Synergistic Catalytic Performance of Toluene Degradation Based on Non-Thermal Plasma and Mn/Ce-Based Bimetal-Organic Frameworks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization and Analysis of MCDx
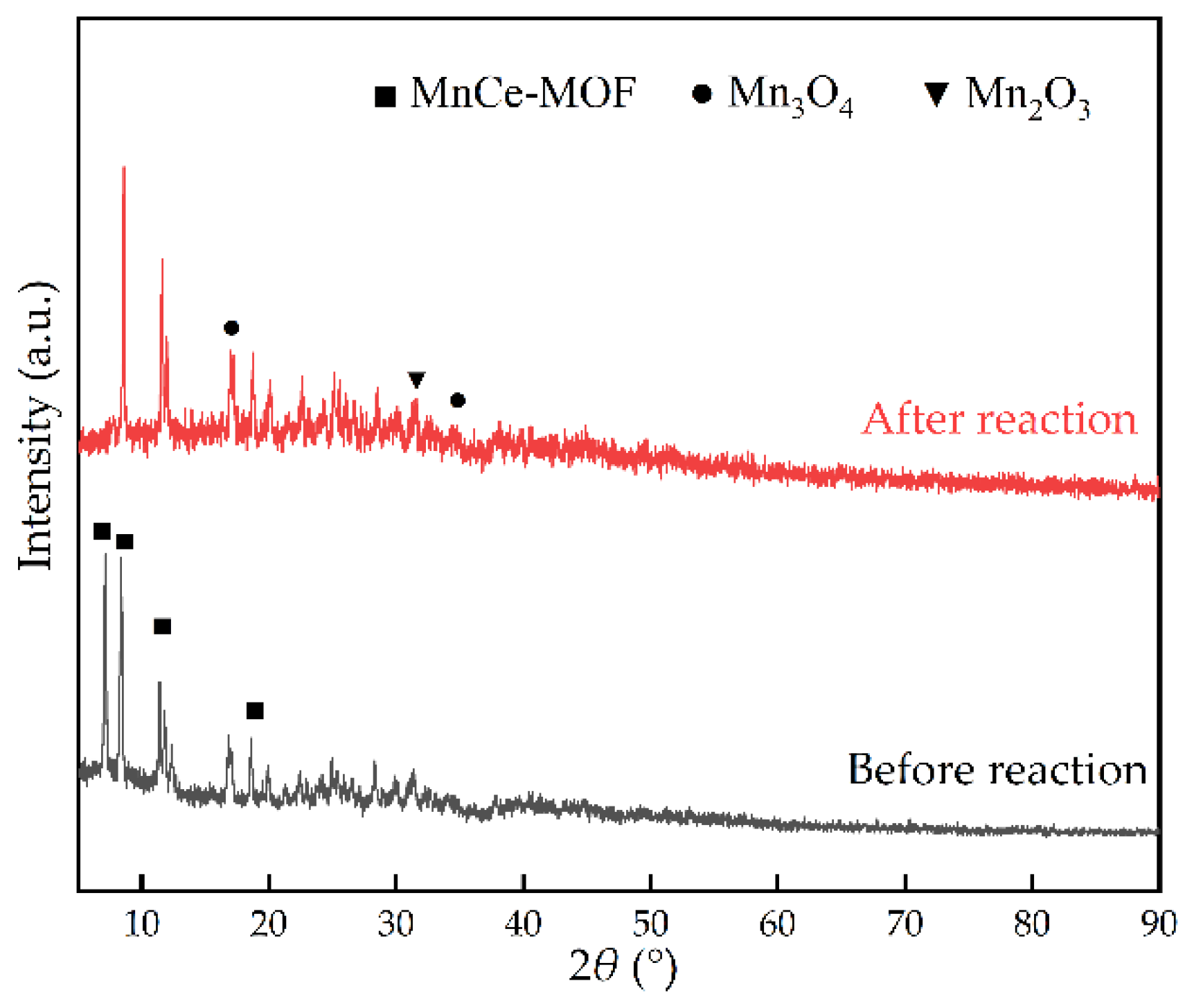
2.1.1. Powder X-ray Diffraction (PXRD) Analysis
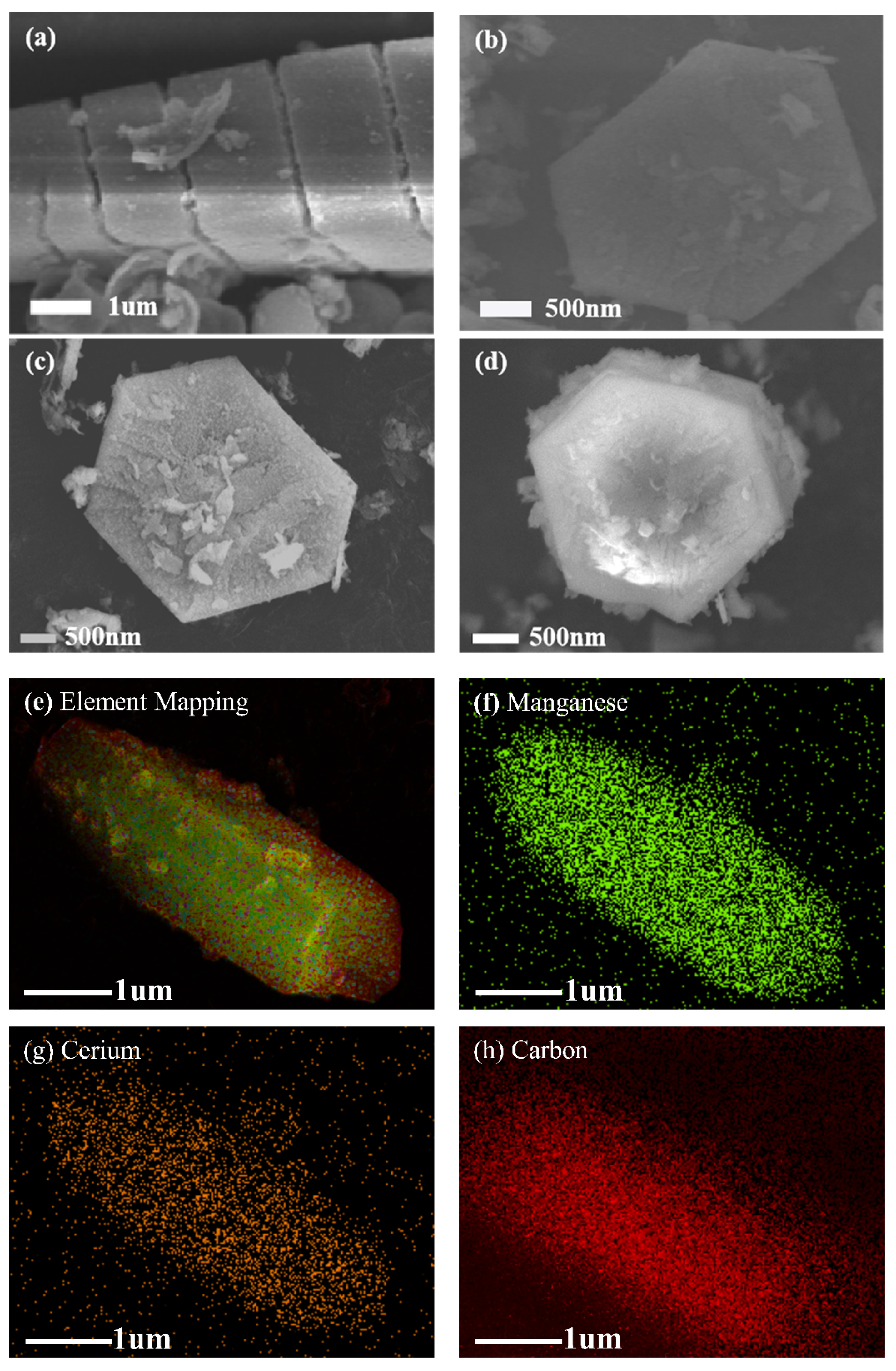
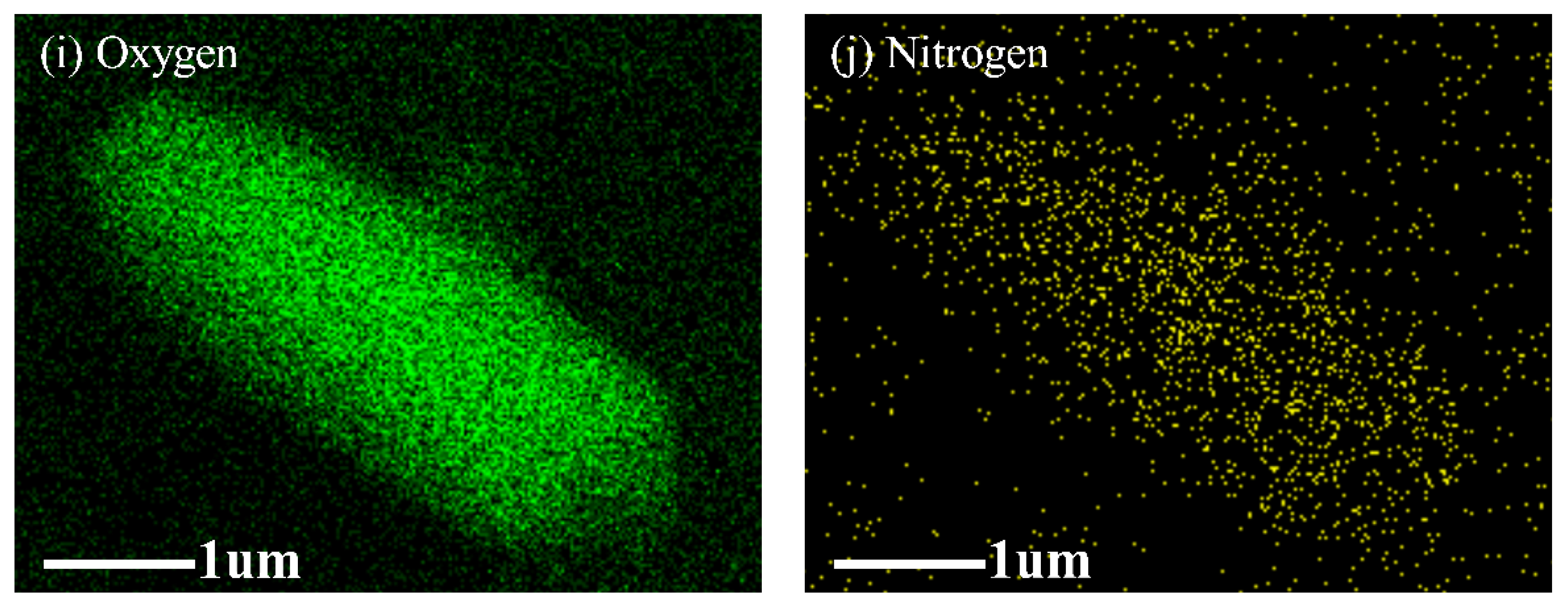
2.1.2. Scanning Electron Microscopy (SEM) Analysis
2.1.3. X-ray Photoelectron Spectroscopy (XPS)
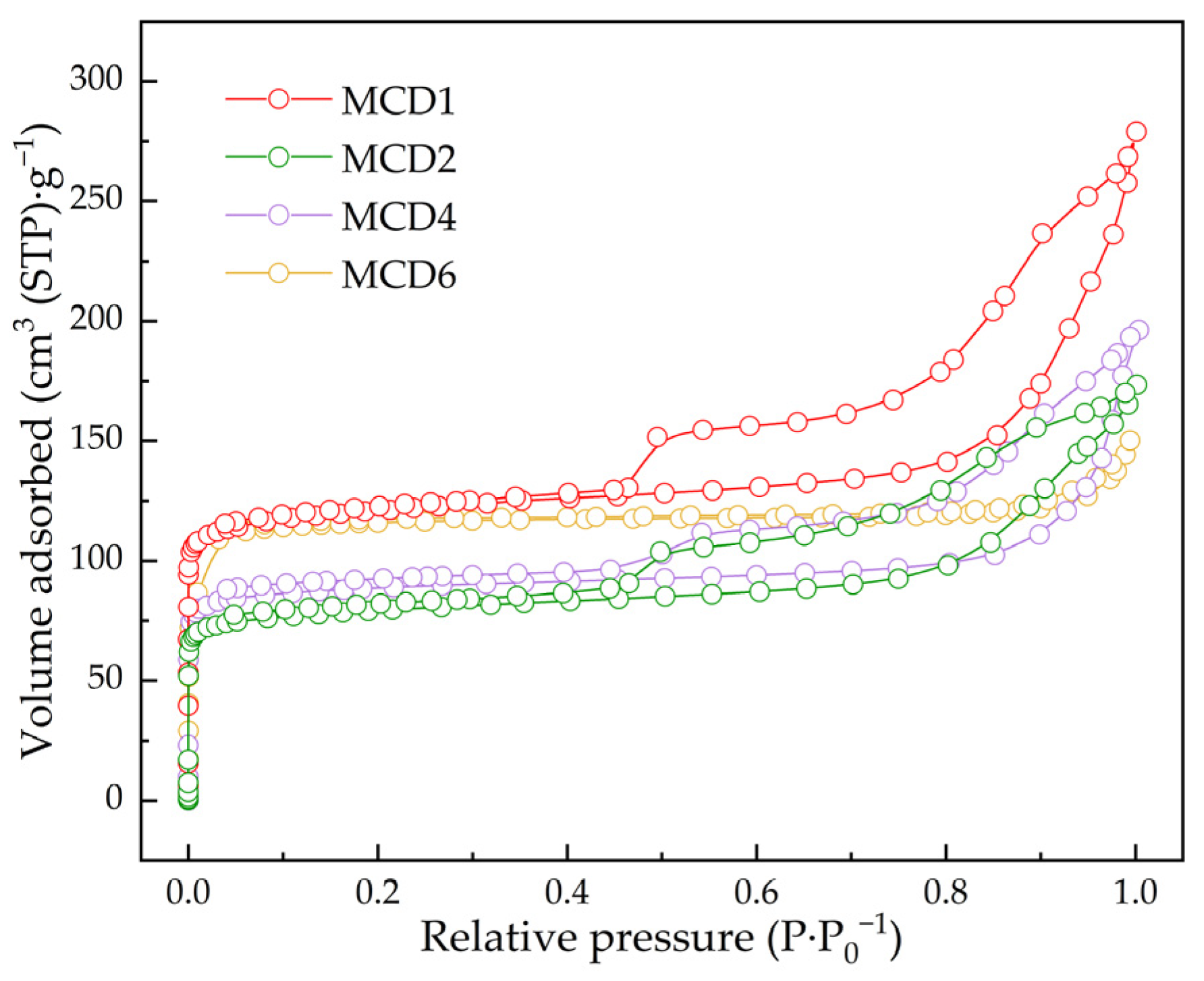
2.1.4. N2 Adsorption–Desorption Measurement
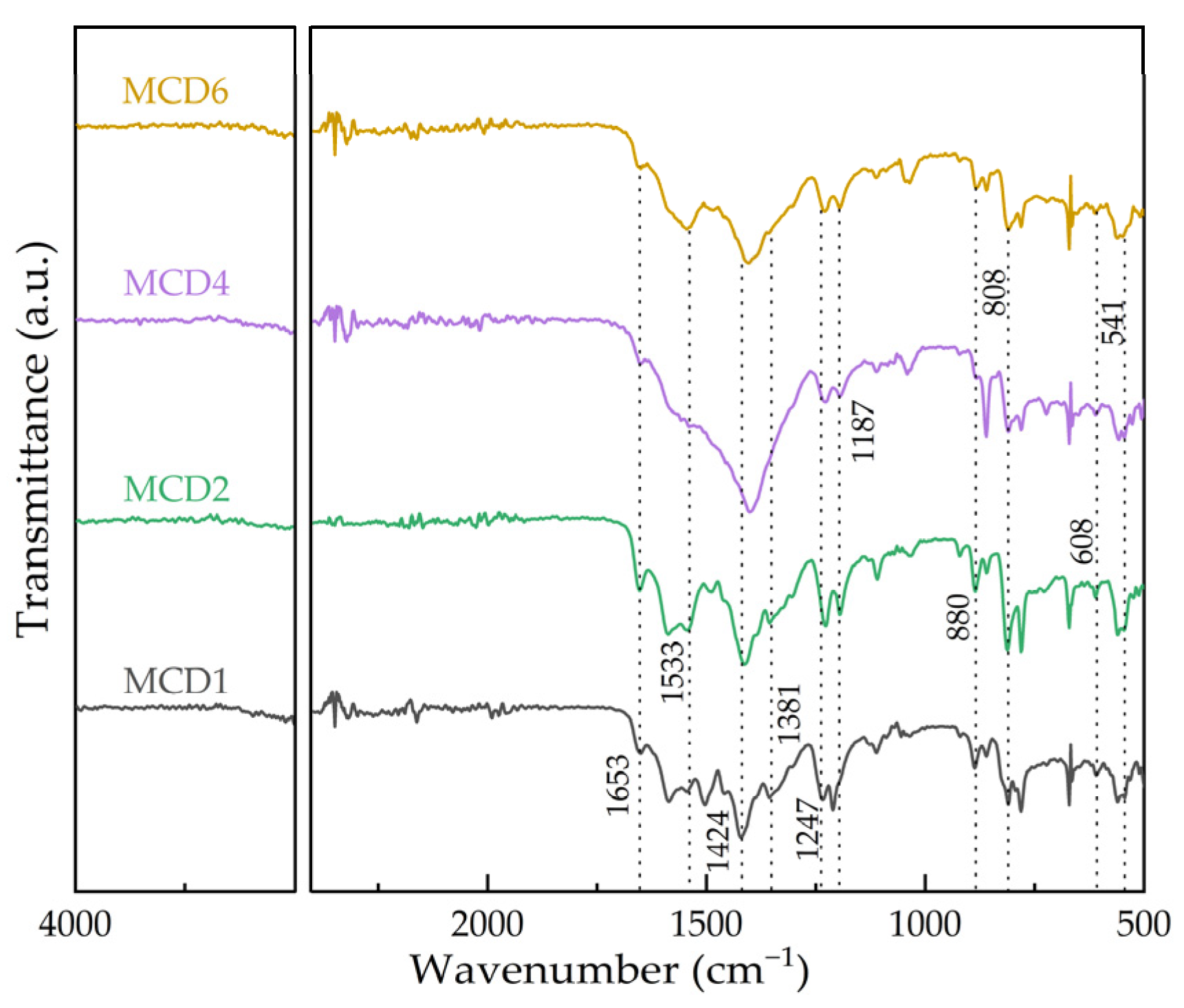
2.1.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.6. Thermogravimetric Analysis (TGA)
2.2. Experimental Analysis of NTP Synergistic MCDx Catalyzing Toluene
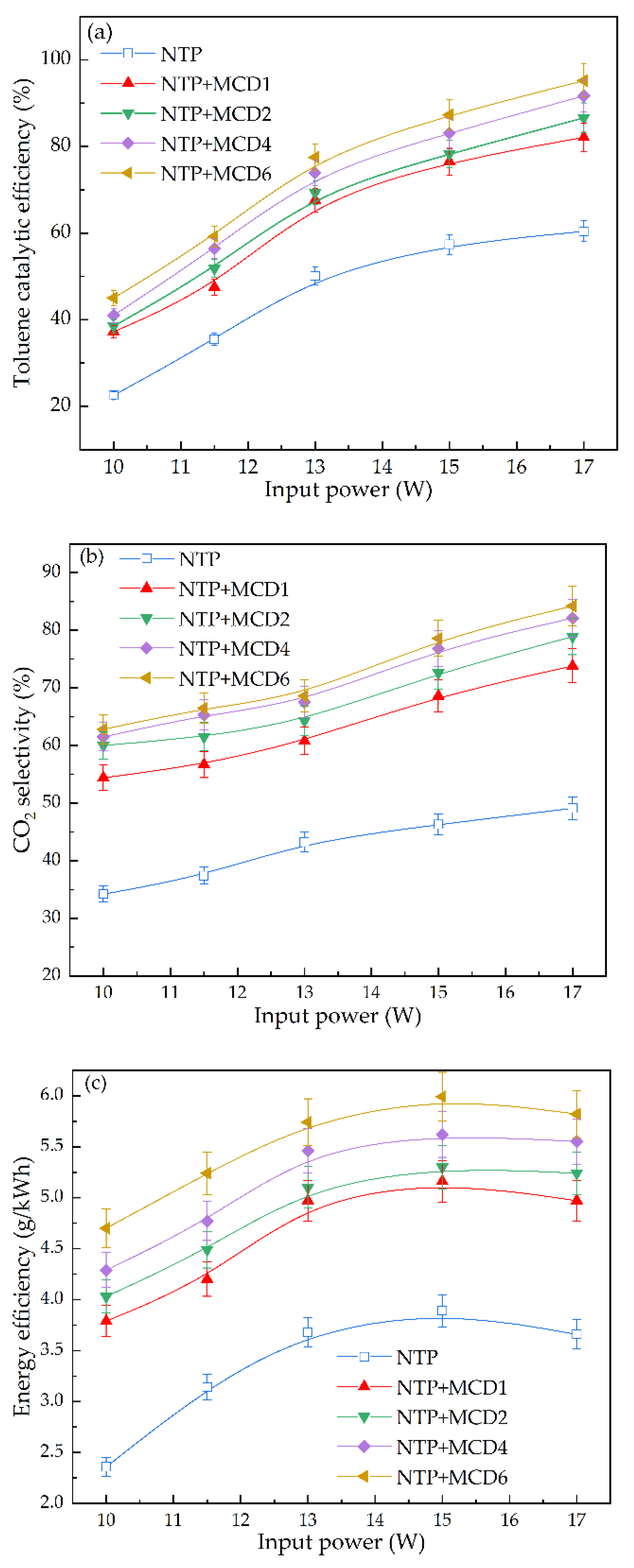
2.2.1. Effect of Input Power on Catalytic Performance of Toluene
- Analysis of catalytic reactivity
- 2.
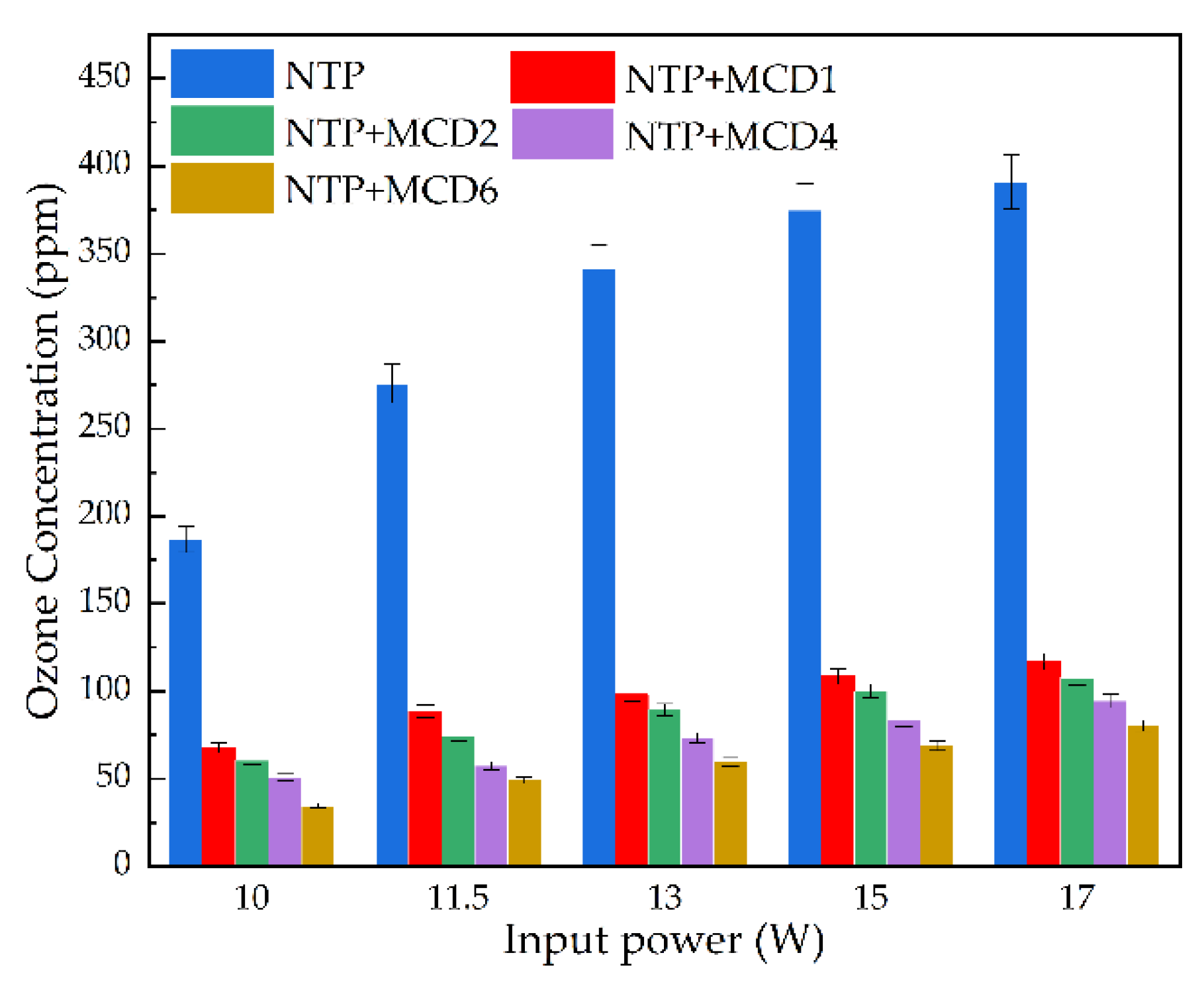
- Analysis of reaction by-products
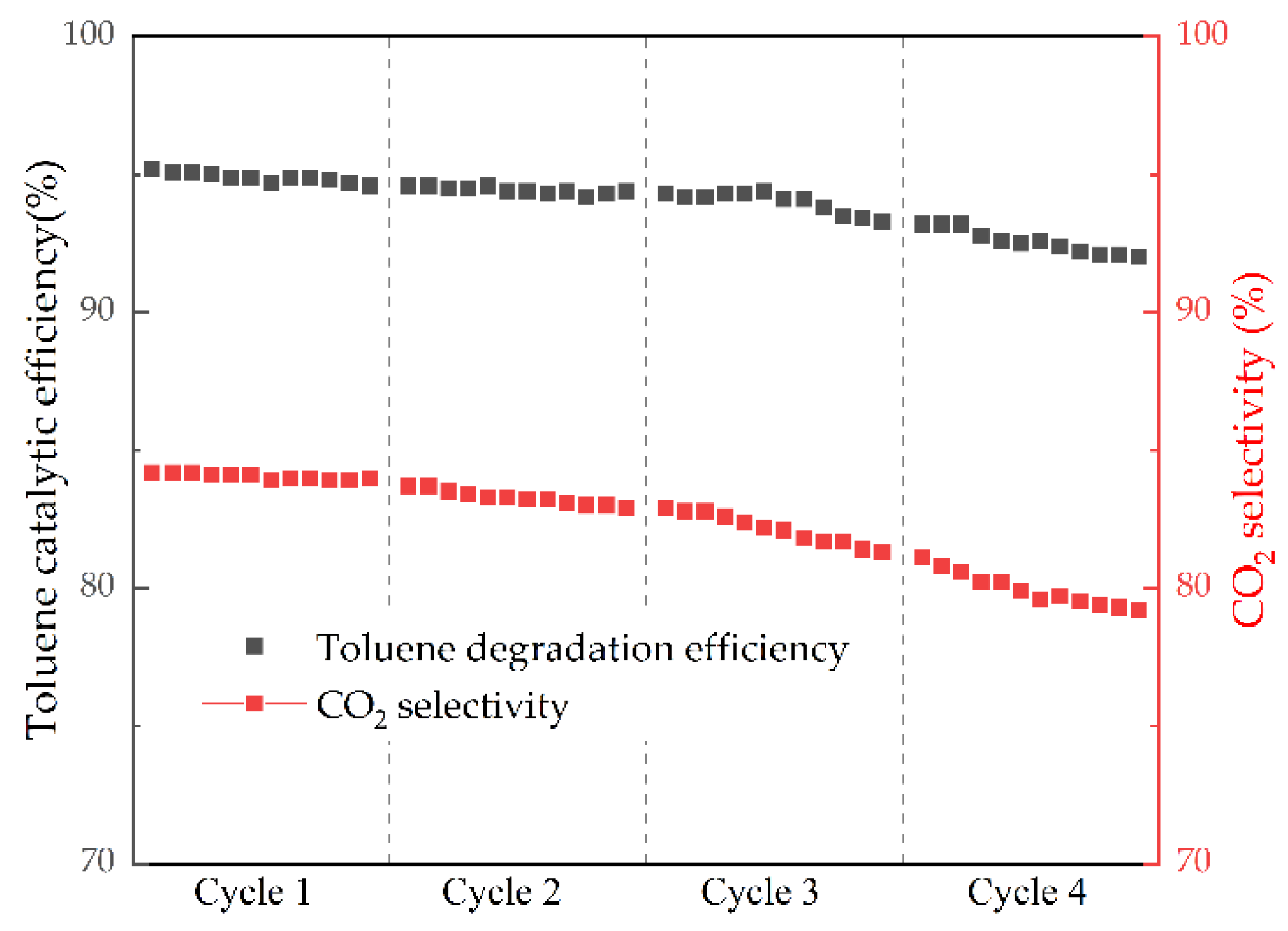
2.2.2. Catalyst Stability Analysis
2.2.3. Mechanism Analysis
3. Materials and Methods
3.1. Material Preparation Method
3.1.1. Experimental Materials
3.1.2. Preparation of MCDx
3.2. Characterization Methods
3.3. NTP and MCDx Synergistic Catalysis of Toluene
3.4. Performance Evaluation Method
4. Conclusions
- (1)
- In the NTP-MCDx synergistic catalytic system, compared with the single NTP, the addition of MCDx not only significantly improved the toluene catalytic efficiency, energy efficiency and CO2 selectivity, but also significantly reduced the emission concentration of by-product O3.
- (2)
- In each system with various MCDx, the order of toluene catalytic performance was: MCD6 > MCD4 > MCD2 > MCD1 > NTP. When the input power was 17 W, MCD6 achieved the highest toluene catalytic efficiency (95.2%) and the highest CO2 selectivity (81%).
- (3)
- MCD6 with the best synergistic catalytic performance was selected for durability test. The results showed that the toluene catalytic efficiency and CO2 selectivity decreased by less than 5% and gradually stabilized, showing good durability, and NTP discharge did not change the structure of the catalyst.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, X.C.; Ma, N.L.; Sonne, C.; Guan, R.R.; Lam, S.S.; van Le, Q.; Chen, X.M.; Yang, Y.F.; Gu, H.P.; Rinklebe, J.; et al. Mitigation of Indoor Air Pollution: A Review of Recent Advances in Adsorption Materials and Catalytic Oxidation. J. Hazard. Mater. 2021, 405, 124138. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wang, Z.H.; Qin, L.; Fu, Y.K.; Li, B.S.; Zhang, M.M.; Liu, S.Y.; Li, L.; Yi, H.; Liu, X.G.; et al. Metal-organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coordin. Chem. Rev. 2021, 427, 213565. [Google Scholar] [CrossRef]
- Roviello, V.; Scognamiglio, P.L.; Caruso, U.; Vicidomini, C.; Roviello, G.N. Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach. Int. J. Environ. Res. Public Health 2022, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luan, T.; Lu, T.; Cheng, K.; Xu, H. Performance of V2O5-WO3-MoO3/TiO2 Catalyst for Selective Catalytic Reduction of NOx by NH3. Chin. J. Chem. Eng. 2013, 21, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.X.; Bi, F.K.; Wang, Y.Y.; Jia, M.H.; Tao, X.F.; Jin, Y.X.; Zhang, X.D. MOF-derived CeO2 supported Ag catalysts for toluene oxidation: The effect of synthesis method. Mol. Catal. 2021, 515, 111922. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, L.; Yang, Z.Q.; Wang, P.; Yan, Y.F.; Ran, J.Y. Adsorption Materials for Volatile Organic Compounds (VOCs) and The Key Factors for VOCs Adsorption Process: A Review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Yang, G.L.; Jiang, X.L.; Xu, H.; Zhao, B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17, 2005327. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chen, X.R.; Chen, H.X.; Niu, Z.; Hirao, H.; Braunstein, P.; Lang, J.P. Ultrafast Luminescent Light-Up Guest Detection Based on the Lock of the Host Molecular Vibration. J. Am. Chem. Soc. 2020, 142, 6690–6697. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, G.Z.; Sun, Q.H.; Jia, H.Z.; Wang, T.C.; Zhu, L.Y. Endogenously Activated Persulfate by Non-Thermal Plasma for Cu(Ii)-Edta Decomplexation: Synergistic Effect and Mechanisms. Chem. Eng. J. 2021, 406, 126774. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, X.X.; AlQahtani, M.S.; Knecht, S.D.; Bilen, S.G.; Chu, W.; Song, C.S. Synergetic effect of non-thermal plasma and supported cobalt catalyst in plasma-enhanced CO2 hydrogenation. Chem. Eng. J. 2023, 451, 138661. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Li, Y.X.; Serrano-Lotina, A.; Han, W.; Portela, R.; Wang, R.X.; Banares, M.A.; Yeung, K.L. Operando Investigation of Toluene Oxidation over 1D Pt@CeO2 Derived from Pt Cluster-Containing MOF. J. Am. Chem. Soc. 2021, 143, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luan, T.; Zhang, S.; Jiang, W.; Feng, W.; Jiang, H. Comprehensive Comparison between Nanocatalysts of Mn-Co/TiO2 and Mn-Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics. Catalysts 2019, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, X.; Cai, S.C.; Chen, J.; Xu, W.J.; Jia, H.P.; Chen, J. Catalytic Combustion of Toluene over Mesoporous Cr2O3-Supported Platinum Catalysts Prepared by in Situ Pyrolysis of MOFs. Chem. Eng. J. 2018, 334, 768–779. [Google Scholar] [CrossRef]
- Zhang, X.D.; Lv, X.T.; Shi, X.Y.; Yang, Y.; Yang, Y.Q. Enhanced Hydrophobic Uio-66 (University of Oslo 66) Metal-Organic Framework with High Capacity and Selectivity for Toluene Capture from High Humid Air. J. Colloid Interface Sci. 2019, 539, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, W.; Luan, T.; Li, H.; Zhang, W.; Feng, W.; Jiang, H. High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species. Catalysts 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.H. Metal-Organic Frameworks for the Adsorption of Gaseous Toluene under Ambient Temperature and Pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, M.; Zhang, W.; Feng, W. Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn-Fe-Ce-Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction. Catalysts 2018, 8, 642. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Peng, X.; Zhang, Z.; Zhang, W.; Li, H.; Chen, B.; Li, S.; Zhang, Y.; Chi, S. Ternary Mixed-Oxide Synergy Effects of Nano TiO2-FexOy-MOk (M=Mn, Ce, Co) On α-Pinene Catalytic Oxidation Process Assisted by Nonthermal Plasma. Mater. Res. Express 2021, 8, 015509. [Google Scholar] [CrossRef]
- Zhang, X.D.; Shi, X.Y.; Chen, J.F.; Yang, Y.; Lu, G. The Preparation of Defective UIO-66 Metal Organic Framework Using MOF-5 as Structural Modifier with High Sorption Capacity for Gaseous Toluene. J. Environ. Chem. Eng. 2019, 7, 103405. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yu, E.Q.; Cai, S.C.; Jia, H.P.; Chen, J.; Liang, P. In Situ Pyrolysis of Ce-MOF to Prepare CeO2 Catalyst with Obviously Improved Catalytic Performance for Toluene Combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional Metal-Organic Frameworks as Effective Sensors of Gases and Volatile Compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-J.; Wang, J.-S.; Jin, F.-Z.; Liu, M.-Y.; Zhao, C.-W.; Li, Y.-A.; Dong, Y.-B. Pd@Cu(II)-MOF-Catalyzed Aerobic Oxidation of Benzylic Alcohols in Air with High Conversion and Selectivity. Inorg. Chem. 2016, 55, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Rad, A.J.; Irani, M. Incorporation of UIO-66-NH2 MOF into the Pan/Chitosan Nanofibers for Adsorption and Membrane Filtration of Pb(Ii), Cd(Ii) and Cr(Vi) Ions from Aqueous Solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cao, Q.; Guan, N.; Zhang, Z.; Fan, G.; Dou, H.; Li, S.; Wang, Q.; Chen, B. Metal-Organic Framework Supporting Fe3O4 Prepared by Microwave in Couple with NTP to Eliminate VOCs from Biofuel. Front. Energy Res. 2022, 10, 936493. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Alam, D.; Zhang, T.; Li, W.; Xia, Y.; Mai-Prochnow, A.; An, H.; Lovell, E.C.; Masood, H.; et al. Plasma catalytic Bubbles Using CeO2 for Organic Pollutant Degradation. Chem. Eng. J. 2021, 403, 126413. [Google Scholar] [CrossRef]
- Huang, H.; Chen, C.; Yang, R.; Yu, Y.; Albilali, R.; He, C. Remarkable Promotion Effect of Lauric Acid on Mn-MIL-100 for Non-Thermal Plasma-Catalytic Decomposition of Toluene. Appl. Surf. Sci. 2020, 503, 144290. [Google Scholar] [CrossRef]
- Bahri, M.; Haghighat, F.; Rohani, S.; Kazemian, H. Metal Organic Frameworks for Gas-Phase VOCs Removal in a NTP-Catalytic Reactor. Chem. Eng. J. 2017, 320, 308–318. [Google Scholar] [CrossRef]
- Zhang, X.D.; Bi, F.K.; Zhu, Z.Q.; Yang, Y.; Zhao, S.H.; Chen, J.F.; Lv, X.T.; Wang, Y.X.; Xu, J.C.; Liu, N. The Promoting Effect of H2O on Rod-Like MnCeOx Derived from MOFs for Toluene Oxidation: A Combined Experimental and Theoretical Investigation. Appl. Catal. B Environ. 2021, 297, 120393. [Google Scholar] [CrossRef]
- Liu, J.R.; Zheng, Y.M.; Zhu, Q.Y.; Dong, Y.X.; Lu, S.H.; Peng, B.; Chen, Y.L.; Zeng, S.H.; Li, K.L. MnOx-CeO2 Derived from Mn-Ce-MOFs with Highly Efficient Removal of Formaldehyde. Catal. Surv. Asia 2020, 24, 207–218. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, B.X.; Zhang, X.Q.; Wang, F.M.; Chi, G.L.; Si, M. A Comparative Study of Manganese-Cerium Doped Metal-Organic Frameworks Prepared via Impregnation and in Situ Methods in the Selective Catalytic Reduction of NO. RSC Adv. 2017, 7, 5928–5936. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, W.; Li, H. The Promotional Effects of Cerium on the Catalytic Properties of Al2O3-Supported MnFeOx for NO Oxidation and Fast SCR Reaction. Res. Chem. Intermed. 2019, 45, 663–686. [Google Scholar] [CrossRef]
- Chavan, S.; Bonino, F.; Valenzano, L.; Civalleri, B.; Lamberti, C.; Acerbi, N.; Cavka, J.H.; Leistner, M.; Bordiga, S. Fundamental Aspects of H2S Adsorption on CPO-27-Ni. J Phys Chem C 2013, 117(30), 15615–15622. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; Available online: https://www.wiley.com/en-us/Spectrometric+Identification+of+Organic+Compounds,+8th+Edition-p-9780470616376 (accessed on 1 September 2014).
- Qiang, H.; Xia, M.Z.; Wang, F.Y.; Lei, W.; Lu, X.; Ni, Y. The highly specific detection and mechanism of Cu-MOF-74 fluorescent probe to amino trimethylene phosphonic acid: Experimental study and theoretical calculation of quantum chemistry. J. Mol. Liq. 2021, 341, 117442. [Google Scholar] [CrossRef]
- Salazar, J.M.; Weber, G.; Simon, J.M.; Bezverkhyy, I.; Bellat, J.P. Characterization of adsorbed water in MIL-53(Al) by FTIR spectroscopy and ab-initio calculations. J. Chem. Phys. 2015, 142, 124702. [Google Scholar] [CrossRef]
- Reynolds, J.E.; Bohnsack, A.M.; Kristek, D.J.; Gutierrez-Alejandre, A.; Dunning, S.G.; Waggoner, N.W.; Sikma, R.E.; Ibarra, I.A.; Humphrey, S.M. Phosphonium Zwitterions for Lighter and Chemically-Robust MOFs: Highly Reversible H2S Capture and Solvent-Triggered Release. J. Mater. Chem. A 2019, 7, 16842–16849. [Google Scholar] [CrossRef]
- Wang, W.R.; He, J.P.; Guo, H.Y.; Dunning, S.G.; Humphrey, S.M.; Jones, R.A. Magnetism and Luminescence of a MOF with Linear Mn-3 Nodes Derived from an Emissive Terthiophene-Based Imidazole Linker. Molecules 2021, 26, 4286. [Google Scholar] [CrossRef]
- Sikma, R.E.; Katyal, N.; Lee, S.K.; Fryer, J.W.; Romero, C.G.; Emslie, S.K.; Taylor, E.L.; Lynch, V.M.; Chang, J.S.; Henkelman, G.; et al. Low-Valent Metal Ions as MOF Pillars: A New Route Toward Stable and Multifunctional MOFs. J. Am. Chem. Soc. 2021, 143, 13710–13720. [Google Scholar] [CrossRef]
- Yuan, K.; Song, T.; Wang, D.; Zou, Y.; Li, J.; Zhang, X.; Tang, Z.; Hu, W. Bimetal–Organic Frameworks for Functionality Optimization: MnFe-MOF-74 as a Stable and Efficient Catalyst for the Epoxidation of Alkenes with H2O2. Nanoscale 2018, 10, 1591–1597. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Craven, M.; Wang, Y.; Kang, D.; Wu, C.; Tu, X. A Review of Non-Thermal Plasma Technology: A Novel Solution for CO2 Conversion and Utilization. Renew. Sustain. Energy Rev. 2021, 135, 109702. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.L.; Ma, X.Y.; Yang, X.; Lin, M.; Ge, M. MnOx-CeO2 Catalyst Derived from Metal-Organic Frameworks for Toluene Oxidation. Catal. Today 2020, 355, 580–586. [Google Scholar] [CrossRef]
- Zeng, X.; Li, B.; Liu, R.; Li, X.; Zhu, T. Investigation of promotion effect of Cu doped MnO2 catalysts on ketone-type VOCs degradation in a one-stage plasma-catalysis system. Chem. Eng. J. 2020, 384, 123362. [Google Scholar] [CrossRef]



| Sample | Element | Mn2+/Mn3+ | Mn3+/Mn4+ | Element | Ce3+/Ce4+ |
|---|---|---|---|---|---|
| MCD1 | Mn 2p3/2 | 0.0342 | 0.6473 | Ce 3d3/2 | 0.2247 |
| MCD2 | Mn 2p3/2 | 0.9125 | 0.6320 | Ce 3d3/2 | 0.2442 |
| MCD4 | Mn 2p3/2 | 3.0948 | 0.2692 | Ce 3d3/2 | 0.2607 |
| MCD6 | Mn 2p3/2 | 4.9645 | 0.1699 | Ce 3d3/2 | 0.3007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, X.; Cao, Q.; Gao, Y.; Luan, T.; Li, Y.; Man, Q.; Zhang, Z.; Chen, B. Synergistic Catalytic Performance of Toluene Degradation Based on Non-Thermal Plasma and Mn/Ce-Based Bimetal-Organic Frameworks. Molecules 2022, 27, 7363. https://doi.org/10.3390/molecules27217363
Rong X, Cao Q, Gao Y, Luan T, Li Y, Man Q, Zhang Z, Chen B. Synergistic Catalytic Performance of Toluene Degradation Based on Non-Thermal Plasma and Mn/Ce-Based Bimetal-Organic Frameworks. Molecules. 2022; 27(21):7363. https://doi.org/10.3390/molecules27217363
Chicago/Turabian StyleRong, Xing, Qing Cao, Yan Gao, Tao Luan, Yanteng Li, Quanyou Man, Zhanchao Zhang, and Baoming Chen. 2022. "Synergistic Catalytic Performance of Toluene Degradation Based on Non-Thermal Plasma and Mn/Ce-Based Bimetal-Organic Frameworks" Molecules 27, no. 21: 7363. https://doi.org/10.3390/molecules27217363





