A Water-Soluble Leggero Pillar[5]arene
Abstract
:1. Introduction
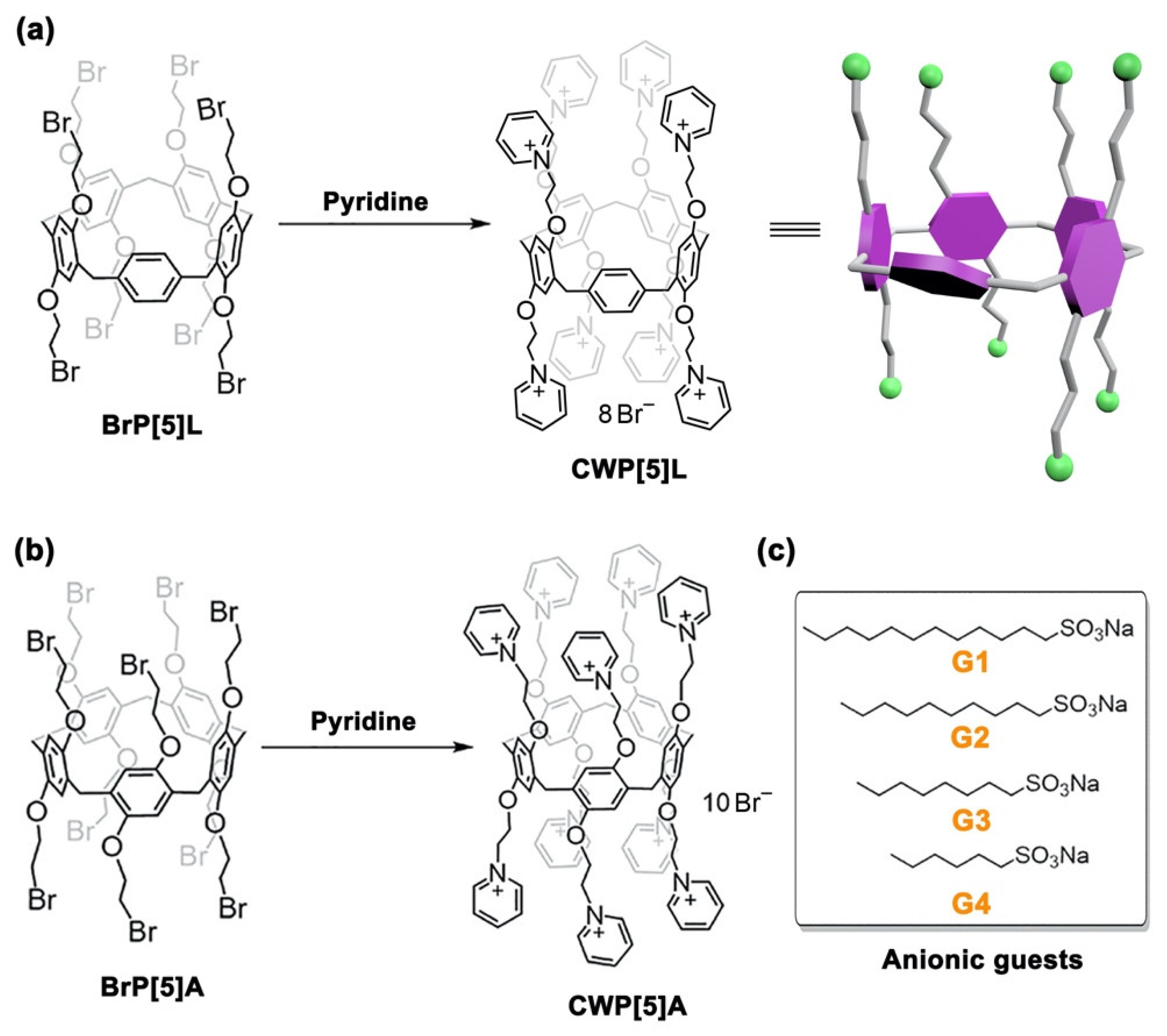
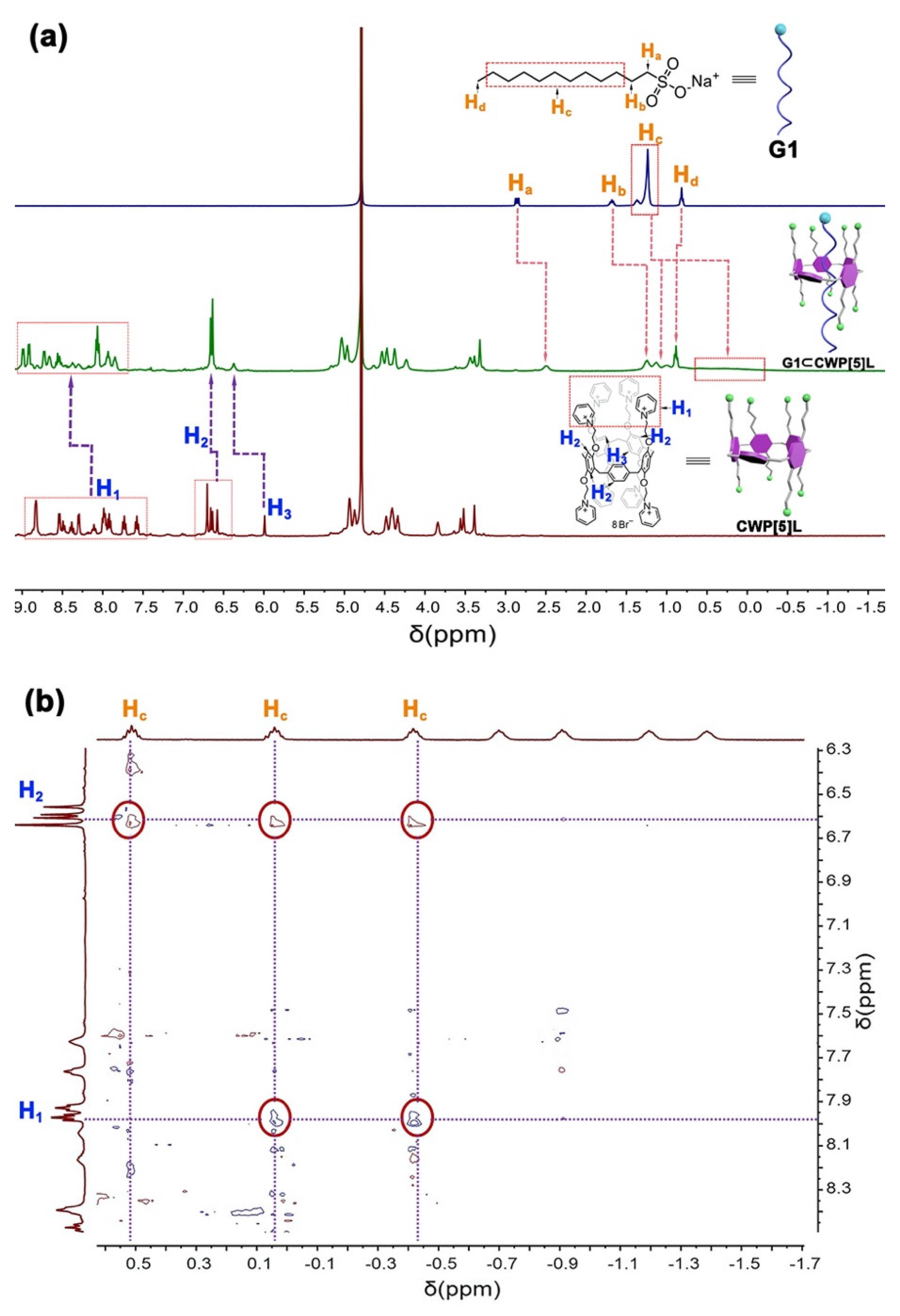
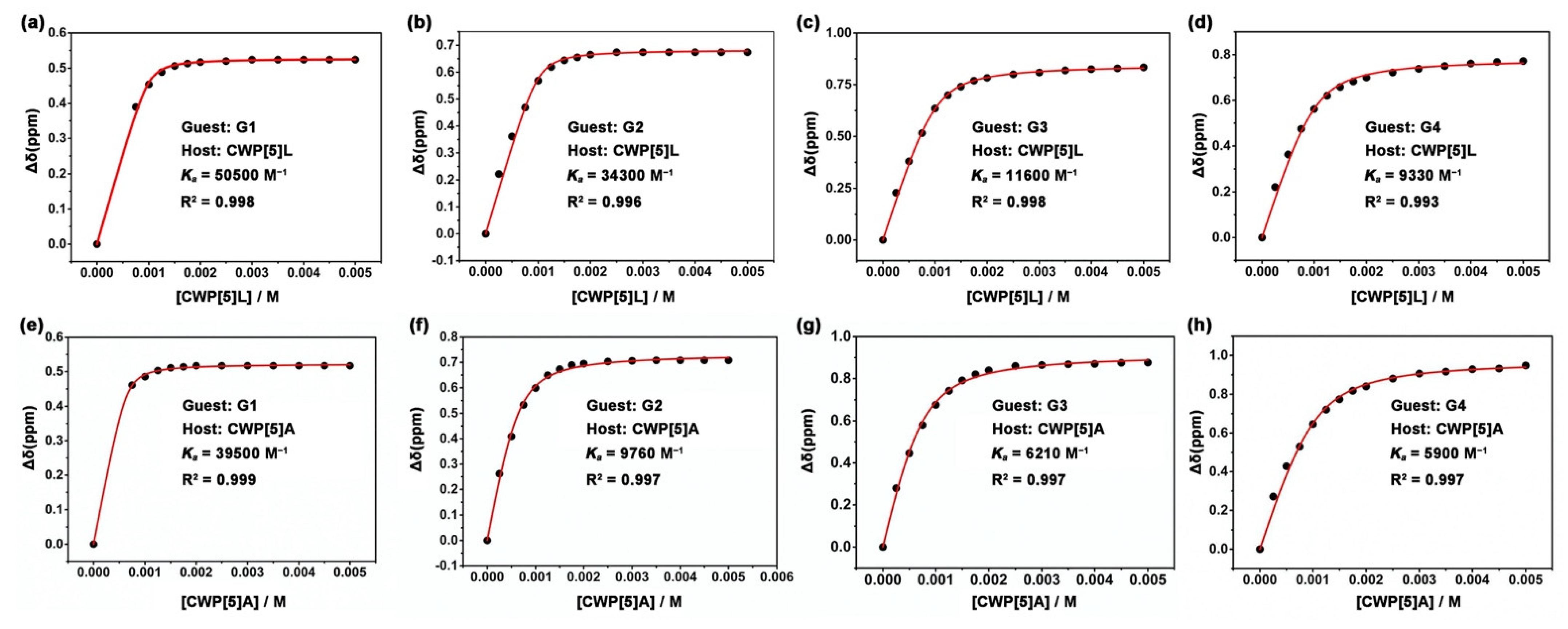
2. Results
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures
3.2.1. Synthesis of CWP[5]L
3.2.2. Synthesis of CWP[5]A
3.3. Determination of Association Constants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Liu, Z.; Nalluri, S.K.M.; Stoddart, J.F. Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 2017, 46, 2459–2478. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-R.; Yang, Y.-W. New opportunities in synthetic macrocyclic arenes. Chem. Commun. 2019, 55, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-A.; Nakamoto, Y. para-Bridged symmetrical pillar[5]arenes: Their Lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Yamagishi, T.-A.; Nakamoto, Y. Pillar-shaped macrocyclic hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Fa, S.; Ohtani, S.; Shi, T.-h.; Brouwer, A.M.; Ogoshi, T. Noncovalently bound and mechanically interlocked systems using pillar[n]arenes. Chem. Soc. Rev. 2022, 51, 3648–3687. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Photoresponsive host–guest functional systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef]
- Yu, G.; Jie, K.; Huang, F. Supramolecular amphiphiles based on host–guest molecular recognition motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.-A.; Ogoshi, T. Stimuli-responsive supramolecular assemblies constructed from pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Wang, D.H.; Wang, J.; Wang, Y.; Yang, Y.W. A fluorescent linear conjugated polymer constructed from pillararene and anthracene. Molecules 2022, 27, 3162. [Google Scholar] [CrossRef]
- Zhao, M.; Li, C.J.; Shan, X.T.; Han, H.J.; Zhao, Q.H.; Xie, M.R.; Chen, J.Z.; Liao, X.J. A stretchable pillararene-containing supramolecular polymeric material with self-healing property. Molecules 2021, 26, 2191. [Google Scholar] [CrossRef]
- Lou, X.Y.; Song, N.; Yang, Y.W. Fluorescence resonance energy transfer systems in supramolecular macrocyclic chemistry. Molecules 2017, 22, 1640. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Yang, Y.-W. Aggregation-induced emission systems involving supramolecular assembly. Aggregate 2020, 1, 19–30. [Google Scholar] [CrossRef]
- Li, M.-H.; Lou, X.-Y.; Yang, Y.-W. Pillararene-based molecular-scale porous materials. Chem. Commun. 2021, 57, 13429–13447. [Google Scholar] [CrossRef]
- Jie, K.; Zhou, Y.; Li, E.; Huang, F. Nonporous adaptive crystals of pillararenes. Acc. Chem. Res. 2018, 51, 2064–2072. [Google Scholar] [CrossRef]
- Wu, J.-R.; Yang, Y.-W. Synthetic macrocycle-based nonporous adaptive crystals for molecular separation. Angew. Chem. Int. Ed. 2021, 60, 1690–1701. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Yang, Y.-W. Pillar[n]arene-based supramolecular switches in solution and on surfaces. Adv. Mater. 2020, 32, 2003263. [Google Scholar] [CrossRef]
- Song, N.; Kakuta, T.; Yamagishi, T.-A.; Yang, Y.-W.; Ogoshi, T. Molecular-scale porous materials based on pillar[n]arenes. Chem 2018, 4, 2029–2053. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.-W. Functional materials with pillarene Struts. Acc. Mater. Res. 2021, 2, 292–305. [Google Scholar] [CrossRef]
- Yao, Y.; Xue, M.; Chi, X.; Ma, Y.; He, J.; Abliz, Z.; Huang, F. A new water-soluble pillar[5]arene: Synthesis and application in the preparation of gold nanoparticles. Chem. Commun. 2012, 48, 6505–6507. [Google Scholar] [CrossRef]
- Li, M.-H.; Yang, Z.; Li, Z.; Wu, J.-R.; Yang, B.; Yang, Y.-W. Construction of hydrazone-linked macrocycle-enriched covalent organic frameworks for highly efficient photocatalysis. Chem. Mater. 2022, 34, 5726–5739. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Yang, Y.-W. Conjugated macrocycle polymer nanoparticles with alternating pillarenes and porphyrins as struts and cyclic nodes. Small 2019, 15, 1805509. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Wang, Y.; Yang, Y.-W. Pillararene-enriched linear conjugated polymer materials with thiazolo[5,4-d]thiazole linkages for photocatalysis. Chem. Commun. 2021, 57, 6546–6549. [Google Scholar] [CrossRef]
- Song, N.; Lou, X.-Y.; Ma, L.; Gao, H.; Yang, Y.-W. Supramolecular nanotheranostics based on pillarenes. Theranostics 2019, 9, 3075–3093. [Google Scholar] [CrossRef]
- Song, N.; Zhang, Z.; Liu, P.; Dai, D.; Chen, C.; Li, Y.; Wang, L.; Han, T.; Yang, Y.-W.; Wang, D.; et al. Pillar[5]arene-modified gold nanorods as nanocarriers for multi-modal imaging-guided synergistic photodynamic-photothermal therapy. Adv. Funct. Mater. 2021, 31, 2009924. [Google Scholar] [CrossRef]
- Li, Z.; Song, N.; Yang, Y.-W. Stimuli-responsive drug delivery systems based on supramolecular nanovalves. Matter 2019, 1, 345–368. [Google Scholar] [CrossRef]
- Schneebeli, S.T.; Cheng, C.; Hartlieb, K.J.; Strutt, N.L.; Sarjeant, A.A.; Stern, C.L.; Stoddart, J.F. Asararenes—A family of large aromatic macrocycles. Chem. Eur. J. 2013, 19, 3860–3868. [Google Scholar] [CrossRef]
- Boinski, T.; Cieszkowski, A.; Rosa, B.; Szumna, A. Hybrid[n]arenes through thermodynamically driven macrocyclization reactions. J. Org. Chem. 2015, 80, 3488–3495. [Google Scholar] [CrossRef]
- Gao, B.; Tan, L.-L.; Song, N.; Li, K.; Yang, Y.-W. A high-yield synthesis of [m]biphenyl-extended pillar[n]arenes for an efficient selective inclusion of toluene and m-xylene in the solid state. Chem. Commun. 2016, 52, 5804–5807. [Google Scholar] [CrossRef]
- Dai, D.; Li, Z.; Yang, J.; Wang, C.; Wu, J.-R.; Wang, Y.; Zhang, D.; Yang, Y.-W. Supramolecular assembly-induced emission enhancement for efficient mercury(II) detection and removal. J. Am. Chem. Soc. 2019, 141, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Yang, J.; Zou, Y.-C.; Wu, J.-R.; Tan, L.-L.; Wang, Y.; Li, B.; Lu, T.; Wang, B.; Yang, Y.-W. Macrocyclic arenes-based conjugated macrocycle polymers for highly selective CO2 capture and iodine adsorption. Angew. Chem. Int. Ed. 2021, 60, 8967–8975. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-R.; Wang, C.-Y.; Tao, Y.-C.; Wang, Y.; Li, C.; Yang, Y.-W. A Water-soluble [2]biphenyl-extended pillar[6]arene. Eur. J. Org. Chem. 2018, 2018, 1321–1325. [Google Scholar] [CrossRef]
- Yang, W.; Samanta, K.; Wan, X.; Thikekar, T.U.; Chao, Y.; Li, S.; Du, K.; Xu, J.; Gao, Y.; Zuilhof, H.; et al. Tiara[5]arenes: Synthesis, solid-state conformational studies, host–guest properties, and Application as Nonporous Adaptive Crystals. Angew. Chem. Int. Ed. 2020, 59, 3994–3999. [Google Scholar] [CrossRef]
- Lei, S.-N.; Xiao, H.; Zeng, Y.; Tung, C.-H.; Wu, L.-Z.; Cong, H. BowtieArene: A dual macrocycle exhibiting stimuli-responsive fluorescence. Angew. Chem. Int. Ed. 2020, 59, 10059–10065. [Google Scholar] [CrossRef]
- Wu, J.-R.; Mu, A.U.; Li, B.; Wang, C.-Y.; Fang, L.; Yang, Y.-W. Desymmetrized leaning pillar[6]arene. Angew. Chem. Int. Ed. 2018, 57, 9853–9858. [Google Scholar] [CrossRef]
- Wu, J.-R.; Li, B.; Yang, Y.-W. Separation of bromoalkanes isomers by nonporous adaptive crystals of leaning pillar[6]arene. Angew. Chem. Int. Ed. 2020, 59, 2251–2255. [Google Scholar] [CrossRef]
- Wu, J.-R.; Yang, Y.-W. Geminiarene: Molecular scale dual selectivity for chlorobenzene and chlorocyclohexane fractionation. J. Am. Chem. Soc. 2019, 141, 12280–12287. [Google Scholar] [CrossRef]
- Wu, J.-R.; Wang, Y.; Yang, Y.-W. Elongated-geminiarene: Syntheses, solid-state conformational investigations, and application in aromatics/cyclic aliphatics separation. Small 2020, 16, 2003490. [Google Scholar] [CrossRef]
- Han, X.-N.; Han, Y.; Chen, C.-F. Pagoda[4]arene and i-Pagoda[4]arene. J. Am. Chem. Soc. 2020, 142, 8262–8269. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Li, C. Biphen[n]arenes: Modular synthesis, customizable cavity sizes, and diverse skeletons. Acc. Chem. Res. 2022, 55, 916–929. [Google Scholar] [CrossRef]
- Wu, J.-R.; Cai, Z.; Wu, G.; Dai, D.; Liu, Y.-Q.; Yang, Y.-W. Bottom-up solid-state molecular assembly via guest-induced intermolecular interactions. J. Am. Chem. Soc. 2021, 143, 20395–20402. [Google Scholar] [CrossRef]
- Wu, J.-R.; Wu, G.; Li, D.; Dai, D.; Yang, Y.-W. Guest-induced amorphous-to-crystalline transformation enables sorting of haloalkane isomers with near-perfect selectivity. Sci. Adv. 2022, 8, eabo2255. [Google Scholar] [CrossRef]
- Joseph, R.; Naugolny, A.; Feldman, M.; Herzog, I.M.; Fridman, M.; Cohen, Y. Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J. Am. Chem. Soc. 2016, 138, 754–757. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, J.; Shen, J.; Tang, G.; Huang, F. Cationic pillar[6]arene/ATP host–guest recognition: Selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem. Sci. 2016, 7, 4073–4078. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J. Discovery of non-classical complex models between a cationic water-soluble pillar[6]arene and naphthalenesulfonate derivatives and their self-assembling behaviors. Soft Matter 2019, 15, 4127–4131. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Zhang, Z.; Yu, G. A cationic water-soluble biphen[3]arene: Synthesis, host–guest complexation and fabrication of a supra-amphiphile. RSC Adv. 2016, 6, 77179–77183. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-R.; Wu, G.; Cai, Z.; Li, D.; Li, M.-H.; Wang, Y.; Yang, Y.-W. A Water-Soluble Leggero Pillar[5]arene. Molecules 2022, 27, 6259. https://doi.org/10.3390/molecules27196259
Wu J-R, Wu G, Cai Z, Li D, Li M-H, Wang Y, Yang Y-W. A Water-Soluble Leggero Pillar[5]arene. Molecules. 2022; 27(19):6259. https://doi.org/10.3390/molecules27196259
Chicago/Turabian StyleWu, Jia-Rui, Gengxin Wu, Zhi Cai, Dongxia Li, Meng-Hao Li, Yan Wang, and Ying-Wei Yang. 2022. "A Water-Soluble Leggero Pillar[5]arene" Molecules 27, no. 19: 6259. https://doi.org/10.3390/molecules27196259
APA StyleWu, J.-R., Wu, G., Cai, Z., Li, D., Li, M.-H., Wang, Y., & Yang, Y.-W. (2022). A Water-Soluble Leggero Pillar[5]arene. Molecules, 27(19), 6259. https://doi.org/10.3390/molecules27196259






