Effect of Wort Boiling on Volatiles Formation and Sensory Properties of Mead
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Aroma-Active Compounds in Mead
| No. 1 | Compound 2 | Odor Quality 3 | RIs 4 | FD Factors 5 | ||
|---|---|---|---|---|---|---|
| DB-FFAP | DB-5 | T | TS | |||
| 1 | ethyl 3-methylbutanoate | fruity, blueberry-like | 1013 | 775 | 1024 | 512 |
| 2 | 2-methyl-1-propanol | malty | 1101 | 640 | 64 | 64 |
| 3 | 3-methylbutyl acetate | banana-like, fruity | 1170 | 878 | 32 | 32 |
| 4 | 1,8-cineol | eucalyptus-like | 1193 | 1036 | 32 | 128 |
| 5 | ethyl hexanoate | fruity, pineapple-like | 1207 | 739 | 512 | 2048 |
| 6 | octanal | citrus-like, green | 1280 | 1003 | 32 | nd 6 |
| 7 | ethyl octanoate | fruity, green | 1425 | 1200 | 512 | 512 |
| 8 | acetic acid | vinegar-like | 1443 | 612 | nd 6 | 32 |
| 9 | methional | cooked potato-like | 1448 | 905 | 64 | 64 |
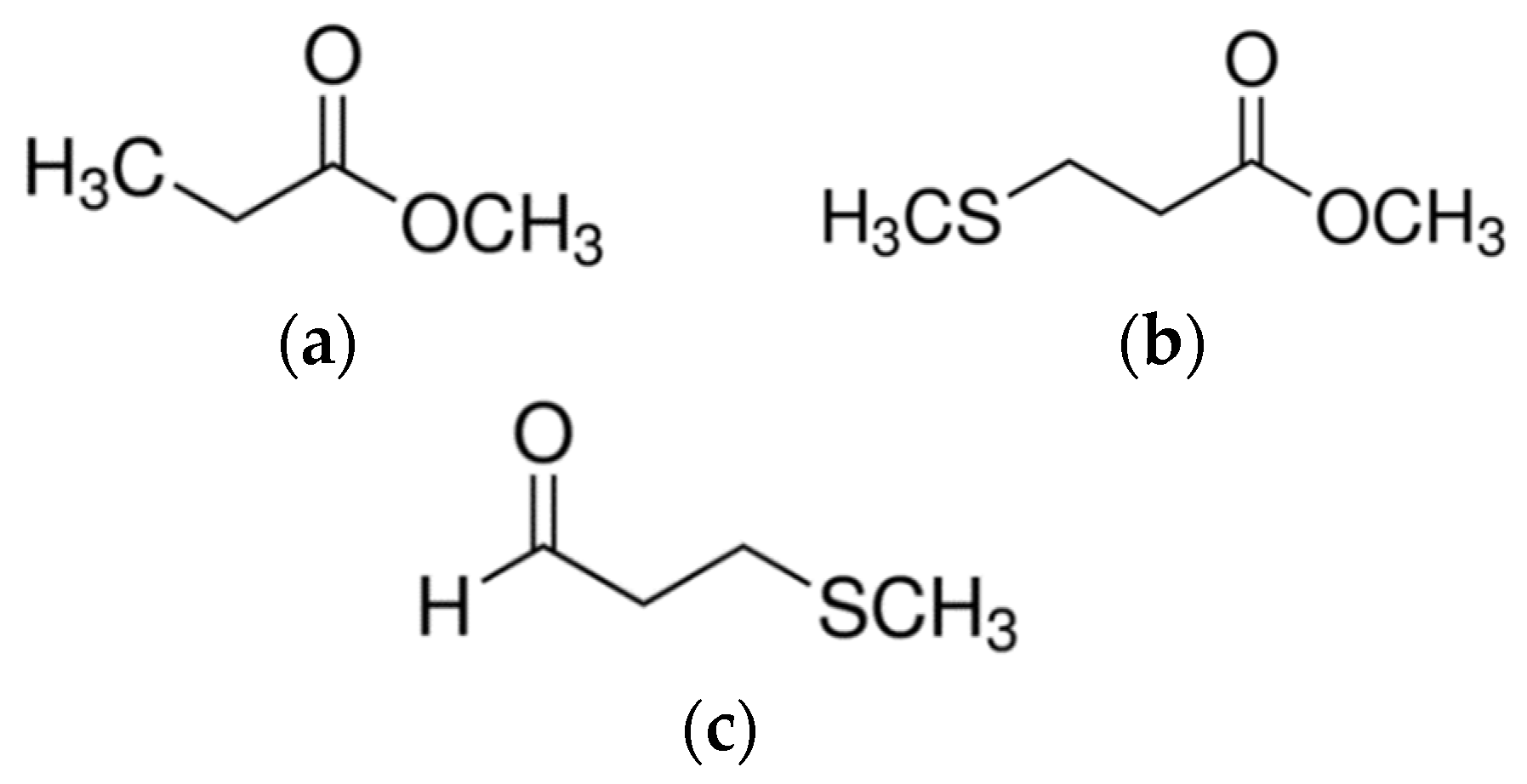
| 10 | methyl 3-(methylthio)propanoate | cabbage-like, earthy | 1517 | 1034 | <32 | 32 |
| 11 | methyl propanoate | cheese-like, sweaty 7 | 1558 | 789 | 32 | nd 6 |
| 12 | 2-acetylpyrazine | popcorn-like, roasty | 1609 | 1024 | nd 6 | 64 |
| 13 | diethyl succinate | etherical 7 | 1665 | 996 | 32 | 256 |
| 14 | 3-methylnonane-2,4-dione | aniseed-like, hay-like, fishy | 1708 | 1251 | <32 | 128 |
| 15 | pentyl acetate | fruity, honey-like 7 | 1814 | 1256 | 128 | nd 6 |
| 16 | hexanoic acid | sweaty | 1836 | 1018 | 32 | 32 |
| 17 | ethyl 3-phenylpropanoate | cinnamon-like, fruity | 1867 | 1418 | nd 6 | 32 |
| 18 | trans-whisky lactone | coconut-like | 1876 | 1303 | 128 | nd 6 |
| 19 | 2-phenylethanol | flowery, honey-like | 1905 | 1160 | 512 | 1024 |
| 20 | 4-hydroxy-2,5-dimethyl-3(2H)-furanone (furaneol®) | caramel-like, sweet | 2030 | 1071 | <32 | 64 |
| 21 | octanoic acid | carrot-like, musty | 2052 | 1279 | nd 6 | 32 |
| 22 | 4-allyl-2-methoxyphenol | clove-like | 2164 | 1359 | 1024 | 2048 |
| 23 | 3-hydroxy-4,5-dimethyl-2(5H)-furanone (sotolon) | seasoning-like, spicy | 2195 | 1108 | 256 | 32 |
| 24 | γ-decalactone | peach-like | 2369 | 1680 | 256 | 32 |
| 25 | dodecanoic acid | wax-like | 2455 | 2169 | 128 | nd 6 |
| 26 | coumarin | woodruff-like, almond paste-like 7 | 2461 | 1442 | <32 | 64 |
| 27 | phenylacetic acid | beeswax-like, honey-like | 2555 | 1261 | 512 | 1024 |
| 28 | vanillin | vanilla-like, sweet | 2569 | 1406 | <32 | 512 |
2.2. Quantitation of Odorants via HS-SPME-HRGC-MS Using SIDA and Calculation of Their OAVs
2.3. Aroma Profiles of Mead ‘Trójniak’ (T) and ‘Trójniak Sycony’ (TS)
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Mead Samples
3.3. Isolation of the Volatiles and Their Analysis by Gas Chromatography-Olfactometry/Flame Ionization Detection (GC-O/FID)
3.4. High-Resolution Gas Chromatography-Mass Spectrometry (HRGC-MS)
3.5. Determination of Mead Volatiles by Headspace-Solid Phase Microextraction-High-Resolution Gas Chromatography-Mass Spectrometry (HS-SPME-HRGC-MS)
3.6. Descriptive Sensory Analysis of Mead Samples—Aroma Profile Analysis (APA)
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McConnell, D.S.; Schramm, K.D. Mead success: Ingredients, processes and techniques. Zygmurgy 1995, 4, 33–39. [Google Scholar]
- Ministry of Agriculture and Rural Development. Dz.U.2013.633 (22 May 2013). Rozporządzenie w Sprawie Rodzajów Fermentowanych Napojów Winiarskich Oraz Szczegółowych Wymagań Organoleptycznych, Fizycznych i Chemicznych, Jakie powinny Spełniać te Napoje. Available online: https://www.prawo.pl/akty/dz-u-2013-633,17997396.html (accessed on 2 September 2021). (In Polish).
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Phenolic profile and antioxidant activity of polish meads. Int. J. Food Prop. 2015, 18, 2713–2725. [Google Scholar] [CrossRef]
- Pascoal, A.; Oliveira, J.M.; Pereira, A.P.; Féas, X.; Anjos, O.; Estevinho, L.M. Influence of fining agents on the sensorial characteristics and volatile composition of mead. J. Inst. Brew. 2017, 123, 562–571. [Google Scholar] [CrossRef]
- Gupta, J.K.; Sharma, R. Production technology and quality characteristics of mead and fruity-honey wines: A review. Nat. Prod. Rad. 2009, 8, 345–355. [Google Scholar]
- Mendes-Ferreira, A.; Cosme, F.; Barbosa, C.; Falco, V.; Inês, A.; Mendes-Faia, A. Optimization of honey-must preparation and alcoholic fermentation by Saccharomyces cerevisiae for mead production. Int. J. Food Microbiol. 2010, 144, 193–198. [Google Scholar] [CrossRef]
- Olaniran, A.; Hiralal, L.; Mokoeana, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Franitza, L.; Granvogl, M.; Schieberle, P. Influence of the production process on the key aroma compounds of rum: From molasses to the spirit. J. Agric. Food Chem. 2016, 64, 9041–9053. [Google Scholar] [CrossRef] [PubMed]
- Zierer, B.; Schieberle, P.; Granvogl, M. Aroma-active compounds in Bartlett pears and their changes during the manufacturing process of Bartlett pear brandy. J. Agric. Food Chem. 2016, 64, 9515–9522. [Google Scholar] [CrossRef] [PubMed]
- Vidrih, R.; Hribar, J. Studies on the sensory properties of mead and the formation of aroma compounds related to the type of honey. Acta Alim. 2007, 36, 151–162. [Google Scholar] [CrossRef]
- Li, R.; Sun, Y. Effects of honey variety and non-Saccharomyces cerevisiae on the flavor volatiles of mead. J. Am. Soc. Brew. Chem. 2019, 77, 40–53. [Google Scholar] [CrossRef]
- Sroka, P.; Tuszyński, T. Changes in organic acid contents during mead wort fermentation. Food Chem. 2007, 104, 1250–1257. [Google Scholar] [CrossRef]
- Pino, J.A.; Fajardo, M. Volatile composition and key flavor compounds of spirits from unifloral honeys. Int. J. Food Sci. Technol. 2011, 46, 994–1000. [Google Scholar] [CrossRef]
- Wintersteen, C.L.; Andrae, L.M.; Engeseth, N.J. Effect of heat treatment on antioxidant capacity and flavor volatiles of mead. J. Food Sci. 2005, 70, C119–C126. [Google Scholar] [CrossRef]
- Gomes, T.; Dias, T.; Cadavez, V.; Verdial, J.; Morais, J.; Ramalhosa, E.; Estevinho, L.M. Influence of sweetness and ethanol content on mead acceptability. Pol. J. Food Nutr. Sci. 2015, 65, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Czabaj, S.; Kawa-Rygielska, J.; Kucharska, A.Z.; Kliks, J. Effects of mead wort heat treatment on the mead fermentation process and antioxidant activity. Molecules 2017, 22, 803. [Google Scholar] [CrossRef] [PubMed]
- Kahoun, D.; Rezková, S.; Královský, J. Effect of heat treatment and storage conditions on mead composition. Food Chem. 2017, 219, 357–363. [Google Scholar] [CrossRef]
- Bednarek, M.; Szwengiel, A. Distinguishing between saturated and unsaturated meads based on their chemical characteristics. LWT-Food Sci. Technol. 2020, 133, 109962. [Google Scholar] [CrossRef]
- Starowicz, M.; Granvogl, M. An overview of mead production and the physicochemical, toxicological, and sensory characteristics of mead with a special emphasis on flavor. Trends Food Sci. Technol. 2020, 106, 402–416. [Google Scholar] [CrossRef]
- Franitza, L.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in two commercial rums by means of the sensomics approach. J. Agric. Food Chem. 2016, 64, 637–645. [Google Scholar] [CrossRef]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Mead production: Effect of nitrogen supplementation on growth, fermentation profile and aroma formation by yeasts in mead fermentation. J. Inst. Brew. 2015, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wand, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC-MS, GC-O, odor threshold and sensory analysis: An insight at the molecular level. Food Chem. 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Available online: www.pherobase.com (accessed on 2 September 2021).
- Jelen, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Šmogrovicová, D.; Nádaský, P.; Tandlich, R.; Wilhelmi, B.S.; Cambray, G. Analytical and aroma profiles of Slovak and South African meads. Czech J. Food Sci. 2012, 30, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Wu, Y.-L.; Lo, D.; Wu, M.C. Physicochemical property changes during the fermentation of logan (Dimocarpus longan) mead and its aroma composition using multiple yeast inoculations. J. Inst. Brew. 2013, 119, 303–308. [Google Scholar] [CrossRef]
- Roldán, A.; van Muiswinkel, G.C.J.; Lasanta, C.; Palacios, V.; Caro, I. Influence of pollen addition on mead elaboration: Physicochemical and sensory characteristics. Food Chem. 2011, 126, 574–582. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Szatkowska, K. Fruit and herbal meads—chemical composition and antioxidant properties. Food Chem. 2019, 283, 19–27. [Google Scholar] [CrossRef]
- Twilley, J.; Jutzi, C.; Tomasino, E. Influence of fermentation temperature and nutrient addition on chemical and sensory characteristics of traditional honey wine. Ann. Food Proc. Preserv. 2018, 3, 1022–1031. [Google Scholar]
- Kime, R.W.; McLellan, M.R.; Lee, C.Y. An improved method of mead production. Am. Bee J. 1991, 131, 394–395. [Google Scholar]
- Pereira, A.P.; Mendes-Ferreira, A.; Dias, L.G.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Volatile composition and sensory properties of mead. Microorganisms 2019, 7, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Senn, K.; Cantu, A.; Heymann, H. Characterizing the chemical and sensory profiles of traditional American meads. J. Food Sci. 2021, 86, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Granvogl, M. Key odor-active compounds in raw green and red Toona sinensis (A. Juss.) Roem. and their changes during blanching. J. Agric. Food Chem. 2020, 68, 7169–7183. [Google Scholar] [CrossRef] [PubMed]

| Compound | Isotope Label | Ions (m/z) 1 | Rf 2 | |
|---|---|---|---|---|
| Analyte | Internal Standard | |||
| 4-allyl-2-methoxyphenol 3 | [2H2] 3 | 165 | 167 3 | 0.80 |
| 1,8-cineol | [2H3] | 155 | 158 | 0.87 |
| diethyl succinate | [2H3] | 175 | 178 | 0.77 |
| ethyl acetate | [2H3] | 89 | 92 | 0.95 |
| ethyl decanoate | [2H3] | 201 | 204 | 0.96 |
| ethyl hexanoate | [2H3] | 145 | 148 | 0.98 |
| ethyl 3-methylbutanoate | [2H9] | 131 | 140 | 1.00 |
| ethyl octanoate | [2H3] | 173 | 176 | 0.98 |
| 2-methyl-1-propanol | [2H3] | 75 | 78 | 0.89 |
| 1-pentanol 4 | [2H2] 4 | 89 | 89 4 | 1.00 |
| phenylacetic acid | [13C2] | 137 | 139 | 0.90 |
| 2-phenylethanol | [2H5] | 105 | 110 | 0.71 |
| Concentrations 1 [µg L−1] | ||
|---|---|---|
| Compound | T | TS |
| ethyl acetate | 16,400 b | 57,000 a |
| ethyl hexanoate | 1220 a | 1230 a |
| 1-pentanol | 980 a | 966 a |
| 2-phenylethanol | 820 a | 551 b |
| phenylacetic acid | 748 a | 770 a |
| 2-methyl-1-propanol | 695 b | 1050 a |
| ethyl decanoate | 610 a | 612 a |
| diethyl succinate | 539 a | 536 a |
| ethyl octanoate | 405 a | 408 a |
| 4-allyl-2-methoxyphenol | 300 b | 560 a |
| ethyl 3-methylbutanoate | 250 a | 160 b |
| 1,8-cineol | 90.2 b | 150 a |
| Total | 23,007 | 63,958 |
| Compound | OT 2 [µg L−1] | OAVs 1 | |
|---|---|---|---|
| T | TS | ||
| ethyl hexanoate | 4 | 305 | 306 |
| ethyl octanoate | 1.6 | 253 | 255 |
| ethyl 3-methylbutanoate | 1.6 | 156 | 100 |
| 1,8-cineol | 3.2 | 28 | 46 |
| 2-methyl-1-propanol | 50 | 14 | 21 |
| 4-allyl-2-methoxyphenol | 50 | 6 | 11 |
| ethyl decanoate | 244 | 3 | 3 |
| ethyl acetate | 7500 | 2 | 8 |
| phenylacetic acid | 6100 | <1 | <1 |
| 2-phenylethanol | 7500 | <1 | <1 |
| 1-pentanol | 30000 | <1 | <1 |
| diethyl succinate | 300000 | <1 | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starowicz, M.; Granvogl, M. Effect of Wort Boiling on Volatiles Formation and Sensory Properties of Mead. Molecules 2022, 27, 710. https://doi.org/10.3390/molecules27030710
Starowicz M, Granvogl M. Effect of Wort Boiling on Volatiles Formation and Sensory Properties of Mead. Molecules. 2022; 27(3):710. https://doi.org/10.3390/molecules27030710
Chicago/Turabian StyleStarowicz, Małgorzata, and Michael Granvogl. 2022. "Effect of Wort Boiling on Volatiles Formation and Sensory Properties of Mead" Molecules 27, no. 3: 710. https://doi.org/10.3390/molecules27030710






