 0.78 ab | 109.3 ± 6.57 bc | 95.0 ± 2.58 b | 6.0 ± 0.26 bc |
| PIP+GAS-NP2 | 0.83 ± 0.01 ab | 5.26 ± 0.36 ab | 54.6 ± 0.85 a | 120.5 ± 6.98 b | 130.8 ± 3.88 a | 6.5 ± 0.67 bc |
| PIP+BA-NP1 | 0.79 ± 0.01 e | 3.88 ± 0.56 cd | 46.4 ± 1.14 e | 86.3 ± 3.52 e | 72.7 ± 3.04 de | 6.3 ± 0.49 bc |
| PIP+BA-NP1.5 | 0.79 ± 0.01 de | 4.53 ± 0.20 bc | 47.8 ± 1.49 de | 91.8 ± 4.00 de | 75.5 ± 3.85 de | 6.8 ± 0.31 bc |
| PIP+BA-NP2 | 0.81 ± 0.01 bcd | 4.07 ± 0.21 cd | 48.8 ± 0.91 bcde | 88.0 ± 2.38 e | 74.5 ± 2.14 de | 4.8 ± 0.54 c |
| NC | 0.84 ± 0.01 a | 5.96 ± 0.14 a | 54.8 ± 0.95 a | 140.7 ± 3.48 a | 134.2 ± 2.36 a | 5.7 ± 0.33 c |
| Treatments | Shoots Fresh Weight (g Plant−1) | Shoots Dry Weight (g Plant−1) | No. of Green Pods Plant−1 | Green Pods Yield (ton ha−1) |
|---|---|---|---|---|
| PC | 216.1 ± 29.8 d | 40.1 ± 3.98 e | 9.7 ± 1.75 f | 14.1 ± 1.00 g |
| PIP+SA-NP1 | 343.5 ± 33.4 c | 62.0 ± 6.19 cde | 17.2 ± 1.82 de | 17.4 ± 1.35 fg |
| PIP+SA-NP1.5 | 348.6 ± 26.6 c | 66.0 ± 4.34 cd | 19.7 ± 0.96 cd | 30.7 ± 1.71 d |
| PIP+SA-NP2 | 262.0 ± 24.5 d | 69.5 ± 10.18 cd | 24.3 ± 1.20 bc | 32.4 ± 0.91 cd |
| PIP+GAS-NP1 | 354.2 ± 14.4 c | 78.1 ± 5.40 bc | 28.3 ± 2.87 ab | 36.1 ± 0.61 abc |
| PIP+GAS-NP1.5 | 427.8 ± 25.1 b | 93.8 ± 5.25 b | 25.2 ± 1.78 bc | 34.1 ± 1.36 bcd |
| PIP+GAS-NP2 | 712.7 ± 20.0 a | 138.0 ± 11.53 a | 29.2 ± 2.56 ab | 39.0 ± 0.91 ab |
| PIP+BA-NP1 | 198.4 ± 22.5 d | 45.6 ± 4.99 de | 12.0 ± 0.82 ef | 19.8 ± 0.89 ef |
| PIP+BA-NP1.5 | 214.3 ± 24.9 d | 47.1 ± 3.98 de | 15.8 ± 2.21 def | 19.7 ± 1.13 ef |
| PIP+BA-NP2 | 205.2 ± 17.7 d | 53.2 ± 1.33 cde | 21.2 ± 1.30 cd | 23.2 ± 1.93 e |
| NC | 719.2 ± 36.7 a | 142.2 ± 4.90 a | 32.8 ± 2.94 a | 39.6 ± 0.93 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, E.A.; Shoala, T.; Abdelkhalik, A.; El-Garhy, H.A.S.; Ismail, I.A.; Farrag, A.A. Nanoinhibitory Impacts of Salicylic Acid, Glycyrrhizic Acid Ammonium Salt, and Boric Acid Nanoparticles against Phytoplasma Associated with Faba Bean. Molecules 2022, 27, 1467. https://doi.org/10.3390/molecules27051467
Ahmed EA, Shoala T, Abdelkhalik A, El-Garhy HAS, Ismail IA, Farrag AA. Nanoinhibitory Impacts of Salicylic Acid, Glycyrrhizic Acid Ammonium Salt, and Boric Acid Nanoparticles against Phytoplasma Associated with Faba Bean. Molecules. 2022; 27(5):1467. https://doi.org/10.3390/molecules27051467
Chicago/Turabian StyleAhmed, Eman A., Tahsin Shoala, Abdelsattar Abdelkhalik, Hoda A. S. El-Garhy, Ismail A. Ismail, and Amro A. Farrag. 2022. "Nanoinhibitory Impacts of Salicylic Acid, Glycyrrhizic Acid Ammonium Salt, and Boric Acid Nanoparticles against Phytoplasma Associated with Faba Bean" Molecules 27, no. 5: 1467. https://doi.org/10.3390/molecules27051467









