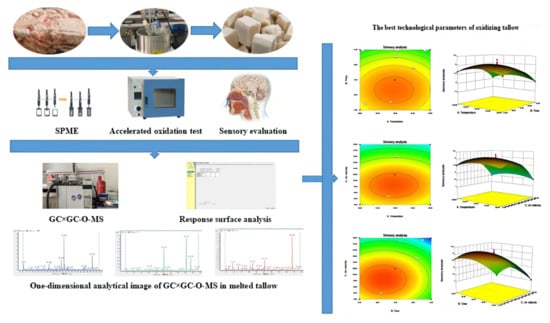
Optimization and Preparation of Tallow with a Strong Aroma by Mild Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Different Factors on AV, PV, and P-AV of Oxidized Melted Tallow
2.2. Sensory Evaluation of the Sample
2.3. SPME-GC–MS Analysis Results of Oxidized Melted Tallow under Different Conditions
- (1)
- Qualitative results
- (2)
- Quantitative results
2.4. Composition Characteristics of Aroma Compounds in Oxidized Melted Tallow under Different Conditions
2.5. Principal Component Analysis
2.6. Response Surface Optimization
2.7. Accelerated Oxidation Test
3. Materials and Methods
3.1. Samples and Chemicals
3.2. Determination of Acid Value
3.3. Determination of Peroxide Value
3.4. Determination of Anisidine Value
3.5. Analysis of Volatile Compounds
3.5.1. Gas Chromatography-Olfactometry–Mass Spectrometry (GC-O–MS) Analysis
3.5.2. SPME-GC–MS Analysis of Volatile Compounds
3.5.3. Qualitative and Quantitative Methods of Volatile Aroma Compounds
3.5.4. Qualitative Analysis
3.6. Sensory Analysis
3.7. Accelerated Oxidation Analysis of Oil
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, W.L. Study on Screening Characteristic Flavor Compounds and Quality Control of Tallow; Xihua University: Chengdu, China, 2018. [Google Scholar]
- Zhou, S.Y. Study on the production technology of refined tallow. Cereals Oils Food Sci. Technol. 2011, 19, 30–32. [Google Scholar] [CrossRef]
- Li, B.B. A preliminary Study on the Flavor and Quality Optimization of Chongqing Tallow Hot Pot Base. Southwest University. 2021. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202201&filename=1021768174.nh (accessed on 17 January 2022).
- Qin, Y.L. Identification Preparation by Maillard Method of Key Aroma Compounds in Tallow; Jiangnan University: Wuxi, China, 2022. [Google Scholar]
- Pearson, A.M.; Wenham, L.M.; Carse, W.A.; McLeod, K.; Davey, C.L.; Kirton, A.H. Observations on the contributions of fat and lean to the aroma of cooked beef and lamb. J. Anim. Sci. 1973, 36, 511–515. [Google Scholar] [CrossRef]
- Xie, J.C.; Sun, B.G.; Zheng, F.P.; Xiao, Y.H.; Liu, J.X. Supercritical CO2 extraction of volatile flavor components in oxidized sheep fat. Food Sci. 2009, 30, 168–171. [Google Scholar]
- Aishima, T.; Nobuhara, A. Beef Flavor Substance, Process for Producing Same and Beef- Flavoring Agent. U.S. Patent 4,094,997, 13 June 1978. [Google Scholar]
- Haring, P.G.M. Process for the preparation of a flavoured foodstuff as well as a foodstuff obtainable by such a process. Eur. Pat. Appl. 1989, 298552. [Google Scholar]
- Haring, P.G.P.R. Process for the preparing a flavour concentrate. Eur. Pat. Appl. 1992, 463668. [Google Scholar]
- Zhong, Q.; Xie, J.C.; Sun, B.G.; Zheng, F.P. “Enzymatic oxidation of chicken fat-thermal reaction” to prepare chicken flavor. Food Sci. 2010, 10, 124–129. [Google Scholar] [CrossRef]
- Li, X. Experimental Report on the Synthesis of Beef Flavor by Maillard Reaction. Chin. Condiments 1993, 6, 20–22. [Google Scholar]
- Xiao, Z.B.; Sun, J. Application of response surface analysis in preparation of beef flavor precursor by controlled oxidation of beef tallow. Food Ind. 2009, 30, 22–24. [Google Scholar]
- Ouyang, J.; Wei, L.Q.; Wu, Y.Z.; Yuan, D.H. Research on the application technology of new fat flavor. China Condiments 2008, 4, 80–82. [Google Scholar]
- Sun, B.G.; Peng, Q.J.; Liang, M.L.; Xie, J.C. Study on the technology of controlled oxidation of tallow. Food Sci. 2005, 04, 133–136. [Google Scholar]
- Yang, G.M.; Xie, J.C.; Sun, B.G. Research progress in preparation of flavoring materials by enzymatic oxidation of oils and fats. Food Sci. Technol. 2006, 31, 5. [Google Scholar]
- Liu, X.Z.; Kong, Y.C.; Li, D. Research progress of fat oxidation in meat and meat products. Meat Ind. 2017, 3, 47–49. [Google Scholar]
- Yan, W.Q.; Tang, B.J. Study on the application of fat degradation and its products in Maillard reaction. Food Sci. 2006, 3, 272–274. [Google Scholar]
- Shi, X.X. Study on the Formation of Characteristic Flavor Precursors by Enzymatic Hydrolysis of Beef Tallow-Mild Heating Oxidation; Jiangnan University: Wuxi, China, 2013. [Google Scholar]
- Shi, X.; Zhang, X.; Song, S.; Tan, C.; Jia, C.; Xia, S. Identification of characteristic flavour precursors from enzymatic hydrolysis-mild thermal oxidation tallow by descriptive sensory analysis and gas chromatography–olfactometry and partial least squares regression. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 2, 69–76. [Google Scholar] [CrossRef] [PubMed]
- National Health and Family Planning Commission (NHFPC). National Food Safety Standards Determination of Acid Value in Food; GB 5009.229; NHFPC: Beijing, China, 31 August 2016. [Google Scholar]
- National Health and Family Planning Commission (NHFPC). National Food Safety Standards Determination of Peroxide Value in Food; GB 5009.227; NHFPC: Beijing, China, 31 August 2016. [Google Scholar]
- National Standardization Administration Committee, General Administration of Quality Supervision, Inspection and Quarantine. Fats and Oils Determination of Anisidine Value; GB T 24304; NHFPC: Beijing, China, 30 September 2009. [Google Scholar]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. 2004. Available online: http://flavornet.org/flavornet.html (accessed on 17 January 2022).
- Cao, J. Study on Oxidation Law and Kinetics of Edible Oils with Different Fatty Acid Structures; Nanchang University: Nanchang, China, 2015. [Google Scholar]









| Different Reaction Conditions | Sample Number | Milky | Muttony | Animal Fat | Sweet | Burnt | Fruit | Sour | Spoiled | Weighted Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | 120 °C | 0.6 | 0.34 | 0.64 | 0.48 | 0.2 | 0.12 | −0.1 | −0.14 | 2.14 |
| 130 °C | 0.72 | 0.3 | 0.72 | 0.48 | 0.2 | 0.12 | −0.1 | −0.2 | 2.24 | |
| 140 °C | 0.78 | 0.34 | 0.8 | 0.48 | 0.2 | 0.12 | −0.16 | −0.16 | 2.4 | |
| 150 °C | 0.94 | 0.24 | 0.68 | 0.58 | 0.1 | 0.24 | −0.1 | −0.14 | 2.54 | |
| 160 °C | 0.64 | 0.34 | 0.9 | 0.48 | 0.24 | 0.12 | −0.22 | −0.14 | 2.36 | |
| Time | 25 min | 0.87 | 0.62 | 0.84 | 0.74 | 0.22 | 0.22 | −0.25 | −0.12 | 3.13 |
| 35 min | 0.85 | 0.65 | 0.89 | 0.71 | 0.20 | 0.27 | −0.22 | −0.06 | 3.29 | |
| 45 min | 0.68 | 0.67 | 0.82 | 0.52 | 0.11 | 0.26 | −0.35 | −0.15 | 2.57 | |
| 55 min | 0.65 | 0.73 | 0.65 | 0.55 | 0.11 | 0.21 | −0.45 | −0.16 | 2.28 | |
| 65 min | 0.55 | 0.78 | 0.58 | 0.46 | 0.15 | 0.25 | −0.69 | −0.25 | 1.83 | |
| Air velocity | 18 L/h | 0.72 | 0.56 | 0.76 | 0.90 | 0.24 | 0.22 | −0.24 | −0.16 | 3.00 |
| 48 L/h | 0.90 | 0.52 | 0.80 | 0.72 | 0.24 | 0.22 | −0.28 | −0.14 | 2.98 | |
| 78 L/h | 0.84 | 0.64 | 0.64 | 0.54 | 0.32 | 0.24 | −0.24 | −0.10 | 2.88 | |
| 108 L/h | 0.84 | 0.68 | 0.72 | 0.84 | 0.24 | 0.22 | −0.28 | −0.14 | 3.12 | |
| 138 L/h | 0.72 | 0.52 | 0.68 | 0.54 | 0.24 | 0.24 | −0.24 | −0.16 | 1.83 |
| Compound Information | Relative Content (ng/g) y | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | CAS | Component | RI x | Odor | 120 °C | 130 °C | 140 °C | 150 °C | 160 °C |
| Aldehydes | |||||||||
| 1 | 110-62-3 | 1-pentanal | 962 | almond | 72.27 ± 12.26 a | 36.91 ± 1.23 b | 33.63 ± 1.28 b | 35.22 ± 1.65 b | 28.91 ± 2.36 b |
| 2 | 66-25-1 | 1-hexanal | 1063 | grassy | 153.27 ± 24.55 a | 97.36 ± 2.85 b | 68.75 ± 1.79 c | 102.44 ± 4.27 b | 88.99 ± 8.96 bc |
| 3 | 111-71-7 | (E)-2-pentenal | 1128 | fruity | 149.27 ± 25.83 a | 49.93 ± 2.93 b | 34.39 ± 0.34 b | 35.92 ± 1.39 b | 42.20 ± 4.36 b |
| 4 | 6728-26-3 | 1-heptanal | 1182 | fatty | 293.27 ± 51.27 a | 154.71 ± 6.97 b | 129.87 ± 5.25 b | 263.81 ± 0.38 a | 173.38 ± 19.54 b |
| 5 | 124-13-0 | 1-octanal | 1274 | fatty | 375.27 ± 46.59 a | 151.56 ± 3.38 c | 144.96 ± 4.71 c | 233.31 ± 8.66 b | 264.61 ± 12.81 b |
| 6 | 18829-55-5 | (E)-2-heptenal | 1309 | fatty | 405.27 ± 59.55 a | 185.16 ± 5.86 bc | 146.09 ± 3.75 c | 195.24 ± 8.71 bc | 230.05 ± 11.42 b |
| 7 | 124-19-6 | 1-nonanal | 1378 | fresh | 1020.27 ± 164.22 a | 407.17 ± 9.12 c | 364.92 ± 16.81 c | 683.57 ± 22.46 b | 558.30 ± 13.32 b |
| 8 | 2548-87-0 | (E)-2-octenal | 1413 | cucumber | 390.27 ± 51.58 a | 112.61 ± 9.53 c | 135.40 ± 2.90 c | 138.41 ± 8.27 c | 209.39 ± 12.43 b |
| 9 | 4313-03-5 | (E,E)-2,4-heptadienal | 1478 | fatty | 248.27 ± 22.28 a | 56.05 ± 2.86 d | 80.43 ± 5.46 c | 87.18 ± 5.40 c | 129.88 ± 2.83 b |
| 10 | 100-52-7 | benzaldehyde | 1504 | nutty | 30.27 ± 7.16 c | 33.00 ± 1.51 c | 43.74 ± 4.08 ab | 51.13 ± 2.35 a | 35.63 ± 2.76 bc |
| 11 | 18829-56-6 | (E)-2-nonenal | 1518 | cucumber | 633.27 ± 57.08 a | 170.32 ± 7.52 c | 193.24 ± 9.74 c | 164.58 ± 7.13 c | 247.69 ± 9.07 b |
| 12 | 122-78-1 | phenylacetaldehyde | 1648 | sweet | ND | ND | 72.32 ± 22.06 a | 46.62 ± 17.78 a | 60.28 ± 5.31 a |
| 13 | 3913-81-3 | (E)-2-decenal | 1639 | fatty | 1551.27 ± 56.24 a | 315.49 ± 15.51 c | 412.00 ± 21.29 b | 297.09 ± 6.37 c | 450.41 ± 6.62 b |
| 14 | 2463-77-6 | 2-undecenal | 1730 | orange peel | 1106.27 ± 54.60 a | 220.72 ± 6.17 bc | 271.66 ± 28.44 b | 172.18 ± 9.53 c | 222.23 ± 6.50 bc |
| 15 | 25152-84-5 | (E,E)-2,4-decadienal | 1744 | fried | 167.27 ± 3.60 b | 10.10 ± 2.05 c | 199.87 ± 34.57 a | 126.04 ± 8.92 c | 205.27 ± 4.74 a |
| Alcohols | |||||||||
| 16 | 71-41-0 | 1-pentanol | 1244 | spicy | 63.27 ± 6.64 a | 38.15 ± 1.16 b | 38.56 ± 5.72 b | 56.06 ± 0.99 a | 35.30 ± 3.50 b |
| 17 | 3391-86-4 | 1-octen-3-ol | 1430 | mushroom | 153.27 ± 23.18 | ND | ND | ND | ND |
| 18 | 111-70-6 | 1-heptanol | 1447 | floral | ND | ND | 56.80 ± 0.71 b | 71.34 ± 3.39 a | 71.09 ± 4.46 a |
| 19 | 143-08-8 | 1-nonanol | 1640 | fatty | 22.27 ± 7.98 | ND | ND | ND | ND |
| Sours | |||||||||
| 20 | 64-19-7 | acetic acid | 1440 | sour | ND | 101.39 ± 2.62 c | 345.80 ± 4.57 a | 107.82 ± 5.67 c | 200.34 ± 1.65 b |
| 21 | 111-14-8 | heptanoic acid | 1940 | sweaty | 57.27 ± 7.61 c | 31.82 ± 4.87 d | 118.43 ± 2.43 b | 173.22 ± 20.81 a | 106.00 ± 8.79 b |
| 22 | 124-07-2 | octanoic acid | 2051 | rot | 49.27 ± 5.10 d | 27.86 ± 24.34 cd | 101.56 ± 1.00 b | 124.82 ± 8.78 a | 68.79 ± 5.47 c |
| 23 | 334-48-5 | decanoic acid | 2262 | putrid | 62.27 ± 8.84 b | 151.38 ± 12.82 b | 152.97 ± 37.80 a | 137.39 ± 16.62 a | 78.87 ± 3.31 a |
| Esters | |||||||||
| 24 | 112-23-2 | heptyl formate | 1448 | cucumber | 145.27 ± 16.90 | 56.40 ± 5.43 | ND | ND | ND |
| 25 | 112-32-3 | octyl formate | 1548 | cucumber | 190.27 ± 17.70 a | 62.97 ± 1.36 c | 59.75 ± 1.88 c | 74.95 ± 3.60 bc | 81.09 ± 2.41 b |
| 26 | 105-21-5 | γ-heptalactone | 1784 | coconut | 29.27 ± 4.02 | 96.75 ± 1.60 | ND | ND | ND |
| Heterocycles | |||||||||
| 27 | 3777-69-3 | 2-pentylfuran | 1215 | fruity | 81.27 ± 12.24 a | 32.42 ± 1.65 c | 33.24 ± 1.36 c | 59.48 ± 2.11 b | 64.06 ± 7.48 b |
| 28 | 109-08-0 | 2-methylpyrazine | 1256 | nutty | ND | ND | 5.87 ± 0.64 c | 18.42 ± 1.65 a | 11.98 ± 1.72 b |
| 29 | 2294-76-0 | 2-pentylpyridine | 1600 | fatty | 66.27 ± 1.40 a | 21.17 ± 1.68 d | 31.85 ± 2.55 c | 44.89 ± 2.91 b | 35.50 ± 2.42 c |
| Compound Information | Relative Content (ng/g) y | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | CAS | Component | RI x | Odor | 25 Min | 35 Min | 45 Min | 55 Min | 65 Min |
| Aldehydes | |||||||||
| 1 | 110-62-3 | 1-pentanal | 962 | almond | 3.72 ± 0.78 e | 14.18 ± 0.25 c | 9.29 ± 1.91 d | 22.13 ± 2.09 b | 33.17 ± 0.51 a |
| 2 | 66-25-1 | 1-hexanal | 1063 | grassy | 12.23 ± 2.76 d | 45.66 ± 0.59 b | 31.02 ± 1.53 c | 40.79 ± 11.90 bc | 68.54 ± 2.98 a |
| 3 | 111-71-7 | (E)-2-pentenal | 1128 | fruity | 0.79 ± 0.17 d | 11.46 ± 1.02 b | 6.97 ± 2.65 c | 14.55 ± 6.88 bc | 30.46 ± 6.62 a |
| 4 | 6728-26-3 | 1-heptanal | 1182 | fatty | 11.78 ± 7.72 c | 36.79 ± 4.01 b | 33.17 ± 12.02 b | 36.62 ± 6.19 b | 86.38 ± 1.41 a |
| 5 | 124-13-0 | 1-octanal | 1274 | fatty | 17.09 ± 0.71 d | 35.96 ± 0.25 c | 24.47 ± 2.11 d | 53.40 ± 4.90 b | 82.43 ± 6.45 a |
| 6 | 18829-55-5 | (E)-2-heptenal | 1309 | fatty | 16.39 ± 3.57 e | 65.45 ± 0.17 c | 41.97 ± 2.50 d | 103.67 ± 1.78 b | 143.25 ± 0.97 a |
| 7 | 124-19-6 | nonanal | 1378 | fresh | 36.64 ± 2.22 e | 97.19 ± 0.97 c | 64.21 ± 8.54 d | 143.24 ± 1.89 b | 201.25 ± 6.89 a |
| 8 | 2548-87-0 | (E)-2-octenal | 1413 | cucumber | 8.31 ± 0.64 e | 27.06 ± 0.77 c | 17.72 ± 0.67 d | 53.35 ± 1.07 b | 87.75 ± 1.18 a |
| 9 | 4313-03-5 | (E,E)-2,4-heptadienal | 1478 | fatty | 1.41 ± 0.60 d | 6.28 ± 1.66 c | 3.11 ± 0.89 d | 27.60 ± 0.93 b | 57.63 ± 2.38 a |
| 10 | 112-31-2 | 1-decanal | 1472 | fatty | 3.90 ± 1.37 a | 5.66 ± 0.74 a | 4.57 ± 1.93 a | 5.81 ± 1.04 a | 5.79 ± 0.97 a |
| 11 | 100-52-7 | benzaldehyde | 1504 | nutty | 6.04 ± 1.41 a | 6.66 ± 0.53 a | 6.10 ± 1.62 a | 5.85 ± 0.03 a | ND |
| 12 | 18829-56-6 | (E)-2-nonenal | 1518 | cucumber | 14.71 ± 3.76 d | 35.54 ± 3.05 c | 29.07 ± 5.13 c | 77.91 ± 8.31 b | 145.95 ± 1.46 a |
| 13 | 112-44-7 | undecanal | 1622 | soap | 2.32 ± 1.39 c | 2.91 ± 0.37 bc | 3.58 ± 1.95 bc | 5.14 ± 0.74 ab | 6.71 ± 0.52 a |
| 14 | 122-78-1 | phenylacetaldehyde | 1648 | sweet | 1.24 ± 0.69 | ND | ND | ND | ND |
| 15 | 3913-81-3 | (E)-2-decenal | 1639 | fatty | 32.13 ± 1.81 d | 74.34 ± 5.01 c | 64.89 ± 1.85 c | 193.20 ± 10.55 b | 344.06 ± 8.67 a |
| 16 | 2463-77-6 | 2-undecenal | 1730 | orange peel | 21.39 ± 1.69 d | 68.24 ± 1.61 c | 44.64 ± 2.39 cd | 140.63 ± 8.83 b | 259.58 ± 29.81 a |
| 17 | 25152-84-5 | (E,E)-2,4-decadienal | 1744 | fried | 5.95 ± 0.29 c | 22.08 ± 1.99 c | 11.03 ± 0.67 c | 52.01 ± 4.59 b | 124.47 ± 18.70 a |
| Sours | |||||||||
| 18 | 64-19-7 | acetic acid | 1440 | sour | 43.85 ± 2.57 a | 43.11 ± 1.14 a | 21.63 ± 0.94 b | 20.49 ± 0.45 b | 20.84 ± 0.69 b |
| 19 | 142-62-1 | hexanoic acid | 1836 | cheese | ND | 10.66 ± 0.32 bc | 7.74 ± 0.43 c | 12.91 ± 3.92 b | 26.07 ± 1.68 a |
| 20 | 111-14-8 | heptanoic acid | 1940 | sweaty | ND | 6.45 ± 0.86 c | 5.25 ± 1.13 bc | 10.02 ± 3.44 b | 16.45 ± 2.08 a |
| 21 | 124-07-2 | octanoic acid | 2051 | rot | 17.87 ± 0.94 c | 19.73 ± 2.09 bc | 18.18 ± 0.98 bc | 22.92 ± 4.42 b | 31.51 ± 0.93 a |
| 22 | 112-05-0 | nonanoic acid | 2144 | waxy | 448.77 ± 2.99 b | 709.50 ± 160.53 a | 623.77 ± 52.50 ab | 664.24 ± 63.45 a | 814.94 ± 31.35 a |
| 23 | 334-48-5 | decanoic acid | 2262 | putrid | 6.62 ± 0.14 a | 12.32 ± 1.83 a | 9.89 ± 2.42 a | 12.30 ± 5.51 a | 9.50 ± 2.27 a |
| Ketones | |||||||||
| 24 | 111-13-7 | 2-octanone | 1271 | earthy | ND | ND | 0.71 ± 0.64 a | 1.48 ± 0.94 a | 2.33 ± 0.89 a |
| 25 | 821-55-6 | 2-nonanone | 1389 | soap | ND | ND | ND | 1.33 ± 0.36 | 2.39 ± 1.21 |
| 26 | 693-54-9 | 2-decanone | 1482 | citrus | ND | ND | ND | 2.90 ± 1.16 | 3.54 ± 1.71 |
| 27 | 593-08-8 | 2-tridecanone | 1814 | creamy | 2.81 ± 0.61 a | 2.35 ± 0.69 a | 2.05 ± 1.18 a | 2.67 ± 0.97 a | 2.64 ± 0.30 a |
| 28 | 21835-01-8 | 3-ethyl-2-hydroxycyclopent-2-enone | 1894 | caramel | ND | ND | ND | 4.30 ± 2.60 | 10.67 ± 0.18 |
| 29 | 2345-28-0 | 2-pentadecanone | 2021 | jasmine | ND | ND | ND | 9.43 ± 1.27 | 12.22 ± 1.68 |
| Esters | |||||||||
| 30 | 112-23-2 | heptyl formate | 1448 | cucumber | 3.79 ± 0.48 d | 11.92 ± 1.10 bc | 6.63 ± 0.52 cd | 16.58 ± 8.75 b | 35.14 ± 0.71 a |
| 31 | 112-32-3 | octyl formate | 1548 | cucumber | 7.90 ± 0.44 c | 13.03 ± 0.30 bc | 8.60 ± 0.93 c | 22.14 ± 1.63 b | 35.11 ± 2.09 a |
| 32 | 96-48-0 | γ-butyrolactone | 1618 | peach | 8.07 ± 1.85 a | 7.16 ± 0.48 a | 5.55 ± 2.28 a | ND | ND |
| 33 | 105-21-5 | γ-heptalactone | 1784 | coconut | ND | ND | ND | 5.20 ± 1.64 | 14.71 ± 0.26 |
| 34 | 104-50-7 | γ-octalactone | 1898 | coconut | ND | ND | ND | 3.81 ± 1.55 | 9.67 ± 0.60 |
| Alcohols | |||||||||
| 35 | 71-41-0 | 1-pentanol | 1244 | spicy | ND | ND | ND | ND | 23.20 ± 1.28 |
| 36 | 3391-86-4 | 1-octen-3-ol | 1430 | mushroom | ND | 4.94 ± 0.30 bc | 2.93 ± 0.12 c | 5.80 ± 2.44 b | 10.42 ± 0.29 a |
| Heterocycles | |||||||||
| 37 | 3777-69-3 | 2-pentylfuran | 1215 | fruity | 3.50 ± 0.25 c | 5.72 ± 0.52 bc | 4.70 ± 0.35 bc | 7.12 ± 2.65 b | 15.52 ± 0.08 a |
| 38 | 536-78-7 | 3-ethylpyridine | 1387 | hazelnut | ND | ND | ND | 2.58 ± 1.09 | 4.96 ± 0.56 |
| 39 | 2294-76-0 | 2-pentylpyridine | 1600 | fatty | 1.60 ± 0.16 c | 4.91 ± 0.39 b | 2.11 ± 0.29 c | 4.07±1.60 b | 7.32±0.61 a |
| Compound Information | Relative Content (ng/g)y | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | CAS | Component | RI x | Odor | 18 L/h | 48 L/h | 78 L/h | 108 L/h | 138 L/h |
| Aldehydes | |||||||||
| 1 | 590-86-3 | 3-methylbutanal | 941 | earthy | 32.37 ± 2.37 a | 33.60 ± 3.96 a | 21.59 ± 0.31 bc | 31.09 ± 0.03 ab | 14.60 ± 0.96 c |
| 2 | 110-62-3 | 1-pentanal | 962 | almond | 30.53 ± 7.07 b | 28.05 ± 5.48 b | 11.90 ± 0.79 c | 45.89 ± 9.92 a | 9.78 ± 1.08 c |
| 3 | 66-25-1 | 1-hexanal | 1063 | grassy | 85.38 ± 14.69 bc | 112.29 ± 24.13 b | 53.94 ± 10.72 c | 186.10 ± 34.37 a | 46.58 ± 6.24 c |
| 4 | 111-71-7 | 1-heptanal | 1182 | fatty | 40.90 ± 3.83 c | 88.09 ± 21.79 b | 22.66 ± 4.08 c | 118.91 ± 9.93 a | 28.81 ± 2.06 c |
| 5 | 124-13-0 | 1-octanal | 1274 | fatty | 21.81 ± 1.19 c | 40.42 ± 11.10 b | 11.41 ± 0.60 c | 62.87 ± 5.90 a | 22.60 ± 2.23 c |
| 6 | 18829-55-5 | (E)-2-heptenal | 1309 | fatty | ND | 23.97 ± 5.34 b | 14.01 ± 0.04 d | 41.31 ± 1.45 a | 21.30 ± 1.54 c |
| 7 | 124-19-6 | 1-nonanal | 1378 | fresh | 33.78 ± 8.71 c | 60.76 ± 0.07 b | 29.24 ± 0.76 c | 112.82 ± 4.04 a | 55.97 ± 5.05 b |
| 8 | 2548-87-0 | (E)-2-octenal | 1413 | cucumber | 2.86 ± 0.64 d | 7.27 ± 1.77 b | 5.40 ± 2.49 c | 14.71 ± 0.37 a | 7.53 ± 0.68 b |
| 9 | 100-52-7 | benzaldehyde | 1504 | nutty | 20.66 ± 6.36 a | 9.94 ± 4.68 b | 6.11 ± 3.31 b | 9.02 ± 0.36 b | 5.97 ± 0.77 b |
| 10 | 18829-56-6 | (E)-2-nonenal | 1518 | cucumber | ND | 7.88 ± 0.26 b | 8.01 ± 2.24 b | 18.68 ± 1.20 a | 9.21 ± 1.91 b |
| 11 | 3913-81-3 | (E)-2-decenal | 1639 | fatty | ND | ND | ND | 20.57 ± 2.17 | 22.21 ± 6.56 |
| 12 | 2463-77-6 | 2-undecenal | 1730 | orange peel | ND | ND | ND | 4.66 ± 3.59 | 7.37 ± 1.38 |
| 13 | 25152-84-5 | (E,E)-2,4-decadienal | 1744 | fried | ND | ND | ND | 5.11 ± 0.28 | 3.32 ± 0.18 |
| 14 | 122-40-7 | α-pentylcinnamaldehyde | 2245 | floral | ND | ND | ND | 30.97 ± 9.81 | 23.82 ± 0.73 |
| Sours | |||||||||
| 15 | 64-19-7 | acetic acid | 1440 | sour | 110.03 ± 5.23 b | 177.73 ± 10.71 a | 63.92 ± 1.65 d | 177.18 ± 13.18 a | 82.10 ± 5.34 c |
| 16 | 79-09-4 | propanoic acid | 1529 | sour | 14.51 ± 0.87 c | 25.38 ± 2.92 a | 12.55 ± 2.76 c | 20.55 ± 1.16 b | 11.99 ± 3.36 c |
| 17 | 503-74-2 | 3-methyl butyric acid | 1680 | sweaty | 109.05 ± 2.33 a | 42.32 ± 2.84 c | 30.63 ± 1.03 d | 60.72 ± 10.95 b | 10.82 ± 0.95 e |
| 18 | 79-31-2 | 2-methyl-hexanoic acid | 1700 | fruity | 128.79 ± 2.10 a | 36.48 ± 2.76 c | 38.40 ± 0.71 c | 59.17 ± 8.99 b | ND |
| 19 | 142-62-1 | hexanoic acid | 1836 | cheese | 9.81 ± 2.39 b | 7.16 ± 1.49 b | 13.41 ± 1.83 a | 15.11 ± 1.22 a | 7.04 ± 0.13 b |
| 20 | 124-07-2 | octanoic acid | 2051 | rot | 7.20 ± 3.07 c | 5.43 ± 0.16 d | 4.04 ± 0.72 e | 7.87 ± 2.23 b | 9.69 ± 0.56 a |
| 21 | 112-05-0 | nonanoic acid | 2144 | waxy | 6.74 ± 1.96 c | 19.20 ± 4.20 ab | 7.43 ± 1.68 c | 17.46 ± 9.66 ab | 21.30 ± 5.90 a |
| 22 | 334-48-5 | decanoic acid | 2262 | putrid | ND | 11.62 ± 3.72 a | 5.91 ± 1.31 c | 5.31 ± 1.57 d | 7.69 ± 1.70 b |
| Alcohols | |||||||||
| 23 | 71-41-0 | 1-pentanol | 1244 | spicy | 35.32 ± 0.87 b | 36.11 ± 5.77 b | 12.84 ± 1.00 c | 52.92 ± 9.17 a | ND |
| 24 | 3391-86-4 | 1-octen-3-ol | 1430 | mushroom | 6.83 ± 0.18 bc | 8.59 ± 1.00 b | 5.64 ± 0.73 cd | 15.42 ± 2.10 a | 3.99 ± 0.17 d |
| 25 | 60-12-8 | benzeneethanol | 1912 | floral | 28.71 ± 5.14 a | 12.20 ± 1.13 c | 17.66 ± 0.21 b | 19.99 ± 0.53 b | 9.58 ± 0.55 c |
| Esters | |||||||||
| 26 | 112-23-2 | heptyl formate | 1448 | cucumber | 8.00 ± 2.10 c | 14.62 ± 1.88 b | 4.62 ± 1.13 d | 19.92 ± 2.29 a | 5.86 ± 0.78 d |
| 27 | 96-48-0 | butyrolactone | 1618 | peach | 16.54 ± 2.65 b | 23.01 ± 3.32 a | 7.56 ± 1.00 c | 18.14 ± 3.22 b | 7.95 ± 0.48 c |
| 28 | 599-04-2 | d-(-)-pantolactone | 1998 | cotton candy | 9.54 ± 1.03 a | 5.89 ± 0.24 cd | 6.76 ± 0.36 bc | 7.20 ± 0.61 b | 4.86 ± 0.91 d |
| Others | |||||||||
| 29 | 128-37-0 | 4-methyl-2,6-di-tert-butylphenol | 1989 | camphor | 2.99 ± 1.12 a | 3.62 ± 1.58 a | 3.26 ± 1.48 a | 2.94 ± 0.97 a | 2.39 ± 0.36 a |
| Condition | Number | Aldehyde | Alcohol | Ester | Sour | Ketone | Heterocycle | Other |
|---|---|---|---|---|---|---|---|---|
| Temperature | 120 °C | 56.00% | 12.00% | 12.00% | 12.00% | 0.00% | 8.00% | 0.00% |
| 130 °C | 58.33% | 4.17% | 12.50% | 16.67% | 0.00% | 8.33% | 0.00% | |
| 140 °C | 60.00% | 4.00% | 4.00% | 16.00% | 0.00% | 12.00% | 0.00% | |
| 150 °C | 60.00% | 4.00% | 4.00% | 16.00% | 0.00% | 12.00% | 0.00% | |
| 160 °C | 60.00% | 4.00% | 4.00% | 16.00% | 0.00% | 12.00% | 0.00% | |
| Time | 25 min | 59.26% | 0.00% | 11.11% | 14.81% | 3.70% | 7.41% | 0.00% |
| 35 min | 51.72% | 3.45% | 10.34% | 20.69% | 3.45% | 6.90% | 0.00% | |
| 45 min | 50.00% | 6.67% | 10.00% | 20.00% | 6.67% | 6.67% | 0.00% | |
| 55 min | 41.67% | 5.56% | 11.11% | 16.67% | 16.67% | 8.33% | 0.00% | |
| 65 min | 38.89% | 8.33% | 11.11% | 16.67% | 16.67% | 8.33% | 0.00% | |
| Air velocity | 18 L/h | 36.36% | 13.64% | 13.64% | 31.82% | 0.00% | 0.00% | 4.55% |
| 48 L/h | 40.00% | 12.00% | 12.00% | 32.00% | 0.00% | 0.00% | 4.00% | |
| 78 L/h | 40.00% | 12.00% | 12.00% | 32.00% | 0.00% | 0.00% | 4.00% | |
| 108 L/h | 48.28% | 10.34% | 10.34% | 27.59% | 0.00% | 0.00% | 3.45% | |
| 138 L/h | 51.85% | 7.41% | 11.11% | 25.93% | 0.00% | 0.00% | 3.70% |
| Level | Factor | ||
|---|---|---|---|
| A Temperature/ °C | B Time/min | C Air Velocity/L·h−1 | |
| 1 | 140 | 25 | 78 |
| 0 | 150 | 35 | 108 |
| −1 | 160 | 45 | 138 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 6.54 | 9 | 0.73 | 27.61 | 0.0001 | significance |
| A: Temperature | 0.0032 | 1 | 0.0032 | 0.12 | 0.7375 | |
| B: Time | 0.63 | 1 | 0.63 | 23.85 | 0.0018 | |
| C: Air velocity | 2.2 | 1 | 2.2 | 83.83 | <0.0001 | |
| AB | 0 | 1 | 0 | 0 | 1 | |
| AC | 0 | 1 | 0 | 0 | 1 | |
| BC | 0 | 1 | 0 | 0 | 1 | |
| A2 | 0.29 | 1 | 0.29 | 10.99 | 0.0129 | |
| B2 | 0.75 | 1 | 0.75 | 28.51 | 0.0011 | |
| C2 | 2.35 | 1 | 2.35 | 89.33 | <0.0001 | |
| Residual | 0.18 | 7 | 0.026 | |||
| Lack of fit | 0 | 3 | 0 | 0 | 1 | no significance |
| Pure error | 0.18 | 4 | 0.046 | |||
| Cor total | 6.72 | 16 | ||||
| R2 = 0.9726; R2Adj = 0.9374 | ||||||
| Compound Information | Relative Content (ng/g) y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | CAS | Component | RI x | Odor | 0 Day | 3 Day | 6 Day | 9 Day | 12 Day | 15 Day |
| Aldehydes | ||||||||||
| 1 | 590-86-3 | 3-methylbutanal | 941 | earthy | 263.63 ± 24.09 b | 299.01 ± 21.64 ab | 308.86 ± 36.75 a | 207.17 ± 11.48 c | 177.67 ± 19.55 cd | 147.43 ± 0.94 d |
| 2 | 110-62-3 | 1-pentanal | 962 | almond | 27.23 ± 4.30 c | 83.19 ± 12.63 ab | 91.50 ± 6.15 a | 92.22 ± 1.41 a | 73.89 ± 5.41 b | 94.86 ± 7.69 a |
| 3 | 66-25-1 | 1-hexanal | 1063 | grassy | 217.41 ± 15.65 bc | 238.44 ± 20.54 bc | 259.96 ± 28.52 b | 217.62 ± 39.70 bc | 315.29 ± 12.72 a | 201.65 ± 1.88 c |
| 4 | 111-71-7 | 1-heptanal | 1182 | fatty | 223.38 ± 38.72 b | 285.09 ± 14.72 b | 387.00 ± 43.87 a | 411.84 ± 33.15 a | 418.97 ± 40.68 a | 439.12 ± 2.26 a |
| 5 | 124-13-0 | 1-octanal | 1274 | fatty | 161.51 ± 11.87 c | 205.33 ± 17.93 b | 264.49 ± 0.83 a | 292.47 ± 18.55 a | 272.33 ± 9.68 a | 291.44 ± 17.48 a |
| 6 | 124-19-6 | 1-nonanal | 1378 | fresh | 85.31 ± 5.89 c | 149.65 ± 20.19 b | 124.06 ± 5.30 bc | 106.56 ± 15.58 c | 219.22 ± 13.82 a | 252.59 ± 26.67 a |
| 7 | 100-52-7 | benzaldehyde | 1504 | nutty | 71.66 ± 15.75 c | 67.19 ± 5.52 c | 70.21 ± 12.28 c | 51.73 ± 9.53 c | 114.50 ± 11.16 b | 148.00 ± 5.25 a |
| 8 | 18829-56-6 | (E)-2-nonenal | 1518 | cucumber | ND | ND | ND | 28.64 ± 3.19 b | 46.07 ± 2.09 a | 48.28 ± 4.05 a |
| 9 | 122-78-1 | phenylacetaldehyde | 1648 | sweet | 28.16 ± 2.13 bc | 31.95 ± 6.09 bc | 50.92 ± 8.44 a | 12.49 ± 0.49 a | 11.93 ± 0.98 b | 20.38 ± 6.25 c |
| 10 | 3913-81-3 | (E)-2-decenal | 1639 | fatty | 22.74 ± 3.15 abc | 11.87 ± 5.96 c | 22.45 ± 5.92 abc | 13.38 ± 4.43 bc | 26.40 ± 0.18 ab | 27.29 ± 8.83 a |
| 11 | 2463-77-6 | 2-undecenal | 1730 | orange peel | ND | 14.92 ± 6.00 b | 12.84 ± 6.12 b | 13.75 ± 4.21 b | 16.32 ± 6.15 a | 13.54 ± 5.78 b |
| Sours | ||||||||||
| 12 | 64-19-7 | acetic acid | 1440 | sour | 1990.12 ± 276.50 ab | 2271.87 ± 95.35 a | 2082.05 ± 16.03 a | 2194.89 ± 61.30 a | 1654.51 ± 40.09 bc | 1528.08 ± 31.3 c |
| 13 | 79-09-4 | propanoic acid | 1529 | sour | 48.65 ± 9.81 b | 79.32 ± 6.34 a | 77.86 ± 5.21 a | 79.80 ± 6.32 a | 70.94 ± 11.79 a | 68.61 ± 6.18 a |
| 14 | 107-92-6 | butanoic acid | 1628 | sour | 32.84 ± 3.76 ab | 30.88 ± 2.64 ab | 32.27 ± 1.14 ab | 34.98 ± 3.49 a | 25.80 ± 1.00 b | 28.24 ± 4.52 ab |
| 15 | 503-74-2 | 3-methylbutyricacid | 1680 | sweaty | 127.84 ± 4.28 bc | 168.12 ± 0.85 a | 146.98 ± 19.08 ab | 144.41 ± 4.81 abc | 128.59 ± 21.52 bc | 115.52 ± 1.67 c |
| 16 | 142-62-1 | hexanoic acid | 1836 | cheese | 23.69 ± 6.23 d | 37.02 ± 2.71 cd | 50.68 ± 9.91 bc | 68.71 ± 4.30 ab | 79.06 ± 13.98 a | 81.04 ± 6.54 a |
| 17 | 111-14-8 | heptanoic acid | 1940 | sweaty | ND | 14.30 ± 1.93 a | 15.81 ± 4.67 a | 14.18 ± 4.68 a | 21.99 ± 10.31 a | 19.11 ± 1.25 a |
| 18 | 124-07-2 | octanoic acid | 2051 | rot | 21.85 ± 5.97 a | 19.77 ± 4.76 a | 27.91 ± 5.92 a | 24.94 ± 6.66 a | 28.22 ± 1.90 a | 28.37 ± 3.64 a |
| Sours | ||||||||||
| 19 | 112-05-0 | nonanoic acid | 2144 | waxy | 17.86 ± 6.85 b | 66.92 ± 5.62 a | 56.05 ± 8.26 a | 21.78 ± 9.12 b | 25.71 ± 0.55 b | 17.31 ± 2.71 b |
| 20 | 334-48-5 | decanoic acid | 2262 | putrid | 21.21 ± 2.45 bc | 7.93 ± 2.58 d | 13.76 ± 3.79 cd | 17.47 ± 6.44 bcd | 31.86 ± 6.59 a | 24.81 ± 5.64 ab |
| Alcohols | ||||||||||
| 21 | 71-41-0 | 1-pentanol | 1244 | spicy | 62.61 ± 8.12 b | 76.58 ± 7.52 a | 65.40 ± 2.83 b | 59.88 ± 2.95 b | 64.89 ± 0.98 b | 44.14 ± 4.34 c |
| 22 | 111-27-3 | 1-hexanol | 1345 | fresh | 30.56 ± 5.78 a | 29.70 ± 7.76 a | 32.04 ± 5.09 a | 33.55 ± 0.27 a | 32.48 ± 8.31 a | 29.88 ± 0.37 a |
| 23 | 3391-86-4 | 1-octen-3-ol | 1430 | mushroom | 11.10 ± 2.87 b | 15.66 ± 1.27 ab | 18.47 ± 4.01 ab | 18.97 ± 6.30 ab | 22.69 ± 3.47 a | 21.42 ± 1.17 a |
| 24 | 513-85-9 | 2,3-butanediol | 1570 | butter | 36.02 ± 0.80 b | 89.88 ± 2.78 a | 81.31 ± 16.25 a | 79.44 ± 9.25 a | 50.38 ± 3.92 b | 53.84 ± 6.28 b |
| 25 | 60-12-8 | benzeneethanol | 1912 | floral | 35.38 ± 18.36 b | 45.98 ± 1.91 ab | 52.89 ± 0.41 a | 60.01 ± 1.10 a | 57.96 ± 6.15 a | 51.97 ± 5.38 ab |
| Esters | ||||||||||
| 26 | 112-23-2 | heptyl formate | 1448 | cucumber | 39.43 ± 4.76 b | 57.00 ± 3.50 ab | 57.40 ± 11.47 ab | 62.09 ± 12.97 a | 60.43 ± 12.68 ab | 52.36 ± 1.06 ab |
| 27 | 112-32-3 | octyl formate | 1548 | cucumber | 29.45 ± 11.21 a | 29.64 ± 3.49 a | 36.26 ± 12.20 a | 31.86 ± 5.65 a | 39.77 ± 1.08 a | 40.49 ± 4.83 a |
| 28 | 96-48-0 | butyrolactone | 1618 | peach | 69.99 ± 9.03 b | 89.14 ± 7.29 a | 83.63 ± 10.08 ab | 85.87 ± 6.07 a | 74.12 ± 6.86 ab | 70.37 ± 2.46 b |
| 29 | 599-04-2 | d-(-)-pantolactone | 1998 | cotton candy | 9.29 ± 0.45 b | 11.64 ± 0.50 ab | 11.37 ± 2.61 ab | 13.90 ± 2.30 a | 12.50 ± 1.04 ab | 11.58 ± 1.30 ab |
| Heterocyclics | ||||||||||
| 30 | 3777-69-3 | 2-pentylfuran | 1215 | fruity | ND | ND | 14.09 ± 5.89 b | 25.42 ± 0.06 ab | 33.88 ± 7.46 a | 39.43 ± 12.59 a |
| 31 | 98-00-0 | 2-furanmethanol | 1635 | burnt | 12.02 ± 2.35 b | 14.88 ± 0.37 a | 12.50 ± 1.82 ab | 52.98 ± 9.53 ab | 35.60 ± 0.49 b | 10.50 ± 1.06 b |
| Odor Type | Weight |
|---|---|
| Milky | 0.3 |
| Muttony | 0.2 |
| Animal fat | 0.2 |
| Sweet | 0.3 |
| Fruit | 0.2 |
| Burnt | 0.1 |
| Spoiled | −0.2 |
| Sour | −0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Raza, J.; Song, H.; Wang, L.; Wang, Q.; Ma, G.; Xiao, Y. Optimization and Preparation of Tallow with a Strong Aroma by Mild Oxidation. Molecules 2022, 27, 9047. https://doi.org/10.3390/molecules27249047
Jin Y, Raza J, Song H, Wang L, Wang Q, Ma G, Xiao Y. Optimization and Preparation of Tallow with a Strong Aroma by Mild Oxidation. Molecules. 2022; 27(24):9047. https://doi.org/10.3390/molecules27249047
Chicago/Turabian StyleJin, Yanjing, Junaid Raza, Huanlu Song, Lijin Wang, Qiaojun Wang, Guoli Ma, and Yang Xiao. 2022. "Optimization and Preparation of Tallow with a Strong Aroma by Mild Oxidation" Molecules 27, no. 24: 9047. https://doi.org/10.3390/molecules27249047
APA StyleJin, Y., Raza, J., Song, H., Wang, L., Wang, Q., Ma, G., & Xiao, Y. (2022). Optimization and Preparation of Tallow with a Strong Aroma by Mild Oxidation. Molecules, 27(24), 9047. https://doi.org/10.3390/molecules27249047