Synthesis, Characterization and Biological Evaluation of Benzothiazole–Isoquinoline Derivative
Abstract
:1. Introduction
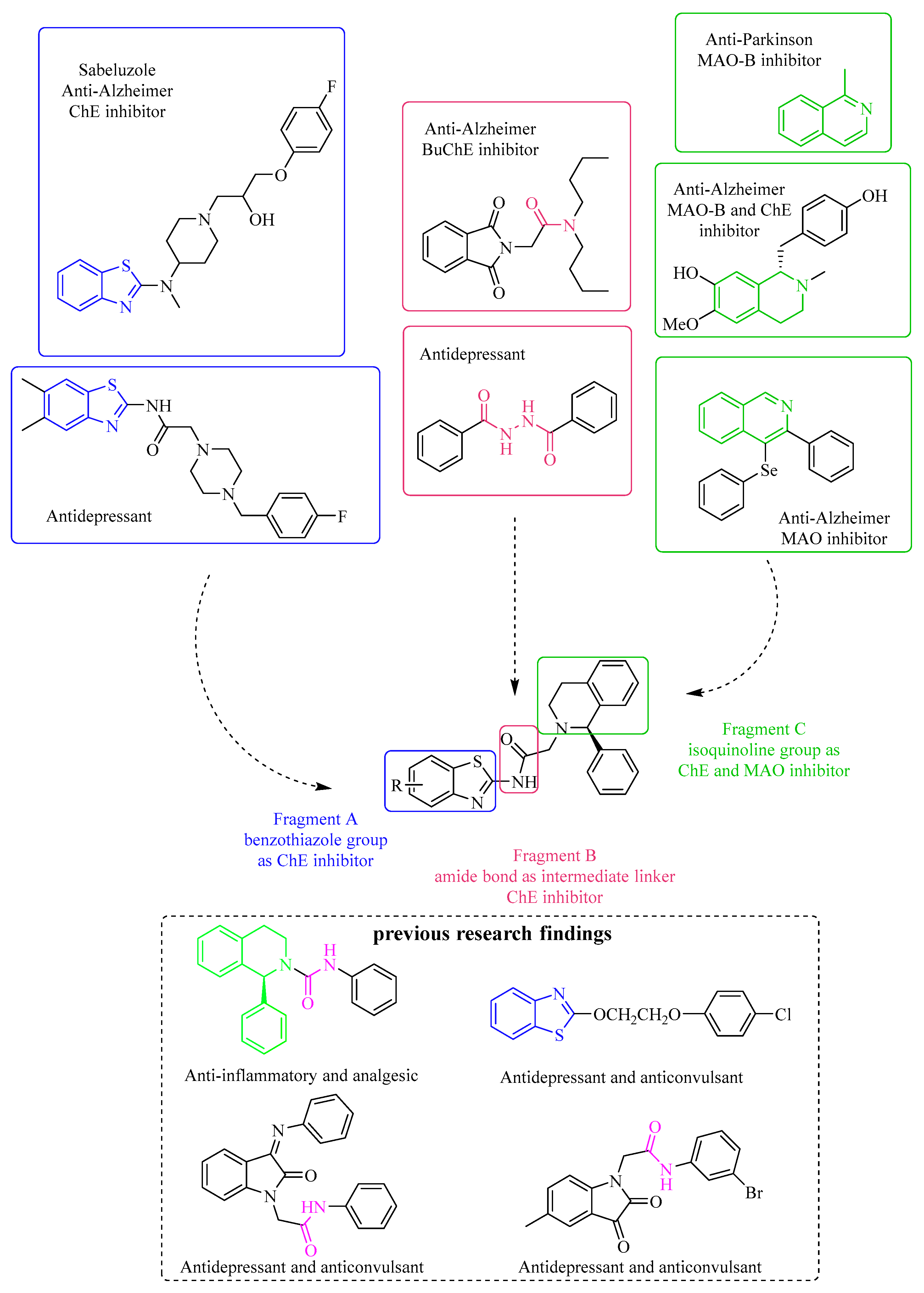
2. Results and Discussion
2.1. Chemistry
Synthetic Route and Method of Novel Benzothiazole–Isoquinoline Derivatives
2.2. In Vitro Biological Evaluation
2.2.1. Inhibitory Activity of Derivatives on MAO
2.2.2. Selective Inhibition of MAO–A and MAO–B by the Derivatives
2.2.3. ChE Inhibition by the Benzothiazole–Isoquinoline Derivatives
2.3. In Vivo Biological Evaluation
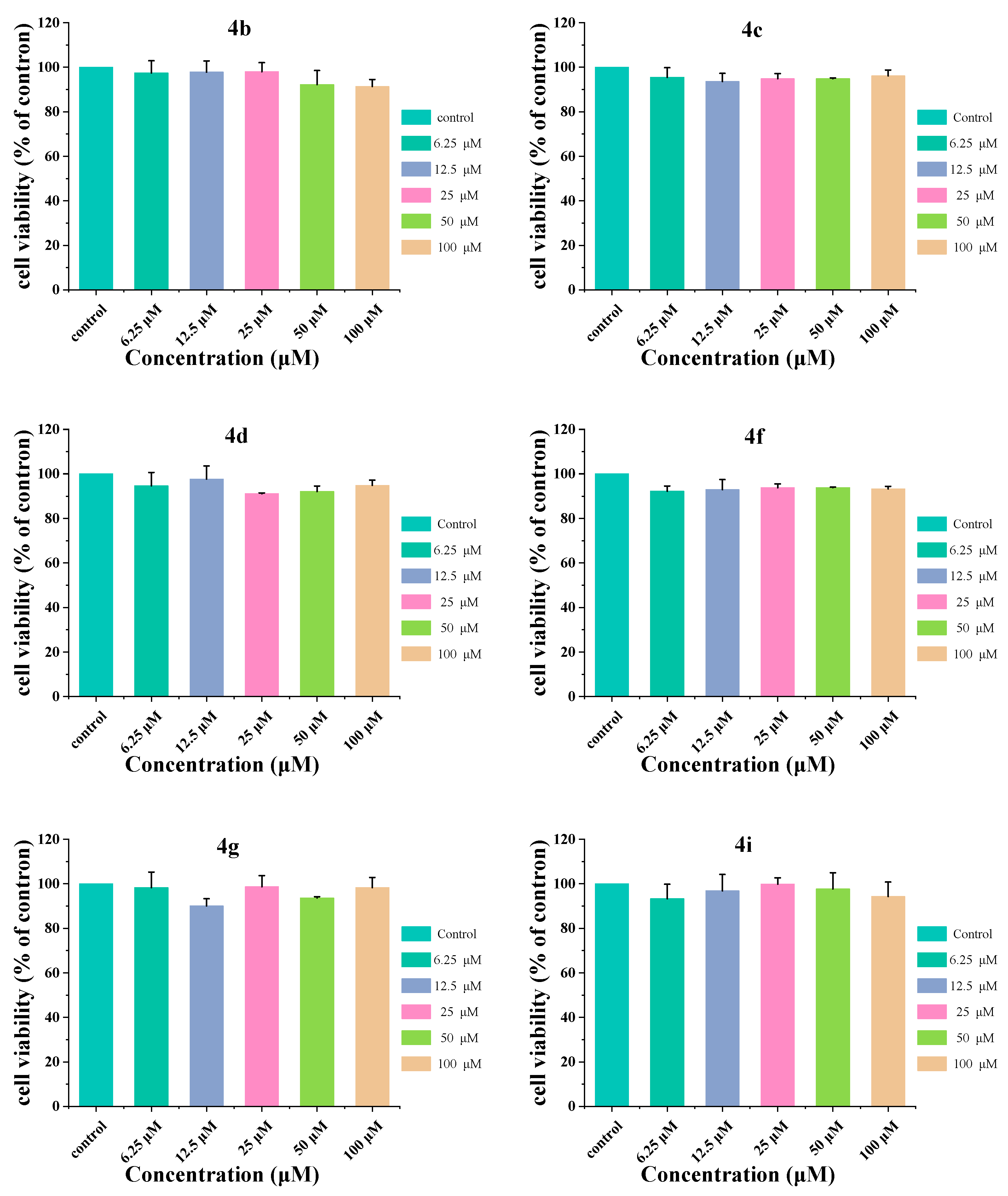
2.4. Cellular Toxicity
2.5. Molecular Docking
3. Experimental Protocols
3.1. The Synthetic Routes of Novel Benzothiazole–Isoquinoline Derivatives
3.1.1. General Procedure for the Synthesis of Substituted Benzo[d]thiazol–2–amine (2a–2p)
3.1.2. General Procedure for the Synthesis of Substituted N–(benzo[d]thiazol–2–yl)–2–chloroacetamide (3a–3p)
3.1.3. General Procedure for the Synthesis of Substituted (R)–N–(benzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4a–4p)
3.2. The Spectral Information of Novel Benzothiazole–Isoquinoline Derivatives
3.2.1. (R)–N–(4–chlorobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4a)
3.2.2. (R)–N–(5–chlorobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4b)
3.2.3. (R)–N–(6–chlorobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4c)
3.2.4. (R)–N–(4–bromobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4d)
3.2.5. (R)–N–(5–bromobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4e)
3.2.6. (R)–N–(6–bromobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4f)
3.2.7. (R)–N–(4–methylbenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4g)
3.2.8. (R)–N–(5–methylbenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4h)
3.2.9. (R)–N–(6–methylbenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4i)
3.2.10. (R)–N–(4–methoxybenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4j)
3.2.11. (R)–N–(5–methoxybenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4k)
3.2.12. (R)–N–(6–methoxybenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4l)
3.2.13. (R)–N–(6–fluorobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4m)
3.2.14. (R)–N–(6–nitrobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4n)
3.2.15. (R)–N–(4,6–dichlorobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4o)
3.2.16. (R)–N–(4,6–dibromobenzo[d]thiazol–2–yl)–2–(1–phenyl–3,4–dihydroisoquinolin–2(1H)–yl)acetamide (4p)
3.3. Determination of the Inhibitory Activity of 4a–4p on MAO by Holts Method
3.3.1. Preparation of MAO
3.3.2. MAO Inhibitory Activity and IC50 Detection
3.3.3. MAO–A Inhibitory Activity and IC50 Detection
3.3.4. MAO–B Inhibitory Activity and IC50 Detection
3.4. Determination of the Inhibitory Activity of Target Compounds 4a–4p on ChE
3.5. Forced Swimming Test
3.6. Cell Culture
3.7. Assessment of Cytotoxicity
3.8. Analyze Cell Viability by AO Staining
3.9. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative Disease: Models, Mechanisms, and a New Hope. DMM Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Tommaso, M.; Arendt–Nielsen, L.; Defrin, R.; Kunz, M.; Pickering, G.; Valeriani, M. Pain in Neurodegenerative Disease: Current Knowledge and Future Perspectives. Behav. Neurol. 2016, 2016, 7576292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2020, 102, 75–90. [Google Scholar] [CrossRef]
- Iqbal, K.; Grundke–Iqbal, I. Alzheimer Disease Is Multifactorial and Heterogeneous. Neurobiol. Aging 2000, 21, 901–902. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s Disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s Disease: Past, Present and Future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The Prevalence of Neuropsychiatric Symptoms in Alzheimer’s Disease: Systematic Review and Meta–Analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Mroueh, M.; Faour, W.H.; Shebaby, W.N.; Daher, C.F.; Ibrahim, T.M.; Ragab, H.M. Synthesis, Biological Evaluation and Modeling of Hybrids from Tetrahydro–1H–Pyrazolo[3,4–b]Quinolines as Dual Cholinestrase and COX–2 Inhibitors. Bioorg. Chem. 2020, 100, 103895. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Liu, X.; Hou, H.; Cao, Y.; Wei, F.; Li, J.; Chen, X.; Shen, Y.; Chen, W. Neurometabolic Characteristics in the Anterior Cingulate Gyrus of Alzheimer’s Disease Patients with Depression: A 1H Magnetic Resonance Spectroscopy Study. BMC Psychiatry 2015, 15, 306. [Google Scholar] [CrossRef]
- Zahodne, L.B.; Gongvatana, A.; Cohen, R.A.; Ott, B.R.; Tremont, G. Are Apathy and Depression Independently Associated with Longitudinal Trajectories of Cortical Atrophy in Mild Cognitive Impairment? Am. J. Geriatr. Psychiatry 2013, 21, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Kumar Waiker, D.; Bhardwaj, B.; Saraf, P.; Shrivastava, S.K. The Molecular Mechanism, Targets, and Novel Molecules in the Treatment of Alzheimer’s Disease. Bioorg. Chem. 2022, 119, 105562. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.E.; Beckman, D.; Ferreira, S.T. Microglial Dysfunction Connects Depression and Alzheimer’s Disease. Brain. Behav. Immun. 2016, 55, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Elsworthy, R.J.; Aldred, S. Depression in Alzheimer’s Disease: An Alternative Role for Selective Serotonin Reuptake Inhibitors? J. Alzheimer Dis. 2019, 69, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Galts, C.P.C.; Bettio, L.E.B.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil–Mohapel, J. Depression in Neurodegenerative Diseases: Common Mechanisms and Current Treatment Options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, W.; Kong, S.Y. Antidepressants for Neuro–Regeneration: From Depression to Alzheimer’s Disease. Arch. Pharm. Res. 2013, 36, 1279–1290. [Google Scholar] [CrossRef]
- Mitić, M.; Lazarević–Pašti, T. Does the Application of Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease Lead to Depression? Expert Opin. Drug Metab. Toxicol. 2021, 17, 841–856. [Google Scholar] [CrossRef]
- Bergantin, L.B. The Interactions Between Alzheimer’s Disease and Major Depression: Role of Ca2+ Channel Blockers and Ca2+/CAMP Signalling. Curr. Drug Res. Rev. 2020, 12, 97–102. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Faster, Better, Stronger: Towards New Antidepressant Therapeutic Strategies. Eur. J. Pharmacol. 2015, 753, 32–50. [Google Scholar] [CrossRef]
- de Picker, L.; van Den Eede, F.; Dumont, G.; Moorkens, G.; Sabbe, B.G.C. Antidepressants and the Risk of Hyponatremia: A Class–by–Class Review of Literature. Psychosomatics 2014, 55, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Spina, E.; Trifirò, G. Antidepressant Use in the Elderly: The Role of Pharmacodynamics and Pharmacokinetics in Drug Safety. Expert Opin. Drug Metab. Toxicol. 2015, 11, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Drye, L.T.; Pollock, B.G.; Devanand, D.P.; Frangakis, C.; Ismail, Z.; Marano, C.; Meinert, C.L.; Mintzer, J.E.; Munro, C.A.; et al. Effect of Citalopram on Agitation in Alzheimer Disease: The CitAD Randomized Clinical Trial. JAMA–J. Am. Med. Assoc. 2014, 311, 682–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahr, M.; Zeiss, R.; Lang, D.; Connemann, B.J.; Hiemke, C.; Muche, R.; Freudenmann, R.W.; Schönfeldt–Lecuona, C. Association between Haemorrhages and Treatment with Selective and Non–Selective Serotonergic Antidepressants: Possible Implications of Quantitative Signal Detection. Psychiatry Res. 2015, 229, 257–263. [Google Scholar] [CrossRef]
- Jasiak, N.M.; Bostwick, J. RRisk of QT/QTc Prolongation Among Newer Non–SSRI Antidepressants. Ann. Pharmacother. 2014, 48, 1620–1628. [Google Scholar] [CrossRef]
- Lozupone, M.; La Montagna, M.; D’Urso, F.; Piccininni, C.; Sardone, R.; Dibello, V.; Giannelli, G.; Solfrizzi, V.; Greco, A.; Daniele, A.; et al. Pharmacotherapy for the Treatment of Depression in Patients with Alzheimer’s Disease: A Treatment–Resistant Depressive Disorder. Expert Opin. Pharmacother. 2018, 19, 823–842. [Google Scholar] [CrossRef]
- Siarkos, K.T.; Katirtzoglou, E.A.; Politis, A.M. A Review of Pharmacological Treatments for Depression in Alzheimer’s Disease. J. Alzheimer Dis. 2015, 48, 15–34. [Google Scholar] [CrossRef]
- Orgeta, V.; Tabet, N.; Nilforooshan, R.; Howard, R. Efficacy of Antidepressants for Depression in Alzheimer’s Disease: Systematic Review and Meta–Analysis. J. Alzheimer Dis. 2017, 58, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Huang, Y.; Gong, Q.; Fu, Y.; Xu, Y.; Huang, J.; You, H.; Zhang, D.; Zhang, D.; et al. The Novel Therapeutic Strategy of Vilazodone–Donepezil Chimeras as Potent Triple–Target Ligands for the Potential Treatment of Alzheimer’s Disease with Comorbid Depression. Eur. J. Med. Chem. 2022, 229, 114045. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.H.; Guan, J.; Ge, S.; Wu, M.B.; Lin, J.P.; Yang, L.R. Advancement of Multi–Target Drug Discoveries and Promising Applications in the Field of Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 169, 200–223. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current Understanding of Alzheimer’s Disease Diagnosis and Treatment. F1000Research 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Zhao, S.; Liu, X.; Han, L.; Wang, R.; Hao, H.; Jiao, Y.; Han, S.; Bai, C. Polygala Tenuifolia: A Source for Anti–Alzheimer’s Disease Drugs. Pharm. Biol. 2020, 58, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Cagide, F.; Valencia, M.E.; Teixeira, J.; Bagetta, D.; Pérez, C.; Uriarte, E.; Oliveira, P.J.; Ortuso, F.; Alcaro, S.; et al. Multi–Target–Directed Ligands for Alzheimer’s Disease: Discovery of Chromone–Based Monoamine Oxidase/Cholinesterase Inhibitors. Eur. J. Med. Chem. 2018, 158, 781–800. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez–Soacha, D.A.; Scheiner, M.; Decker, M. Multi–Target–Directed–Ligands Acting as Enzyme Inhibitors and Receptor Ligands. Eur. J. Med. Chem. 2019, 180, 690–706. [Google Scholar] [CrossRef]
- Rossi, M.; Freschi, M.; De Camargo Nascente, L.; Salerno, A.; De Melo Viana Teixeira, S.; Nachon, F.; Chantegreil, F.; Soukup, O.; Prchal, L.; Malaguti, M.; et al. Sustainable Drug Discovery of Multi–Target–Directed Ligands for Alzheimer’s Disease. J. Med. Chem. 2021, 64, 4972–4990. [Google Scholar] [CrossRef]
- Guan, L.P.; Xia, Y.N.; Jin, Q.H.; Liu, B.Y.; Wang, S.H. Synthesis, Potential Anti–Inflammatory and Analgesic Activities Study of (S)–N–Substituted–1–Phenyl–3,4–Dihydroisoquinoline–2(1H)–Carboxamides. Bioorg. Med. Chem. Lett. 2017, 27, 3378–3381. [Google Scholar] [CrossRef]
- Zhen, X.; Peng, Z.; Zhao, S.; Han, Y.; Jin, Q.; Guan, L. Synthesis, Potential Anticonvulsant and Antidepressant Effects of 2–(5–Methyl–2,3–Dioxoindolin–1–Yl)Acetamide Derivatives. Acta Pharm. Sin. B 2015, 5, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Fu, Z.; Guan, L.; Jiang, H. Syntheses of Benzo[d]Thiazol–2(3H)–One Derivatives and Their Antidepressant and Anticonvulsant Effects. Mar. Drugs 2019, 17, 430. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.-P.; Liu, B.-Y.; Quan, Y.-C.; Yang, L.-Y.; Zhen, X.-H.; Wang, S.-H. Synthesis and Evaluation of Phenyliminoindolin–Containing Phenylacetamide Derivatives with the Antidepressant and Anticonvulsant Effects. Med. Chem. 2016, 12, 786–794. [Google Scholar] [CrossRef]
- Haider, K.; Rehman, S.; Pathak, A.; Najmi, A.K.; Yar, M.S. Advances in 2–Substituted Benzothiazole Scaffold–Based Chemotherapeutic Agents. Arch. Pharm. 2021, 354, 2100246. [Google Scholar] [CrossRef]
- Rouf, A.; Tanyeli, C. Bioactive Thiazole and Benzothiazole Derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.; Kilic, B.; Sagkan, R.I.; Aksakal, F.; Ercetin, T.; Gulcan, H.O.; Dogruer, D.S. Design, Synthesis and Biological Evaluation of New Benzoxazolone/Benzothiazolone Derivatives as Multi–Target Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2021, 212, 113124. [Google Scholar] [CrossRef] [PubMed]
- Wichur, T.; Więckowska, A.; Więckowski, K.; Godyń, J.; Jończyk, J.; Valdivieso, Á.D.R.; Panek, D.; Pasieka, A.; Sabaté, R.; Knez, D.; et al. 1–Benzylpyrrolidine–3–Amine–Based BuChE Inhibitors with Anti–Aggregating, Antioxidant and Metal–Chelating Properties as Multifunctional Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 187, 111916. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cho, H.J.; Bandara, N.; Sun, L.; Tran, D.; Rogers, B.E.; Mirica, L.M. Metal–Chelating Benzothiazole Multifunctional Compounds for the Modulation and 64Cu PET Imaging of Aβ Aggregation. Chem. Sci. 2020, 11, 7789–7799. [Google Scholar] [CrossRef]
- Özkay, Ü.D.; Kaya, C.; Çevik, U.A.; Can, Ö.D. Synthesis and Antidepressant Activity Profile of Some Novel Benzothiazole Derivatives. Molecules 2017, 22, 1490. [Google Scholar] [CrossRef]
- Geerts, H.; Nuydens, R.; De Jong, M.; Cornelissen, F.; Nuyens, R.; Wouters, L. Sabeluzole Stabilizes the Neuronal Cytoskeleton. Neurobiol. Aging 1996, 17, 573–581. [Google Scholar] [CrossRef]
- Abe, K.; Saitoh, T.; Horiguchi, Y.; Utsunomiya, I.; Taguchi, K. Synthesis and Neurotoxicity of Tetrahydroisoquinoline Derivatives for Studying Parkinson’s Disease. Biol. Pharm. Bull. 2005, 28, 1355–1362. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, T.B.; Da Rocha, J.T.; Prigol, M.; Saraiva, R.A.; Nogara, P.F.; Stein, A.L.A.; da Rocha, J.B.T.; Zeni, G.; Nogueira, C.W. 4–Organoseleno–Isoquinolines Selectively and Reversibly Inhibit the Cerebral Monoamine Oxidase B Activity. J. Mol. Neurosci. 2016, 59, 135–145. [Google Scholar] [CrossRef]
- Shang, X.F.; Yang, C.J.; Morris–Natschke, S.L.; Li, J.C.; Yin, X.D.; Liu, Y.Q.; Guo, X.; Peng, J.W.; Goto, M.; Zhang, J.Y.; et al. Biologically Active Isoquinoline Alkaloids Covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef]
- Liu, P.; Chen, X.; Zhou, H.; Wang, L.; Zhang, Z.; Ren, X.; Zhu, F.; Guo, Y.; Huang, X.; Liu, J.; et al. The Isoquinoline Alkaloid Dauricine Targets Multiple Molecular Pathways to Ameliorate Alzheimer–like Pathological Changes in Vitro. Oxid. Med. Cell. Longev. 2018, 2018, 2025914. [Google Scholar] [CrossRef]
- Możdżeń, E.; Wąsik, A.; Romańska, I.; Michaluk, J.; Antkiewicz–Michaluk, L. Antidepressant–like Effect of 1,2,3,4–Tetrahydroisoquinoline and Its Methyl Derivative in Animal Models of Depression. Pharmacol. Rep. 2017, 69, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Abdel–Aziz, M.; Abuo–Rahma, G.E.D.A.; Hassan, A.A. Synthesis of Novel Pyrazole Derivatives and Evaluation of Their Antidepressant and Anticonvulsant Activities. Eur. J. Med. Chem. 2009, 44, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, M.R.; Kanhed, A.M.; Kulkarni, V.M.; Bhosale, S.H.; Yadav, M.R. Design, Synthesis, and Computational Studies of Phenylacetamides as Antidepressant Agents. Mol. Divers. 2022, 26, 3157–3172. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Nizami, S.S.; Mahmood, U.; Masood, S.; Iftikhar, S.; Saied, S. In–Vitro Evaluation and in–Silico Studies Applied on Newly Synthesized Amide Derivatives of N–Phthaloylglycine as Butyrylcholinesterase (BChE) Inhibitors. Comput. Biol. Chem. 2018, 74, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Abiri, A.; Eskandari, K.; Haghighijoo, Z.; Edraki, N.; Asadipour, A. Phenoxyethyl Piperidine/Morpholine Derivatives as PAS and CAS Inhibitors of Cholinesterases: Insights for Future Drug Design. Sci. Rep. 2019, 9, 19855. [Google Scholar] [CrossRef] [Green Version]
- Abd El–Gaber, M.K.; Hassan, H.Y.; Mahfouz, N.M.; Farag, H.H.; Bekhit, A.A. Synthesis, Biological Investigation and Molecular Docking Study of N–Malonyl–1,2–Dihydroisoquinoline Derivatives as Brain Specific and Shelf–Stable MAO Inhibitors. Eur. J. Med. Chem. 2015, 93, 481–491. [Google Scholar] [CrossRef]


| Compounds | R | Inhibition Rate of MAO (%) | IC50 of MAO (μM) |
|---|---|---|---|
| 4a | o–Cl | 14.93 | >150 |
| 4b | m–Cl | 39.27 | 38.82 ± 3.76 |
| 4c | p–Cl | 21.59 | 45.90 ± 2.66 |
| 4d | o–Br | 43.37 | 64.83 ± 4.20 |
| 4e | m–Br | 9.29 | 128.08 ± 1.27 |
| 4f | p–Br | 24.15 | 41.78 ± 2.94 |
| 4g | o–CH3 | 57.11 | 14.80 ± 5.45 |
| 4h | m–CH3 | 16.72 | 76.37 ± 2.58 |
| 4i | p–CH3 | 26.46 | 18.53 ± 1.69 |
| 4j | o–OCH3 | 5.70 | >150 |
| 4k | m–OCH3 | 17.75 | 65.48 ± 3.51 |
| 4l | p–OCH3 | 11.34 | 76.32 ± 2.94 |
| 4m | p–F | 9.76 | >150 |
| 4n | p–NO2 | 12.51 | >150 |
| 4o | 2,4–Cl2 | 10.19 | 67.80 ± 1.26 |
| Compounds | R | Inhibition Rate (%) | IC50 (μM) | ||
|---|---|---|---|---|---|
| MAO–A | MAO–B | Compounds | R | ||
| 4b | m–Cl | 3.01 | 39.15 | 4b | m–Cl |
| 4c | p–Cl | 12.03 | 48.45 | 4c | p–Cl |
| 4d | o–Br | 18.05 | 46.00 | 4d | o–Br |
| 4f | p–Br | 15.04 | 46.49 | 4f | p–Br |
| 4g | o–CH3 | 9.03 | 51.39 | 4g | o–CH3 |
| 4i | p–CH3 | 2.51 | 61.17 | 4i | p–CH3 |
| Clorgyline | — | 84.21 | — | Clorgyline | — |
| Pargyline | — | — | 93.47 | Pargyline | — |
| Compounds | R | Inhibition Rate (%) | IC50 (μM) | ||
|---|---|---|---|---|---|
| AChE | BuChE | AChE | BuChE | ||
| 4a | o–Cl | 6.08 | 38.08 | >150 | >150 |
| 4b | m–Cl | 4.42 | 69.44 | >150 | 17.59 ± 1.78 |
| 4c | p–Cl | 1.10 | 68.80 | >150 | >150 |
| 4d | o–Br | 0.55 | 77.76 | >150 | 14.61 ± 5.81 |
| 4e | m–Br | 4.97 | 68.80 | >150 | 18.27 ± 2.68 |
| 4f | p–Br | 1.10 | 32.32 | >150 | >150 |
| 4g | o–CH3 | 1.10 | 72.64 | >150 | 30.35 ± 4.59 |
| 4h | m–CH3 | 3.31 | 37.44 | >150 | 11.52 ± 3.22 |
| 4i | p–CH3 | 2.21 | 47.68 | >150 | 19.17 ± 2.72 |
| 4j | o–OCH3 | 12.71 | 51.52 | >150 | >150 |
| 4k | m–OCH3 | 3.31 | 57.92 | >150 | 10.25 ± 2.91 |
| 4l | p–OCH3 | 3.31 | 41.92 | >150 | 3.86 ± 0.82 |
| 4m | p–F | 2.76 | 22.33 | >150 | 50.26 ± 1.68 |
| 4n | p–NO2 | 2.76 | 20.31 | >150 | 12.34 ± 3.76 |
| 4o | 2,4–Cl2 | 3.87 | 52.16 | >150 | 21.76 ± 2.85 |
| 4p | 2,4–Br2 | 6.63 | 43.84 | >150 | 27.12 ± 1.73 |
| Tacrine | — | — | 99.41 | — | 12.43 ± 2.49 |
| Donepezil | — | 69.06 | — | 16.75 ± 1.32 | — |
| Compounds | R | Antidepressant Effects | |
|---|---|---|---|
| Duration of Immobility (s) | DID (%) a | ||
| 4b | m–Cl | 85.7 ± 8.1 *** | 51.98 |
| 4c | p–Cl | 77.0 ± 18.3 *** | 56.84 |
| 4d | o–Br | 62.3 ± 14.0 ** | 65.06 |
| 4f | p–Br | 71.7 ± 4.9 *** | 59.83 |
| 4g | o–CH3 | 69.0 ± 15.7 ** | 61.32 |
| 4i | p–CH3 | 83.8 ± 13.6 ** | 53.05 |
| Fluoxetine | — | 31.7 ± 7.7 *** | 82.20 |
| Control | — | 178.4 ± 5.9 | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhao, D.; He, Z.; Hu, Y.; Zhu, Y.; Zhang, L.; Jin, L.; Guan, L.; Wang, S. Synthesis, Characterization and Biological Evaluation of Benzothiazole–Isoquinoline Derivative. Molecules 2022, 27, 9062. https://doi.org/10.3390/molecules27249062
Liu W, Zhao D, He Z, Hu Y, Zhu Y, Zhang L, Jin L, Guan L, Wang S. Synthesis, Characterization and Biological Evaluation of Benzothiazole–Isoquinoline Derivative. Molecules. 2022; 27(24):9062. https://doi.org/10.3390/molecules27249062
Chicago/Turabian StyleLiu, Weihua, Donghai Zhao, Zhiwen He, Yiming Hu, Yuxia Zhu, Lingjian Zhang, Lianhai Jin, Liping Guan, and Sihong Wang. 2022. "Synthesis, Characterization and Biological Evaluation of Benzothiazole–Isoquinoline Derivative" Molecules 27, no. 24: 9062. https://doi.org/10.3390/molecules27249062
APA StyleLiu, W., Zhao, D., He, Z., Hu, Y., Zhu, Y., Zhang, L., Jin, L., Guan, L., & Wang, S. (2022). Synthesis, Characterization and Biological Evaluation of Benzothiazole–Isoquinoline Derivative. Molecules, 27(24), 9062. https://doi.org/10.3390/molecules27249062







