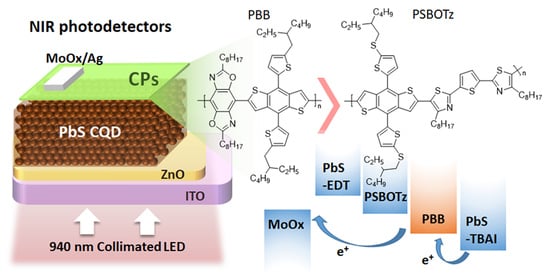
Improvement of Dynamic Performance and Detectivity in Near-Infrared Colloidal Quantum Dot Photodetectors by Incorporating Conjugated Polymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.2. Static Properties of QPDs
2.3. Dynamic Properties of QPDs
2.4. Morphological Characterization
3. Materials and Methods
3.1. Materials Synthesis
3.2. Device Fabrication
3.3. Device Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lan, Z.; Lei, Y.; Chan, W.K.E.; Chen, S.; Luo, D.; Zhu, F. Near-infrared and visible light dual-mode organic photodetectors. Sci. Adv. 2020, 6, eaaw8065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Guo, J.; Yu, L.; Li, J.; Zhang, M.; Li, H.; Shi, Y.; Dai, D. Silicon/2D-material photodetectors: From near-infrared to mid-infrared. Light Sci. Appl. 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Wang, S.; Li, T.; Jing, X.; Liu, Y.; Wang, Y.; Wen, H.; Yao, M.; Zhan, X.; et al. Fast Response Organic Tandem Photodetector for Visible and Near-Infrared Digital Optical Communications. Small 2021, 17, e2101316. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, C.; Guo, Y.; Yang, Y.; Xing, Y.; Que, W. PbS QD-based photodetectors: Future-oriented near-infrared detection technology. J. Mater. Chem. C 2021, 9, 417–438. [Google Scholar] [CrossRef]
- Ma, N.; Jiang, J.; Zhao, Y.; He, L.; Ma, Y.; Wang, H.; Zhang, L.; Shan, C.; Shen, L.; Hu, W. Stable and sensitive tin-lead perovskite photodetectors enabled by azobenzene derivative for near-infrared acousto-optic conversion communications. Nano Energy 2021, 86, 106113. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Liu, Y. Exploration of Near-Infrared Organic Photodetectors. Chem. Mater. 2019, 31, 6359–6379. [Google Scholar] [CrossRef]
- Zhao, X.; Song, L.; Zhao, R.; Tan, M.C. High-Performance and Flexible Shortwave Infrared Photodetectors Using Composites of Rare Earth-Doped Nanoparticles. ACS App. Mater. Interfaces 2019, 11, 2344–2351. [Google Scholar] [CrossRef]
- Kang, J.; Oh, J.T.; Kim, H.; Kim, H.; Choi, H.; Jung, I.H. Environmentally friendly AgBiS2 nanocrystal-based high-performance quantum-dot photodetectors. Appl. Surf. Sci. 2022, 597, 153661. [Google Scholar] [CrossRef]
- Virgili, T.; Ganzer, L.; Botta, C.; Squeo, B.M.; Pasini, M. Asymmetric AZA-BODIPY with Optical Gain in the Near-Infrared Region. Molecules 2022, 27, 4538. [Google Scholar] [CrossRef]
- Huo, Z.; Zhang, Y.; Han, X.; Wu, W.; Yang, W.; Wang, X.; Zhou, M.; Pan, C. Piezo-phototronic effect enhanced performance of a p-ZnO NW based UV–Vis–NIR photodetector. Nano Energy 2021, 86, 106090. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Yue, C.; Tang, X.; Gao, Z.; Jiang, Y.; Du, C.; Deng, Z.; Jia, H.; Wang, W.; et al. High performance visible-SWIR flexible photodetector based on large-area InGaAs/InP PIN structure. Sci. Rep. 2022, 12, 7681. [Google Scholar] [CrossRef] [PubMed]
- Vafaie, M.; Fan, J.Z.; Morteza Najarian, A.; Ouellette, O.; Sagar, L.K.; Bertens, K.; Sun, B.; García de Arquer, F.P.; Sargent, E.H. Colloidal quantum dot photodetectors with 10-ns response time and 80% quantum efficiency at 1,550 nm. Matter 2021, 4, 1042–1053. [Google Scholar] [CrossRef]
- Ren, H.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent Progress in Organic Photodetectors and their Applications. Adv. Sci. 2021, 8, 2002418. [Google Scholar] [CrossRef]
- Jeong, W.; Kang, J.; Lim, S.Y.; Ahn, H.; Kim, H.M.; Won, J.H.; Jung, I.H. Spontaneously Induced Hierarchical Structure by Surface Energy in Novel Conjugated Polymer-Based Ultrafast-Response Organic Photodetectors. Adv. Opt. Mater. 2022, 10, 2102607. [Google Scholar] [CrossRef]
- Jang, H.; Seok, Y.; Choi, Y.; Cho, S.-H.; Watanabe, K.; Taniguchi, T.; Lee, K. High-Performance Near-Infrared Photodetectors Based on Surface-Doped InSe. Adv. Funct. Mater. 2021, 31, 2006788. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; Smeets, D.; De Muynck, D.; Nollet, T.; Martins, J.C.; Vanhaecke, F.; Vantomme, A.; Delerue, C.; Allan, G.; et al. Size-Dependent Optical Properties of Colloidal PbS Quantum Dots. ACS Nano 2009, 3, 3023–3030. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Osedach, T.P.; Chang, L.-Y.; Geyer, S.M.; Wanger, D.; Binda, M.T.; Arango, A.C.; Bawendi, M.G.; Bulovic, V. Colloidal PbS Quantum Dot Solar Cells with High Fill Factor. ACS Nano 2010, 4, 3743–3752. [Google Scholar] [CrossRef]
- Tang, J.; Kemp, K.W.; Hoogland, S.; Jeong, K.S.; Liu, H.; Levina, L.; Furukawa, M.; Wang, X.; Debnath, R.; Cha, D.; et al. Colloidal-quantum-dot photovoltaics using atomic-ligand passivation. Nat. Mater. 2011, 10, 765–771. [Google Scholar] [CrossRef]
- Klem, E.J.D.; MacNeil, D.D.; Cyr, P.W.; Levina, L.; Sargent, E.H. Efficient solution-processed infrared photovoltaic cells: Planarized all-inorganic bulk heterojunction devices via inter-quantum-dot bridging during growth from solution. Appl. Phys. Lett. 2007, 90, 183113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Du, S.; Tian, F.; Long, X.; Xie, S.; Tang, S.; Bao, L. Silver Nanoparticle-Functionalised Nitrogen-Doped Carbon Quantum Dots for the Highly Efficient Determination of Uric Acid. Molecules 2022, 27, 4586. [Google Scholar] [CrossRef]
- Yaghmur, A.; Hamad, I. Microfluidic Nanomaterial Synthesis and In Situ SAXS, WAXS, or SANS Characterization: Manipulation of Size Characteristics and Online Elucidation of Dynamic Structural Transitions. Molecules 2022, 27, 4602. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ren, Z.; Zhang, A.; Mao, P.; Li, H.; Zhong, X.; Li, W.; Yang, S.; Wang, J. Hybrid Organic/PbS Quantum Dot Bilayer Photodetector with Low Dark Current and High Detectivity. Adv. Funct. Mater. 2018, 28, 1706690. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, J.; Qin, R.; Yang, G.; Yu, J. Efficient Visible-Near-Infrared Hybrid Perovskite: PbS Quantum Dot Photodetectors Fabricated Using an Antisolvent Additive Solution Process. Adv. Opt. Mater. 2018, 6, 1800979. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xu, J.L.; Chen, T.; Gao, X.; Wang, S.D. Toward Broadband Imaging: Surface-Engineered PbS Quantum Dot/Perovskite Composite Integrated Ultrasensitive Photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 44430–44437. [Google Scholar] [CrossRef]
- Kang, J.; Park, D.; Jeong, M.-K.; Kim, J.; Eom, S.H.; Jang, S.-Y.; Yim, S.; Jung, I.H. Improved Performance of Quantum-Dot Photodetectors Using Cheap and Environmentally Friendly Polyethylene Glycol. Adv. Mater. Interfaces 2019, 6, 1801666. [Google Scholar] [CrossRef]
- Jeong, M.-K.; Kang, J.; Park, D.; Yim, S.; Jung, I.H. A conjugated polyelectrolyte interfacial modifier for high performance near-infrared quantum-dot photodetectors. J. Mater. Chem. C 2020, 8, 2542–2550. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, J.; Li, H.; Mao, P.; Wei, Y.; Zhong, X.; Hu, J.; Yang, S.; Wang, J. Bilayer PbS Quantum Dots for High-Performance Photodetectors. Adv. Mater. 2017, 29, 1702055. [Google Scholar] [CrossRef]
- Chuang, C.H.; Brown, P.R.; Bulovic, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Zhu, Y.; Huang, J.; Xu, X.; Cui, S.; Lu, Z. Hole transport layer selection toward efficient colloidal PbS quantum dot solar cells. Opt. Express 2019, 27, A1338–A1349. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, J.; Choi, M.J.; Ryu, S.U.; Choi, K.; Lee, S.; Hoogland, S.; Arquer, F.P.G.; Sargent, E.H.; Park, T. Efficient and Stable Colloidal Quantum Dot Solar Cells with a Green-Solvent Hole-Transport Layer. Adv. Energy Mater. 2020, 10, 2002084. [Google Scholar] [CrossRef]
- Kim, H.I.; Baek, S.W.; Cheon, H.J.; Ryu, S.U.; Lee, S.; Choi, M.J.; Choi, K.; Biondi, M.; Hoogland, S.; de Arquer, F.P.G.; et al. A Tuned Alternating D-A Copolymer Hole-Transport Layer Enables Colloidal Quantum Dot Solar Cells with Superior Fill Factor and Efficiency. Adv. Mater. 2020, 32, e2004985. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yang, F.; Yuan, J.; Zhang, Y.; Gu, M.; Xu, Y.; Ling, X.; Wang, Y.; Li, F.; Zhai, T.; et al. Toward Scalable PbS Quantum Dot Solar Cells Using a Tailored Polymeric Hole Conductor. ACS Energy Lett. 2019, 4, 2850–2858. [Google Scholar] [CrossRef]
- Wu, J.-S.; Cheng, S.-W.; Cheng, Y.-J.; Hsu, C.-S. Donor–acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 2015, 44, 1113–1154. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Z.; Sun, Y. Polymer Donors for High-Performance Non-Fullerene Organic Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 4442–4453. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jeong, M.-K.; Suh, E.H.; Jeong, W.; Oh, J.G.; Jang, J.; Jung, I.H. Rational Design of Highly Soluble and Crystalline Conjugated Polymers for High-Performance Field-Effect Transistors. Adv. Electron. Mater. 2022, 8, 2101105. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Harbuzaru, A.; Donoso, B.; Prieto, P.; Ponce Ortiz, R.; Díaz-Ortiz, Á. Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors. Molecules 2022, 27, 4340. [Google Scholar] [CrossRef]
- Mubarok, M.A.; Wibowo, F.T.A.; Aqoma, H.; Vamsi Krishna, N.; Lee, W.; Ryu, D.Y.; Cho, S.; Jung, I.H.; Jang, S.-Y. PbS-Based Quantum Dot Solar Cells with Engineered π-Conjugated Polymers Achieve 13% Efficiency. ACS Energy Lett. 2020, 5, 3452–3460. [Google Scholar] [CrossRef]
- Son, S.Y.; Choi, K.; Lee, J.; Kim, H.I.; Park, T.; Kim, M. Backbone Randomization in Conjugated Polymer-Based Hole-Transport Materials to Enhance the Efficiencies of Perovskite Solar Cells. Chem. Mater. 2022, 34, 4856–4864. [Google Scholar] [CrossRef]
- Zha, J.-W.; Zheng, M.-S.; Fan, B.-H.; Dang, Z.-M. Polymer-based dielectrics with high permittivity for electric energy storage: A review. Nano Energy 2021, 89, 106438. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, K.; Han, L.; Liu, Z.; Shi, G.; Fang, H.; Chen, S.; Wu, T.; Yang, F.; Gu, M.; et al. In Situ Passivation for Efficient PbS Quantum Dot Solar Cells by Precursor Engineering. Adv. Mater. 2018, 30, e1704871. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.; Won, J.H.; Ahn, H.; Kim, J.; Yoon, S.C.; Lim, E.; Jung, I.H. Enhanced Static and Dynamic Properties of Highly Miscible Fullerene-Free Green-Selective Organic Photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 25164–25174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, J.; Park, Y.-S.; Ahn, H.; Eom, S.H.; Jang, S.-Y.; Jung, I.H. Alkylthiazole-based semicrystalline polymer donors for fullerene-free organic solar cells. Polym. Chem. 2019, 10, 4314–4321. [Google Scholar] [CrossRef]
- Song, J.H.; Mai, X.D.; Jeong, S.; Kim, Y.H. Hysteresis and Photoinstability Caused by Mobile Ions in Colloidal Quantum Dot Photovoltaics. J. Phys. Chem. Lett. 2017, 8, 5259–5263. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.-T.; Duong, N.-H.; Mai, X.-D. Effect of chloride treatment on optical and electrical properties of PbS quantum dots. Chem. Phys. 2020, 538, 110895. [Google Scholar] [CrossRef]
- Rohr, J.A.; Moia, D.; Haque, S.A.; Kirchartz, T.; Nelson, J. Exploring the validity and limitations of the Mott-Gurney law for charge-carrier mobility determination of semiconducting thin-films. J. Phys. Condens. Matter. 2018, 30, 105901. [Google Scholar] [CrossRef] [Green Version]
- Simone, G.; Dyson, M.J.; Weijtens, C.H.L.; Meskers, S.C.J.; Coehoorn, R.; Janssen, R.A.J.; Gelinck, G.H. On the Origin of Dark Current in Organic Photodiodes. Adv. Opt. Mater. 2019, 8, 1901568. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xing, X.; Zheng, L.; Chen, Z.; Xiao, L.; Qu, B.; Gong, Q. Vertical phase separation in bulk heterojunction solar cells formed by in situ polymerization of fulleride. Sci. Rep. 2014, 4, 5071. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, J.; Ullah, M.I.; Xia, Y.; Liang, G.; Wang, S.; Zhang, J.; Hsu, H.Y.; Song, H.; Tang, J. Enhanced Passivation and Carrier Collection in Ink-Processed PbS Quantum Dot Solar Cells via a Supplementary Ligand Strategy. ACS Appl. Mater. Interfaces 2020, 12, 42217–42225. [Google Scholar] [CrossRef]
- Sun, C.; Pan, F.; Chen, S.; Wang, R.; Sun, R.; Shang, Z.; Qiu, B.; Min, J.; Lv, M.; Meng, L.; et al. Achieving Fast Charge Separation and Low Nonradiative Recombination Loss by Rational Fluorination for High-Efficiency Polymer Solar Cells. Adv. Mater. 2019, 31, e1905480. [Google Scholar] [CrossRef]
- Cui, F.Z.; Chen, Z.H.; Qiao, J.W.; Wang, T.; Lu, G.H.; Yin, H.; Hao, X.T. Ternary-Assisted Sequential Solution Deposition Enables Efficient All-Polymer Solar Cells with Tailored Vertical-Phase Distribution. Adv. Funct. Mater. 2022, 32, 2200478. [Google Scholar] [CrossRef]
- Kim, H.; Kang, J.; Ahn, H.; Jung, I.H. Contribution of dark current density to the photodetecting properties of thieno[3,4-b]pyrazine-based low bandgap polymers. Dyes Pigm. 2022, 197, 109910. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Zhao, Z.; Sun, Y.; Liu, M.; Zhou, Z.; Zhang, X.; Zhang, F. Highly sensitive photomultiplication type polymer photodetectors by manipulating interfacial trapped electron density. Chem. Eng. J. 2022, 435, 134973. [Google Scholar] [CrossRef]
- He, J.; Liu, H.; Huang, C.; Jia, Y.; Li, K.; Mesli, A.; Yang, R.; He, Y.; Dan, Y. Analytical Transient Responses and Gain-Bandwidth Products of Low-Dimensional High-Gain Photodetectors. ACS Nano 2021, 15, 20242–20252. [Google Scholar] [CrossRef] [PubMed]
- Dolatyari, M.; Rostami, A.; Mathur, S.; Klein, A. Trap engineering in solution processed PbSe quantum dots for high-speed MID-infrared photodetectors. J. Mater. Chem. C 2019, 7, 5658–5669. [Google Scholar] [CrossRef]
- Xu, K.; Sun, H.; Ruoko, T.-P.; Wang, G.; Kroon, R.; Kolhe, N.B.; Puttisong, Y.; Liu, X.; Fazzi, D.; Shibata, K.; et al. Ground-state electron transfer in all-polymer donor–acceptor heterojunctions. Nat. Mater. 2020, 19, 738–744. [Google Scholar] [CrossRef]






| Pin a (mW/cm2) | Jd (A/cm2) | Jph (A/cm2) | R (A/W) | D* (Jones) | Bias (V) | |
|---|---|---|---|---|---|---|
| PSBOTz/PbS-TBAI | 0.007 | 3.05 × 10−8 | 1.22 × 10−6 | 0.175 | 1.77 × 1012 | −1 |
| 0.103 | 3.05 × 10−8 | 1.49 × 10−5 | 0.144 | 1.46 × 1012 | ||
| 0.500 | 3.05 × 10−8 | 6.21 × 10−5 | 0.124 | 1.26 × 1012 | ||
| 1.00 | 3.05 × 10−8 | 1.17 × 10−4 | 0.117 | 1.18 × 1012 | ||
| 5.00 | 3.05 × 10−8 | 5.04 × 10−4 | 0.101 | 1.02 × 1012 | ||
| PBB/PbS-TBAI | 0.007 | 1.90 × 10−8 | 1.54 × 10−6 | 0.220 | 2.82 × 1012 | −1 |
| 0.102 | 1.90 × 10−8 | 1.88 × 10−5 | 0.183 | 2.34 × 1012 | ||
| 0.500 | 1.90 × 10−8 | 8.13 × 10−5 | 0.163 | 2.08 × 1012 | ||
| 1.00 | 1.90 × 10−8 | 1.56 × 10−4 | 0.156 | 2.00 × 1012 | ||
| 5.00 | 1.90 × 10−8 | 6.89 × 10−4 | 0.138 | 1.77 × 1012 | ||
| PbS-EDT/PbS-TBAI | 0.007 | 2.19 × 10−7 | 1.52 × 10−6 | 0.217 | 8.24 × 1011 | −1 |
| 0.102 | 2.19 × 10−7 | 1.72 × 10−5 | 0.169 | 6.37 × 1011 | ||
| 0.500 | 2.19 × 10−7 | 7.18 × 10−5 | 0.145 | 5.46 × 1011 | ||
| 1.00 | 2.19 × 10−7 | 1.32 × 10−4 | 0.132 | 4.97 × 1011 | ||
| 5.00 | 2.19 × 10−7 | 5.82 × 10−4 | 0.116 | 4.39 × 1011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.I.; Kang, J.; Park, J.; Jeong, W.; Kim, J.; Yim, S.; Jung, I.H. Improvement of Dynamic Performance and Detectivity in Near-Infrared Colloidal Quantum Dot Photodetectors by Incorporating Conjugated Polymers. Molecules 2022, 27, 7660. https://doi.org/10.3390/molecules27217660
Kim MI, Kang J, Park J, Jeong W, Kim J, Yim S, Jung IH. Improvement of Dynamic Performance and Detectivity in Near-Infrared Colloidal Quantum Dot Photodetectors by Incorporating Conjugated Polymers. Molecules. 2022; 27(21):7660. https://doi.org/10.3390/molecules27217660
Chicago/Turabian StyleKim, Myeong In, Jinhyeon Kang, Jaehee Park, WonJo Jeong, Junho Kim, Sanggyu Yim, and In Hwan Jung. 2022. "Improvement of Dynamic Performance and Detectivity in Near-Infrared Colloidal Quantum Dot Photodetectors by Incorporating Conjugated Polymers" Molecules 27, no. 21: 7660. https://doi.org/10.3390/molecules27217660