Silica Aerogel-Rubber Composite: A Sustainable Alternative for Buildings’ Thermal Insulation
Abstract
:1. Introduction
2. Results and Discussion
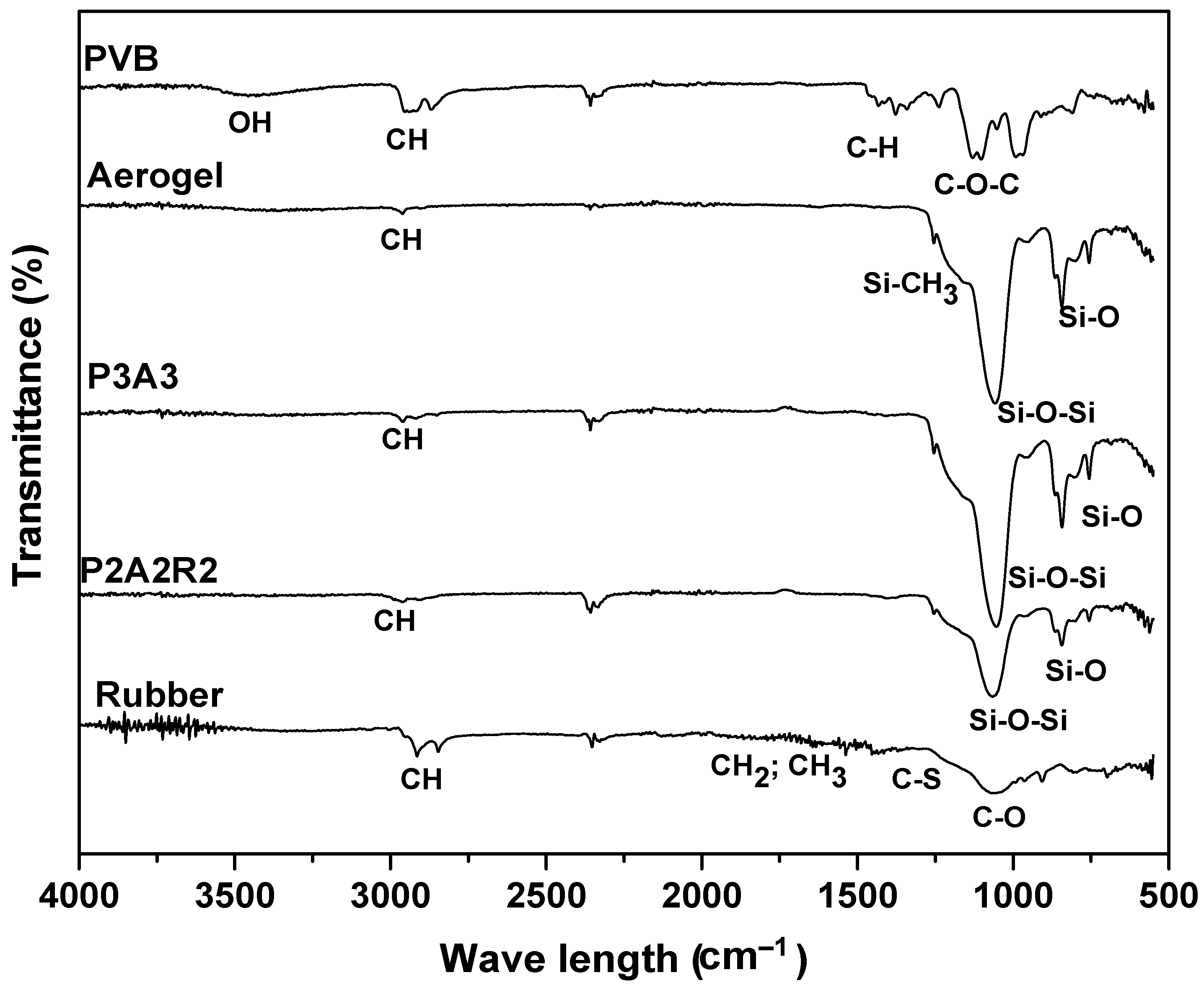
2.1. Chemical Characterization
2.2. Structural and Morphological Features
2.3. Surface Hydrophobicity
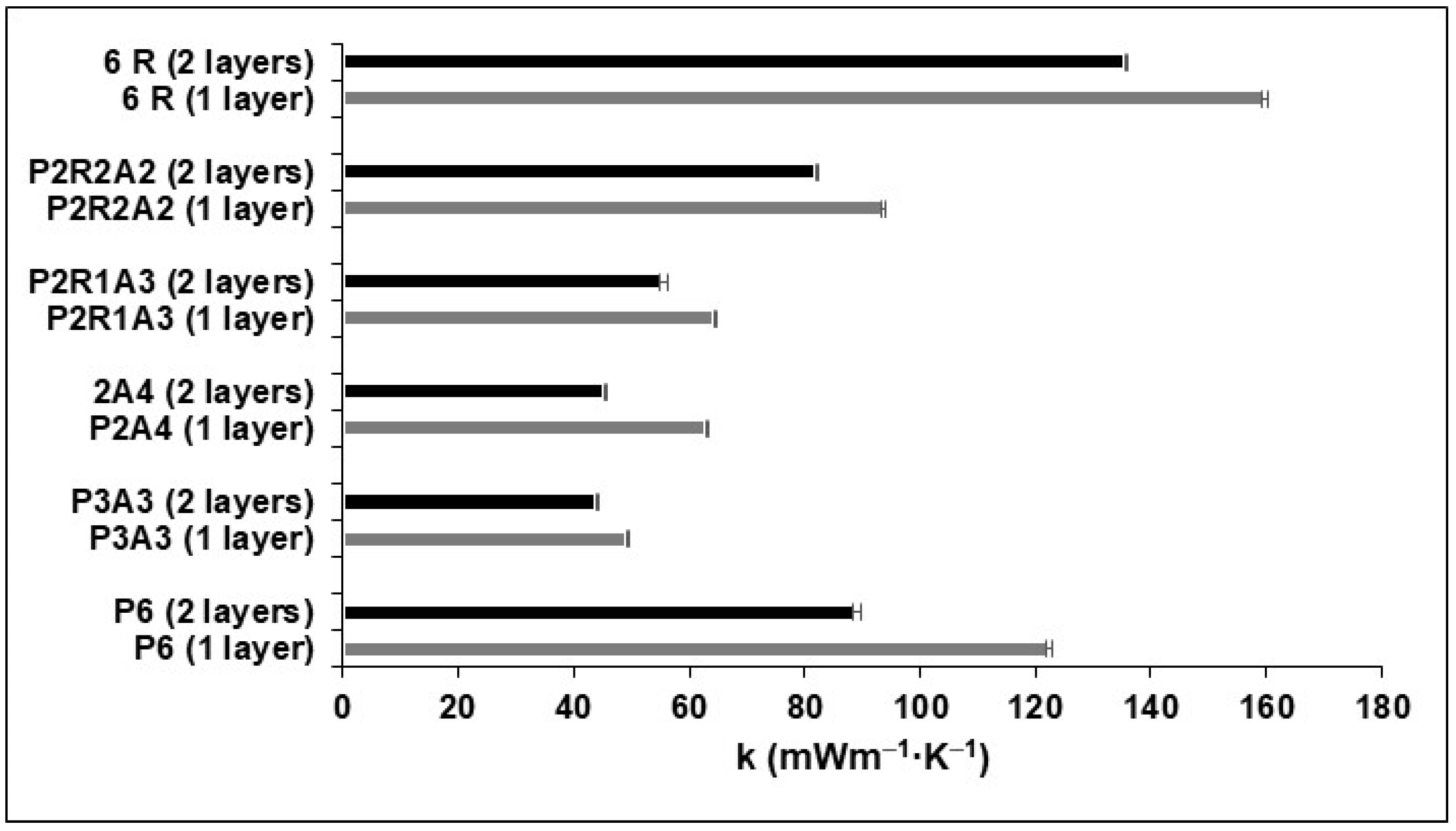
2.4. Density and Thermal Conductivity
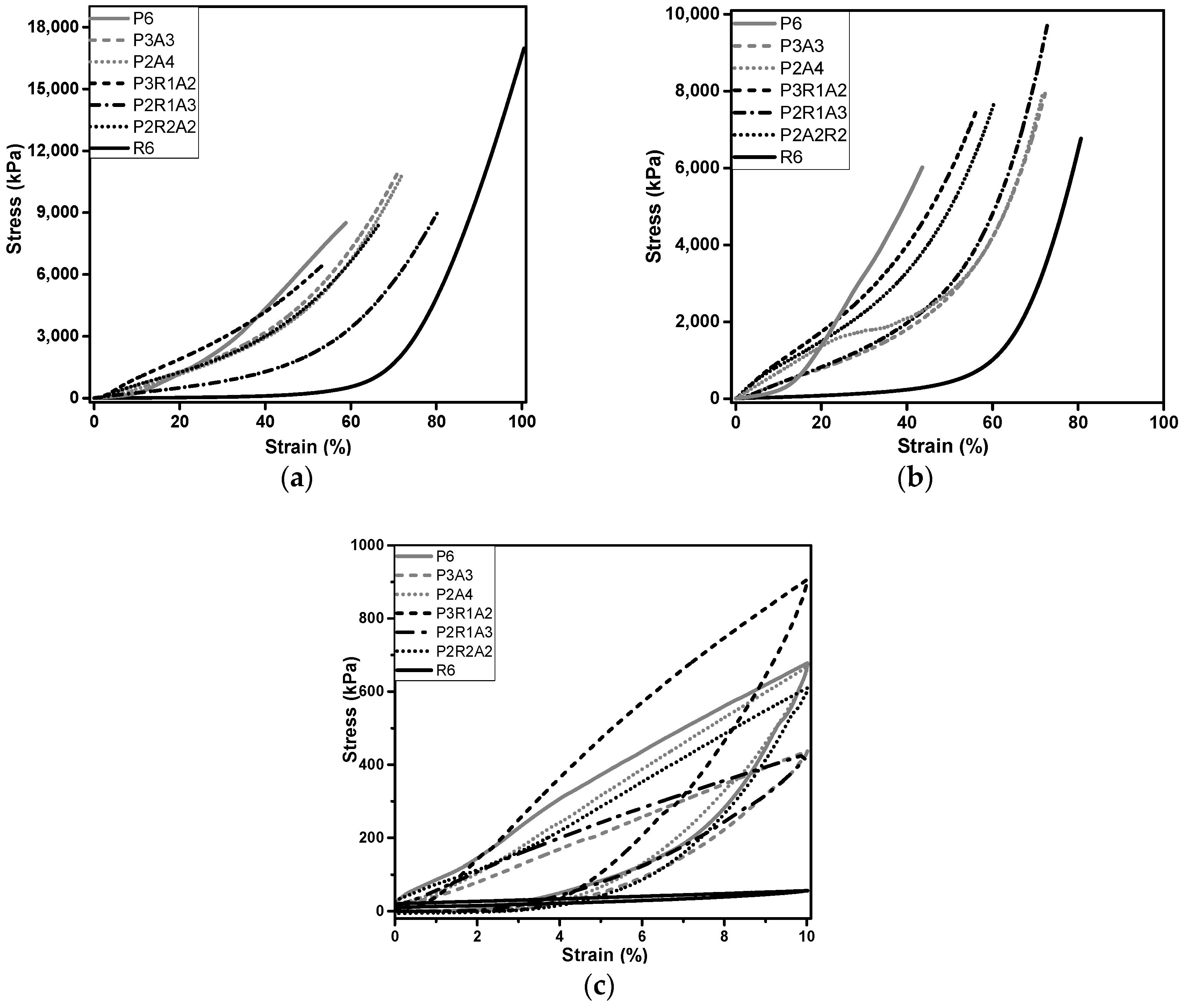
2.5. Mechanical Characterization
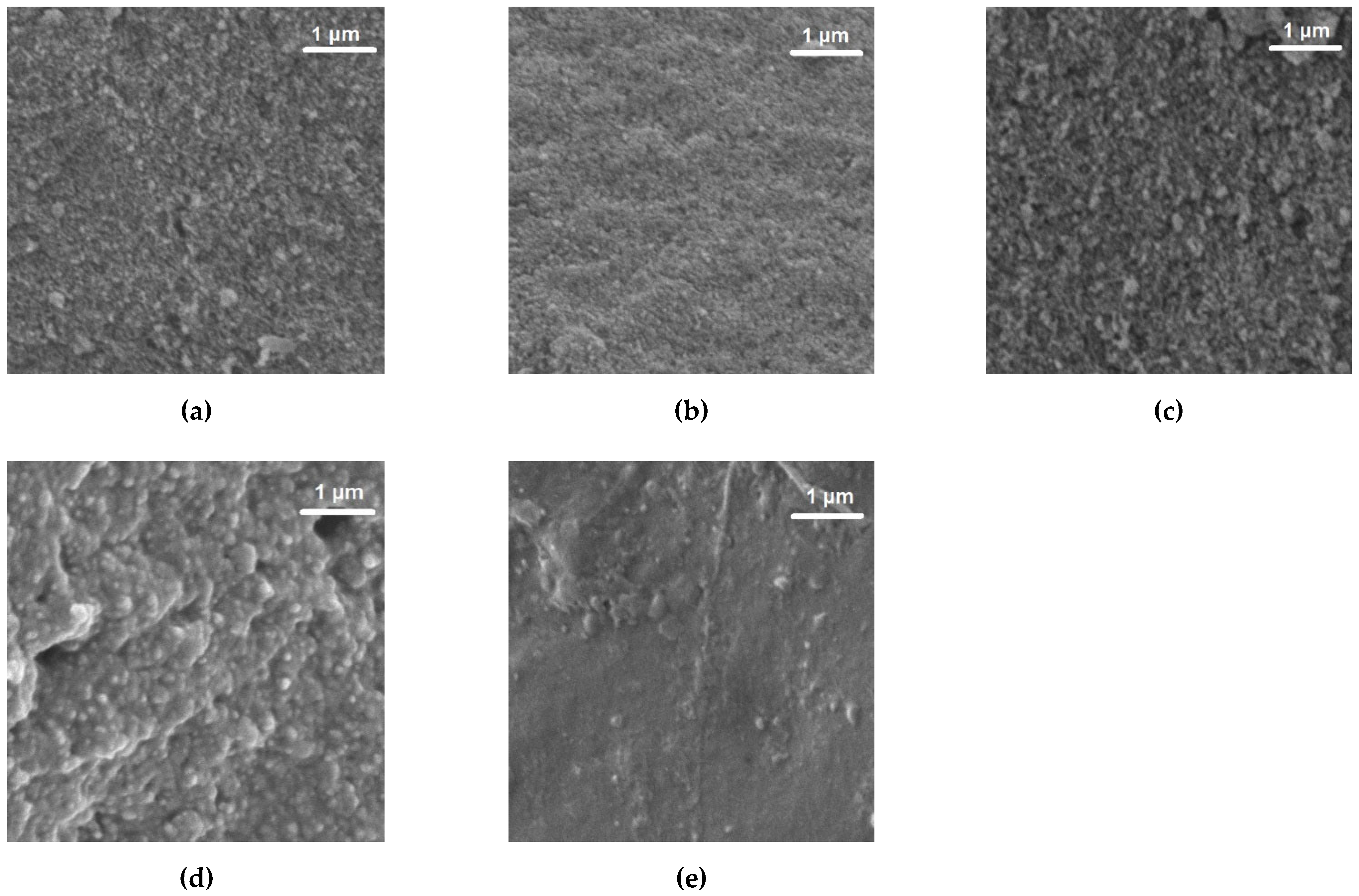
2.6. Surface Morphology
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Aerogel
3.3. Synthesis of the Aerogel–Rubber Composites
3.4. Characterization of the Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mazrouei-Sebdani, Z.; Begum, H.; Schoenwald, S.; Horoshenkov, K.V.; Malfait, W.J. A review on silica aerogel-based materials for acoustic applications. J. Non-Cryst. Solids 2021, 562, 120770. [Google Scholar] [CrossRef]
- Ratke, L. Monoliths and Fibrous Cellulose Aerogels. In Aerogels Handbook. Advances in Sol-Gel Derived Materials and Technologies; Aegerter, M., Leventis, N., Koebel, M., Eds.; Springer: New York, NY, USA, 2011; pp. 173–190. [Google Scholar]
- Feng, Q.; Chen, K.; Ma, D.; Lin, H.; Liu, Z.; Qin, S.; Luo, Y. Synthesis of high specific surface area silica aerogel from rice husk ash via ambient pressure drying. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 539, 399–406. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Xu, D.; Hai, O.; Zheng, W. Low density and hydrophobic silica aerogels dried under ambient pressure using a new co-precursor method. J. Non-Cryst. Solids 2016, 452, 187–193. [Google Scholar] [CrossRef]
- Schultz, J.M.; Jensen, K.I.; Kristiansen, F.H. Super insulating aerogel glazing. Sol. Energy Mater. Sol. Cells 2005, 89, 275–285. [Google Scholar] [CrossRef]
- Carraher, C.E., Jr. Silica aerogels—Properties and uses. Polym. News 2005, 30, 386–388. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Soares, N.; Santos, P.; Gervásio, H.; Costa, J.J.; Simões da Silva, L. Energy efficiency and thermal performance of lightweight steel-framed (LSF) construction: A review. Renew. Sustain. Energy Rev. 2017, 78, 194–209. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. Synthesis of lightweight polymer-reinforced silica aerogels with improved mechanical and thermal insulation properties for space applications. Microporous Mesoporous Mater. 2014, 197, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhang, L.; Li, J.; Zhao, X.; Yang, C. Organic-inorganic hybridization for the synthesis of robust in situ hydrophobic polypropylsilsesquioxane aerogels with fast oil absorption properties. RSC Adv. 2018, 8, 5695–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhagat, S.D.; Rao, A.V. Surface chemical modification of TEOS based silica aerogels synthesized by two step (acid–base) sol–gel process. Appl. Surf. Sci. 2006, 252, 4289–4297. [Google Scholar] [CrossRef]
- Linhares, T.; Pessoa De Amorim, M.T.; Durães, L. Silica aerogel composites with embedded fibres: A review on their preparation, properties and applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering Strong Silica Aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click Synthesis of Monolithic Silicon Carbide Aerogels from Polyacrylonitrile-Coated 3D Silica Networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Boday, D.J.; Keng, P.Y.; Muriithi, B.; Pyun, J.; Loy, D.A. Mechanically reinforced silica aerogel nanocomposites via surface initiated atom transfer radical polymerizations. J. Mater. Chem. 2010, 20, 6863–6865. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Smith, D.M.; Maskara, A.; Boes, U. Aerogel-based thermal insulation. J. Non-Cryst. Solids 1998, 225, 254–259. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P.; Gustavsen, A.; Jacobsen, S. Aerogel-incorporated concrete: An experimental study. Constr. Build. Mater. 2014, 52, 130–136. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P.; Ihara, T.; Gustavsen, A. Insulating glazing units with silica aerogel granules: The impact of particle size. Appl. Energy 2014, 128, 27–34. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P.; Gustavsen, A.; He, J. Lightweight and thermally insulating aerogel glass materials. Appl. Phys. A 2014, 117, 799–808. [Google Scholar] [CrossRef]
- Roque, E.; Santos, P.; Pereira, A.C. Thermal and sound insulation of lightweight steel-framed façade walls. Sci. Technol. Built Environ. 2018, 25, 156–176. [Google Scholar] [CrossRef]
- Roque, E.; Santos, P. The Effectiveness of Thermal Insulation in Lightweight Steel-Framed Walls with Respect to Its Position. Buildings 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Grammelis, P.; Margaritis, N.; Dallas, P.; Rakopoulos, D.; Mavrias, G. A Review on Management of End of Life Tires (ELTs) and Alternative Uses of Textile Fibers. Energies 2021, 14, 571. [Google Scholar] [CrossRef]
- Thai, Q.B.; Siang, T.E.; Le, D.K.; Shah, W.A.; Phan-Thien, N.; Duong, H.M. Advanced fabrication and multi-properties of rubber aerogels from car tire waste. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 577, 702–708. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Janik, H.; Borzędowska-Labuda, K.; Kucińska-Lipka, J. Environmentally friendly polymer-rubber composites obtained from waste tyres: A review. J. Clean. Prod. 2017, 147, 560–571. [Google Scholar] [CrossRef]
- Bowles, A.; Fowler, G.; O'Sullivan, C.; Parker, K. Sustainable rubber recycling from waste tyres by waterjet: A novel mechanistic and practical analysis. Sustain. Mater. Technol. 2020, 25, e00173. [Google Scholar] [CrossRef]
- Walters, M.H.; Keyte, D.N. Heterogeneous Structure in Blends of Rubber Polymers. Rubber Chem. Technol. 1965, 38, 62–75. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, H.; Shi, Y.; Cui, J. The mechanical properties of Polyvinyl Butyral (PVB) at high strain rates. Constr. Build. Mater. 2015, 93, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Chen, X.; Wu, X. The mechanical behaviour of polyvinyl butyral at intermediate strain rates and different temperatures. Constr. Build. Mater. 2018, 182, 66–79. [Google Scholar] [CrossRef]
- Ismail, A.A.; van de Voort, F.R.; Sedman, J. Chapter 4 Fourier transform infrared spectroscopy: Principles and applications. Tech. Instrum. Anal. Chem. 1997, 18, 93–139. [Google Scholar] [CrossRef]
- Hajian, M.; Reisi, M.R.; Koohmareh, G.A.; Jam, A.R.Z. Preparation and characterization of Polyvinylbutyral/Graphene Nanocomposite. J. Polym. Res. 2012, 19, 9966. [Google Scholar] [CrossRef]
- Yang, B.; Ni, H.; Huang, J.; Luo, Y. Effects of Poly(vinyl butyral) as a Macromolecular Nucleating Agent on the Nonisothermal Crystallization and Mechanical Properties of Biodegradable Poly(butylene succinate). Macromolecules 2013, 47, 284–296. [Google Scholar] [CrossRef]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 323–330. [Google Scholar] [CrossRef]
- Al-Oweini, R.; Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2020, 919, 140–145. [Google Scholar] [CrossRef]
- Chen, F.; Qian, J. Studies of the thermal degradation of waste rubber. Waste Manag. 2003, 23, 463–467. [Google Scholar] [CrossRef]
- Kang, S.-K.; Choi, S.-Y. Synthesis of low-density silica gel at ambient pressure: Effect of heat treatment. J. Mater. Sci. 2000, 35, 4971–4976. [Google Scholar] [CrossRef]
- Malfait, W.J.; Zhao, S.; Verel, R.; Iswar, S.; Rentsch, D.; Fener, R.; Zhang, Y.; Milow, B.; Koebel, M.M. The Surface Chemistry of Hydrophobic Silica Aerogels. Chem. Mater. 2015, 27, 6737–6745. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in carbon nanostructure–silica aerogel composites: A review. J. Mater. Chem. A 2017, 6, 1340–1369. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Crystalline Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Sun, Y.; Li, Y.; Wang, Y.; Ge, D.; Xu, J. Systematic experimental study on mechanical behavior of PVB (polyvinyl butyral) material under various loading conditions. Polym. Eng. Sci. 2012, 52, 1137–1147. [Google Scholar] [CrossRef]
- Hosford, W.F. Mechanical Behavior of Materials, 2nd ed.; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Ishida, T.; Kakushima, K.; Mizoguchi, T.; Fujita, H. Role of dislocation movement in the electrical conductance of nanocontacts. Sci. Rep. 2012, 2, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajonk, G.M.; Rao, A.V.; Parvathy, N.N.; Elaloui, E. Microstructural characterization of silica aerogels using scanning electron microscopy. J. Mater. Sci. 1996, 31, 5683–5689. [Google Scholar] [CrossRef]


| Samples/Composites | Water Contact Angle (°) |
|---|---|
| P6 | 116.7 ± 2.4 |
| P3A3 | 151.7 ± 4.6 |
| P2A4 | 156.8 ± 3.1 |
| P3R1A2 | 158.8 ± 2.1 |
| P2R1A3 | 160.3 ± 3.6 |
| P2R2A2 | 158.2 ± 4.8 |
| R6 | 150.0 ± 4.5 |
| Samples and Composites | Young’s Modulus (kPa) | Compressive Stress after 10% Strain (kPa) | Recovery after 10% of Compression |
|---|---|---|---|
| P6 | 79.3 | 978 | 69 |
| P3A3 | 75.4 | 587 | 84 |
| P2A4 | 61.9 | 812 | 87 |
| P3R1A2 | 12.9 | 855 | 84 |
| P2R1A3 | 40.8 | 614 | 91 |
| P2R2A2 | 93.2 | 652 | 90 |
| R6 | 4.6 | 91 | 98 |
| Samples | Images | Composition |
|---|---|---|
| P6 |  | 6 g PVB |
| P3A3 |  | 3 g PVB, 3 g Aerogel |
| P2A4 |  | 2 g PVB, 4 g Aerogel |
| P3R1A2 |  | 3 g PVB 1 g Rubber 2 g Aerogel |
| P2R1A3 |  | 2 g PVB 1 g Rubber 3 g Aerogel |
| P2R2A2 |  | 2 g PVB 2 g Rubber 2 g Aerogel |
| R6 |  | 6 g Rubber |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.; Dias, D.A.; Pontinha, A.D.R. Silica Aerogel-Rubber Composite: A Sustainable Alternative for Buildings’ Thermal Insulation. Molecules 2022, 27, 7127. https://doi.org/10.3390/molecules27207127
Alves P, Dias DA, Pontinha ADR. Silica Aerogel-Rubber Composite: A Sustainable Alternative for Buildings’ Thermal Insulation. Molecules. 2022; 27(20):7127. https://doi.org/10.3390/molecules27207127
Chicago/Turabian StyleAlves, Patrícia, Diogo Azeiteiro Dias, and Ana Dora Rodrigues Pontinha. 2022. "Silica Aerogel-Rubber Composite: A Sustainable Alternative for Buildings’ Thermal Insulation" Molecules 27, no. 20: 7127. https://doi.org/10.3390/molecules27207127





