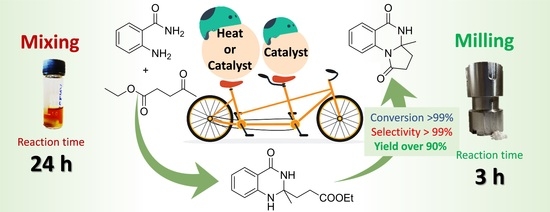
Synthesis of a Pyrrolo[1,2-a]quinazoline-1,5-dione Derivative by Mechanochemical Double Cyclocondensation Cascade
Abstract
:1. Introduction
2. Results and Discussions
2.1. Catalytic Cascade Reaction of Anthranilamide and Ethyl Levulinate in the Batch System
2.2. Mechanochemical Catalytic Cascade Reaction of Anthranilamide and Ethyl Levulinate
3. Materials and Methods
3.1. Reaction of Anthranilamide and Ethyl Levulinate in the Batch System: General Procedure
3.2. Reaction of Anthranilamide and Ethyl Levulinate by Ball Milling: General Procedure


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Frank, É.; Szőllősi, G. Nitrogen-Containing Heterocycles as Significant Molecular Scaffolds for Medicinal and Other Applications. Molecules 2021, 26, 4617. [Google Scholar] [CrossRef]
- Alaimo, R.J. Antibacterial 2,3-Dihydro-2-(5-nitro-2-thienyl)quinazolin-4-(1H)-ones. J. Med. Chem. 1972, 15, 335–336. [Google Scholar] [CrossRef]
- Parish, H.A., Jr.; Gilliom, R.D. Syntheses and Diuretic Activity of 1,2-Dihydro-2-(3-pyridyl)-3H-pyrido[2,3-d]pyrimidin-4-one and Related Compounds. J. Med. Chem. 1982, 25, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hour, M.-J.; Huang, L.-J.; Kuo, S.-C.; Xia, Y.; Bastow, K.; Nakanishi, Y.; Hamel, E.; Lee, K.-H. 6-Alkylamino- and 2,3-Dihydro-3’-methoxy-2-phenyl-4-quinazolinones and Related Compounds: Their Synthesis, Cytotoxicity, and Inhibition of Tubulin Polymerization. J. Med. Chem. 2000, 43, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Kaur, G.; Pahwa, R.; Sharma, A.; Rajak, H. Quinazolinone Analogs as Potential Therapeutic Agents. Curr. Med. Chem. 2011, 18, 4786–4812. [Google Scholar] [CrossRef]
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Patents 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Wahan, S.K.; Sharma, B.; Chawla, P.A. Medicinal perspective of quinazolinone derivatives: Recent developments and structure-activity relationship studies. J. Heterocycl. Chem. 2022, 59, 239–257. [Google Scholar] [CrossRef]
- Badolato, M.; Aiello, F.; Neamatti, N. 2,3-Dihydroquinazolin-4(1H)-one as a privileged scaffold in drug design. RSC Adv. 2018, 8, 20894–20921. [Google Scholar] [CrossRef] [PubMed]
- Yale, H.L.; Kalkstein, M. Substituted 2,3-Dihydro-4(1H)-quinazolinones. A New Class of Inhibitors of Cell Multiplication. J. Med. Chem. 1967, 10, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.P.; Sivappa, R. A short and efficient general synthesis of luotonin A, B and E. Tetrahedron 2004, 60, 9931–9935. [Google Scholar] [CrossRef]
- Klemm, L.H.; Weakly, T.J.R.; Gilbertson, R.D.; Song, Y.-H. Definitive Structural Assignment of Condensation Products from Anthranilamide and 3-Amino-2-carbamoylthiophene with Ketones. Formation of Tetrahydroquinazolinones and Their Thiophene Isosteres. J. Heterocycl. Chem. 1998, 35, 1269–1273. [Google Scholar] [CrossRef]
- Bunce, R.A.; Nammalwar, B. New Conditions for Synthesis of (±)-2-Monosubstituted and (±)-2,2-Disubstituted 2,3-Dihydro-4(1H)-quinazolinones from 2-Nitro and 2-Aminobenzamide. J. Heterocycl. Chem. 2011, 48, 991–997. [Google Scholar] [CrossRef]
- Esfandiari, S.; Maghsoodlou, M.T.; Habibi-Khorassani, S.M.; Kiaee, S.; Aboonajmi, J. Malonic acid as a catalyst for efficient and simple synthesis of 2,3-dihydroquinazolin-4(1H)-one in green solvent. Iranian J. Org. Chem. 2012, 4, 827–830. [Google Scholar]
- Zhaleh, S.; Hazeri, N.; Maghsoodlou, M.T. Green protocol for synthesis of 2,3-dihydroquinazolin-4(1H)-ones: Lactic acid as catalyst under solvent-free condition. Res. Chem. Intermed. 2016, 42, 6381–6390. [Google Scholar] [CrossRef]
- Sarfraz, M.; Sultana, N.; Rashid, U.; Akram, M.S.; Sadiq, A.; Tariq, M.I. Synthesis, biological evaluation and docking studies of 2,3-dihydroquinazolin-4(1H)-one derivatives as inhibitors of cholinesterases. Bioorg. Chem. 2017, 70, 237–244. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kaur, V. Synthesis of 3-Oxa- and 3-Aza-1-dethiacepham Analogs. Synthesis 1989, 677–680. [Google Scholar] [CrossRef]
- Bhavani, A.K.D.; Reddy, P.S.N. Synthesis of Some Tetrahydro-3,3’-bisquinazolin-4,4’-diones. Org. Prep. Proc. Int. 1992, 24, 1–5. [Google Scholar] [CrossRef]
- Naleway, J.J.; Fox, C.M.J.; Robinhold, D.; Terpetschnig, E.; Olson, N.A.; Haugland, R.P. Synthesis and Use of New Fluorogenic Precipitating Substrates. Tetrahedron Lett. 1994, 35, 8569–8572. [Google Scholar] [CrossRef]
- Zappalà, M.; Grasso, S.; Micale, N.; Zuccalà, G.; Menniti, F.S.; Ferreri, G.; Di Sarro, G.; De Micheli, C. 1-Aryl-6,7-methylenedioxy-3H-quinazolin-4-ones as Anticonvulsant Agents. Bioorg. Med. Chem. Lett. 2003, 13, 4427–4430. [Google Scholar] [CrossRef]
- Labade, V.B.; Shinde, P.V.; Shingare, M.S. A facile and rapid access towards the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Tetrahedron Lett. 2013, 54, 5778–5780. [Google Scholar] [CrossRef]
- Chen, J.X.; Wu, H.Y.; Su, W.K. A facile synthesis of 2,3-dihydro-2-aryl-4(1H)-quinazolinones catalyzed by scandium(III) triflate. Chin. Chem. Lett. 2007, 18, 536–538. [Google Scholar] [CrossRef]
- Wang, X.-S.; Yang, K.; Zhou, J.; Tu, S.-J. Facile Method for the Combinatorial Synthesis of 2,2-Disubstituted Quinazolin-4(1H)-one Derivatives Catalyzed By Iodine in Ionic Liquids. J. Comb. Chem. 2010, 12, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi-Alibeik, M.; Shabani, E. Synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by zirconium (IV) chloride as a mild and efficient catalyst. Chin. Chem. Lett. 2011, 22, 1163–1166. [Google Scholar] [CrossRef]
- Englund, E.E.; Neumann, S.; Eliseeva, E.; McCoy, J.G.; Titus, S.; Zheng, W.; Southall, N.; Shinn, P.; Leister, W.; Thomas, C.J.; et al. The synthesis and evaluation of dihydroquinazolin-4-ones and quinazolin-4-ones as thyroid stimulating hormone receptor agonists. Med. Chem. Commun. 2011, 2, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.-H.; Fan, L.-Y.; Li, X.-X.; Liu, M.-X. Y(OTf)3-catalyzed heterocyclic formation via aerobic oxygenation: An approach to dihydro quinazolinones and quinazolinones. Chin. Chem. Lett. 2015, 26, 1355–1358. [Google Scholar] [CrossRef]
- Rajaka, L.; Penumati, N.R.; Nagaiah, K.; Poornachandra, Y.; Kumar, C.G. Convenient and Scalable Synthesis of 2,3-Dihydroquinazolin-4(1H)-one Derivatives and Their Anticancer Activities. Synth. Commun. 2015, 45, 1893–1901. [Google Scholar] [CrossRef]
- Ramesh, N.; Rao, M.G.; Varala, R.; Rao, V.U.; Babu, B.H. Mercuric chloride catalyzed synthesis of some anticancer 2-aryl-2,3-dihydroquinazolin-4(1H)-ones. Med. Chem. Res. 2016, 25, 1945–1951. [Google Scholar] [CrossRef]
- Sivaguru, P.; Parameswaran, K.; Lalitha, A. Synthesis of novel eight-membered dibenzo[b,f][1,5]oxazocin-6-ones. Tetrahedron Lett. 2016, 57, 2549–2553. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Wang, Y.; Sun, H.; Xie, Z.; Zhang, W.; Gao, Z. Ethanol promoted titanocene Lewis acid catalyzed synthesis of quinazoline derivatives. RSC Adv. 2016, 6, 66074–66077. [Google Scholar] [CrossRef]
- Shaabani, A.; Maleki, A.; Mofakham, H. Click Reaction: Highly Efficient Synthesis of 2,3-Dihydroquinazolin-4(1H)-ones. Synth. Commun. 2008, 38, 3751–3759. [Google Scholar] [CrossRef]
- Li, S.-C.; Jhang, W.-F.; Liou, T.-J.; Yang, D.-Y. Photochemical synthesis of indazolo[3,2-b]quinazolines and their redox-switching properties. Dyes Pigm. 2015, 114, 259–266. [Google Scholar] [CrossRef]
- Qiao, R.Z.; Xu, B.L.; Wang, Y.H. A facile synthesis of 2-substituted-2,3-dihydro-4(1H)-quinazolinones in 2,2,2-trifluoroethanol. Chin. Chem. Lett. 2007, 18, 656–658. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Amani, A.M.; Aryan, R.; Ghaieni, H.R.; Shadjou, N. Synthesis of New 2-Aryl Substituted 2,3-Dihydroquinazolin-4(1H)-ones Under Solvent-Free Conditions, Using Molecular Iodine as a Mild and Efficient Catalyst. Synth. Commun. 2008, 38, 3567–3576. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Zarei, L. Rapid Synthesis of 2-Substituted-2,3-dihydro-4(1H)-quinazolinones using Boric Acid or Sodium Dihydrogen Phosphate under Solvent-Free Conditions. S. Afr. J. Chem. 2012, 65, 36–38. [Google Scholar]
- Xie, Z.-B.; Zhang, S.-G.; Jiang, G.-F.; Sun, D.-Z.; Le, Z.-G. The green synthesis of 2,3-dihydroquinazolin-4(1H)-ones via direct cyclocondensation reaction under catalyst-free conditions. Green Chem. Lett. Rev. 2015, 8, 95–98. [Google Scholar] [CrossRef]
- Roy, A.D.; Jayalakshmi, K.; Dasgupta, S.; Roy, R.; Mukhopadhyay, B. Real time HR-MAS NMR: Application in reaction optimization, mechanism elucidation and kinetic analysis for heterogeneous reagent catalyzed small molecule chemistry. Magn. Reson. Chem. 2008, 46, 1119–1126. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Oveisi, A.R. PPA-SiO2 as a Heterogeneous Catalyst for Efficient Synthesis of 2-Substituted-1,2,3,4-tetrahydro-4-quinazolinones under Solvent-free Conditions. Chin. J. Chem. 2009, 27, 2418–2422. [Google Scholar] [CrossRef]
- Dar, B.A.; Sahu, A.K.; Patidar, P.; Sharma, P.R.; Mupparapu, N.; Vyas, D.; Maity, S.; Sharma, M.; Singh, B. Heteropolyacid-clay nano-composite as a novel heterogeneous catalyst for the synthesis of 2,3-dihydroquinazolinones. J. Ing. Eng. Chem. 2013, 19, 407–412. [Google Scholar] [CrossRef]
- Dindulkar, S.D.; Oh, J.; Arole, V.M.; Jeong, Y.T. Supported ceric ammonium nitrate: A highly efficient catalytic system for the synthesis of diversified 2,3-substituted 2,3-dihydroquinazolin-4(1H)-ones. Comptes Rendus Chim. 2014, 17, 971–979. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. Efficient synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones in the presence of nanocomposites under microwave irradiation. J. Mol. Catal. A: Chem. 2014, 390, 1–6. [Google Scholar] [CrossRef]
- Havasi, F.; Ghorbani-Choghamarani, A.; Nikpour, F. Synthesis and characterization of nickel complex anchored onto MCM-41 as a novel and reusable nanocatalyst for the efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Microporous Mesoporous Mat. 2016, 24, 26–35. [Google Scholar] [CrossRef]
- Kausar, N.; Roy, I.; Chattopadhyay, D.; Das, A.R. Synthesis of 2,3-dihydroquinazolinones and quinazolin-4(3H)-ones catalyzed by graphene oxide nanosheets in an aqueous medium: “on-water” synthesis accompanied by carbocatalysis and selective C-C bond cleavage. RSC Adv. 2016, 6, 22320–22330. [Google Scholar] [CrossRef]
- Shiri, L.; Ghorbani-Choghamarani, A.; Kazemi, M. Cu(II) immobilized on Fe3O4‒diethylenetriamine: A new magnetically recoverable catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones and oxidative coupling of thiols. Appl. Organometal. Chem. 2017, 31, e3596. [Google Scholar] [CrossRef]
- Mirjalili, B.B.F.; Bamoniri, A.; Azad, S. Synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by nano-Fe3O4/TiCl2/cellulose as a bio-based magnetic catalyst. J. Iran. Chem. Soc. 2017, 14, 47–55. [Google Scholar] [CrossRef]
- Radfar, I.; Miraki, M.K.; Ghandi, L.; Esfandiary, N.; Abbasi, A.; Karimi, M.; Heydar, A. BF3-grafted Fe3O4@Sucrose nanoparticles as a highly-efficient acid catalyst for syntheses of Dihydroquinazolinones (DHQZs) and Bis 3-Indolyl Methanes (BIMs). Appl. Organometal. Chem. 2018, 32, e4431. [Google Scholar] [CrossRef]
- Efran, M.A.; Akhlaghinia, B.; Ghodsinia, S.S.E. An Efficient Green Protocol for Synthesis of 2,3-Dihyroquinazolin-4(1H)-ones Using SBA-16/GPTMS-TSC-CuI under Solvent-Free Conditions. ChemistrySelect 2020, 5, 2306–2316. [Google Scholar] [CrossRef]
- Subba Reddy, B.V.; Venkateswarlu, A.; Madan, C.; Vinu, A. Cellulose-SO3H: An efficient and biodegradable solid acid for the synthesis of quinazolin-4(1H)-ones. Tetrahedron Lett. 2011, 52, 1891–1894. [Google Scholar] [CrossRef]
- Ghashang, M.; Mansoor, S.S.; Aswin, K. Synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by succinimide-N-sulfonic acid as a mild and efficient catalyst. Res. Chem. Intermed. 2015, 41, 3447–3460. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Azadi, G. Synthesis, characterization, and application of Fe3O4-SA-PPCA as a novel nanomagnetic reusable catalyst for the efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones and polyhydroquinolines. RSC Adv. 2015, 5, 9752–9758. [Google Scholar] [CrossRef]
- Rao, A.V.D.; Vykunteswararao, B.P.; Bhaskarkumar, T.; Jogdand, N.R.; Kalita, D.; Lilakar, J.K.D.; Siddaiah, V.; Sanasi, P.D.; Raghunadh, A. Sulfonic acid functionalized Wang resin (Wang-OSO3H) as polymeric acidic catalyst for the eco-friendly synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Tetrahedron Lett. 2015, 56, 4714–4717. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Tahmasbi, B. The first report on the preparation of boehmite silica sulfuric acid and its application in some multicomponent organic reactions. New J. Chem. 2016, 40, 1205–1212. [Google Scholar] [CrossRef]
- Hajjami, M.; Ghorbani-Choghamarani, A.; Ghafouri-Nejad, R.; Tahmasbi, B. Efficient preparation of boehmite silica dopamine sulfamic acid as a novel nanostructured compound and its application as a catalyst in some organic reactions. New J. Chem. 2016, 40, 3066–3074. [Google Scholar] [CrossRef]
- Bharate, S.B.; Mupparapu, N.; Manda, S.; Bharate, J.B.; Mudududdla, R.; Yadav, R.R.; Vishwakarma, R.A. Efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones using heterogeneous solid acid catalysts: Unexpected formation of 2,3-dihydro-2-(4-(tetrahydro-2H-pyran-2-yloxy)butyl)quinazolin-4(1H)-one. Arkivoc 2012, viii, 308–318. [Google Scholar] [CrossRef]
- Murthy, P.V.N.S.; Rambabu, D.; Krishna, G.R.; Reddy, C.M.; Prasad, K.R.S.; Rao, M.V.B.; Pal, M. Amberlyst-15 mediated synthesis of 2-substituted 2,3-dihydroquinazolin-4(1H)-ones and their crystal structure analysis. Tetrahedron Lett. 2012, 53, 863–867. [Google Scholar] [CrossRef]
- Rambabu, D.; Kumar, S.K.; Sreenivas, B.Y.; Sandra, S.; Kandaale, A.; Misra, P.; Rao, M.V.B.; Pal, M. Ultrasound-based approach to spiro-2,3-dihydroquinazolin-4(1H)-ones: Their in vitro evaluation against chorismate mutase. Tetrahedron Lett. 2013, 54, 495–501. [Google Scholar] [CrossRef]
- Cai, G.; Xu, X.; Li, Z.; Weber, W.P.; Lu, P. A One-Pot Synthesis of 2-Aryl-2,3-dihydro-4(1H)-quinazolinones by use of Samarium Iodide. J. Heterocycl. Chem. 2002, 39, 1271–1272. [Google Scholar] [CrossRef]
- Su, W.; Yang, B. Reductive Cyclization of Nitro and Azide Compounds with Aldehydes and Ketones Promoted by Metallic Samarium and Catalytic Amount of Iodine. Aust. J. Chem. 2002, 55, 695–697. [Google Scholar] [CrossRef]
- Shi, D.; Rong, L.; Wang, J.; Zhuang, Q.; Wang, X.; Hu, H. Synthesis of quinazolin-4(3H)-ones and 1,2-dihydroquinazolin-4(3H)-ones with the aid of a low-valent titanium reagent. Tetrahedron Lett. 2003, 44, 3199–3201. [Google Scholar] [CrossRef]
- Surpur, M.P.; Singh, P.R.; Patil, S.B.; Samant, S.D. Expeditious One-Pot and Solvent-Free Synthesis of Dihydroquinazolin-4(1H)-ones in the Presence of Microwaves. Synth. Commun. 2007, 37, 1965–1970. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Amani, A.M.; Mahdavinia, G.H.; Sepehrian, H.; Ebrahimi, S. Synthesis of Some Novel 2-Aryl-Substituted 2,3-Dihydroquinazolin-4(1H)-ones under Solvent-Free Conditions Using MCM-41-SO3H as a Highly Efficient Sulfonic Acid. Synthesis 2010, 1356–1360. [Google Scholar] [CrossRef]
- Tamaddon, F.; Pouramini, F. Amberlyst A26 OH as a Recyclable Catalyst for Hydration of Nitriles and Water-Based Synthesis of 4(1H)-Quinazolinones from 2-Aminobenzonitrile and Carbonyl Compounds. Synlett 2014, 25, 1127–1131. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Rigi, F. An efficient synthesis of quinazoline and xanthene derivatives using starch sulfate as a biodegradable solid acid catalyst. Res. Chem. Intermed. 2015, 41, 721–738. [Google Scholar] [CrossRef]
- Wang, X.-S.; Yang, K.; Zhang, M.-M.; Yao, C.-S. Synthesis of 2-Arylquinazolin-4(3H)-one Derivatives Catalyzed by Iodine in [bmim+][BF4‒]. Synth. Commun. 2010, 40, 2633–2646. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Matsusaki, Y.; Nobuta, T.; Tada, N.; Miura, T.; Itoh, A. Aerobic photooxidative synthesis of 2-aryl-4-quinazolinones from aromatic aldehydes and aminobenzamide using catalytic amounts of molecular iodine. RSC Adv. 2015, 5, 63952–63954. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhang, D.; Zhang, L.; Sun, H.; Jiang, H.; Liu, H. Gold(I)-Catalyzed Tandem Transformation: A Simple Approach for the Synthesis of Pyrrolo/Pyrido[2,1-a][1,3]benzoxazinones and Pyrrolo/Pyrido[2,1-a]quinazolinones. J. Org. Chem. 2010, 75, 3274–3282. [Google Scholar] [CrossRef]
- Wang, M.; Dou, G.; Shi, D. Efficient and Convenient Synthesis of Pyrrolo[1,2-a]quinazoline Derivatives with the Aid of Tin(II) Chloride. J. Comb. Chem. 2010, 12, 582–586. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, D.-Q. An Efficient Synthesis of Pyrrolo[1,2-a]quinazolin-5(1H)-one Derivatives with the Aid of Low-Valent Titanium Reagent. J. Heterocycl. Chem. 2011, 48, 634–638. [Google Scholar] [CrossRef]
- Safai, H.R.; Shekouhy, M.; Khademi, S.; Rahmanian, V.; Safaei, M. Diversity-oriented synthesis of quinazoline derivatives using zirconium tetrakis(dodecylsulfate) [Zr(DS)4] as a reusable Lewis acid-surfactant-combined catalyst in tap water. J. Ind. Eng. Chem. 2014, 20, 3019–3024. [Google Scholar] [CrossRef]
- Lu, L.; Yang, K.; Zhang, M.-M.; Wang, X.-S. An Efficient Synthesis of Pyrrolo[1,2-a]quinazoline Derivatives in Ionic Liquid Catalyzed by Iodine. J. Heterocycl. Chem. 2014, 51, 841–845. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, C.; Wang, Q.; Yang, G. Catalytic dehydration of hexose sugars to 5-hydroxymethylfural. Biofuels Bioprod. Biorefining 2019, 13, 153–173. [Google Scholar] [CrossRef]
- Nzediegwu, E.; Dumont, M.-J. Chemo-Catalytic Transformation of Cellulose and Cellulosic-Derived Waste Materials into Platform Chemicals. Waste Biomass Valorization 2021, 12, 2825–2851. [Google Scholar] [CrossRef]
- Castro, G.A.D.; Fernandes, S.A. Microwave-assisted green synthesis of levulinate esters as biofuel precursors using calix[4]arene as an organocatalyst under solvent-free conditions. Sustain. Energ. Fuels 2021, 5, 108–111. [Google Scholar] [CrossRef]
- Zhang, W.; Cue, B.W. (Eds.) Green Techniques for Organic Synthesis and Medicinal Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Protti, S.; Palmieri, A. (Eds.) Sustainable Organic Synthesis: Tools and Strategies; Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef]
- Ranu, B.; Stolle, A. (Eds.) Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; RSC Green Chemistry, No. 31; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Margetić, D.; Štrukil, V. Mechanochemical Organic Synthesis; Elsevier Inc.: Amsterdam, The Netherland, 2016. [Google Scholar]
- Avila-Ortiz, C.G.; Juaristi, E. Novel Methodologies for Chemical Activation in Organic Synthesis under Solvent-Free Reaction Conditions. Molecules 2020, 25, 3579. [Google Scholar] [CrossRef]
- Prasad, D.V.N.; Theuerkauf, J. Effect of Grinding Media Size And Chamber Length on Grinding in a Spex Mixer Mill. Chem. Eng. Technol. 2009, 32, 1102–1106. [Google Scholar] [CrossRef]
- Prasad, D.V.N.; Theuerkauf, J. Improvement in the Collision Intensity of Grinding Media in High Energy Impact Mills. Chem. Eng. Technol. 2010, 33, 1433–1437. [Google Scholar] [CrossRef]
- Fulmer, D.A.; Shearouse, W.C.; Medonza, S.T.; Mack, J. Solvent-Free Sonogashira coupling reaction via high speed ball milling. Green Chem. 2009, 11, 1821–1825. [Google Scholar] [CrossRef]
- Sawama, Y.; Yasukawa, N.; Ban, K.; Goto, R.; Niikawa, M.; Monguchi, Y.; Itoh, M.; Sajiki, H. Stainless Steel-Mediated Hydrogen Generation from Alkanes and Diethyl Ether and Its Application for Arene Reduction. Org. Lett. 2018, 20, 2892–2896. [Google Scholar] [CrossRef]
- Bolm, C.; Hernández, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chem. Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef]
- Gérard, E.M.C.; Sahin, H.; Encinas, A.; Bräse, S. Systematic Study of a Solvent-Free Mechanochemically Induced Domino Oxa-Michael‒Aldol Reaction in a Ball Mill. Synlett 2008, 2702–2704. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Eslami, M. Highly efficient organocatalytic synthesis of diverse and densely functionalized 2-amino-3-cyano-4H-pyrans under mechanochemical ball milling. Green Chem. 2014, 16, 4914–4921. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Tagliapierta, S.; Mantegna, S.; Cravotto, G. Mechanochemical and sonochemical heterocyclizations. Chem. Heterocycl. Compds. 2016, 52, 856–865. [Google Scholar] [CrossRef]
- El-Sayed, T.H.; Aboelnaga, A.; El-Atawy, M.A.; Hagar, M. Ball Milling Promoted N-Heterocycles Synthesis. Molecules 2018, 23, 1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, J.J.; Song, Z.G.; Wang, L. Cerium(IV) ammonium nitrate catalyzed green synthesis of 2,3-dihydroquinazolin-4(1H)-ones using a grinding technique. Chem. Heterocycl. Compds. 2011, 47, 851–855. [Google Scholar] [CrossRef]
- Ding, Q.-S.; Zhang, J.-L.; Chen, J.-X.; Liu, M.-C.; Ding, J.-C.; Wu, H.-Y. Tandem Synthesis of 2,3-Dihydroquinazolin-4(1H)-ones on Grinding under Solvent-Free Conditions. J. Heterocycl. Chem. 2012, 49, 375–380. [Google Scholar] [CrossRef]
- Wang, M.; Gao, J.; Song, Z.; Wang, L. Cerous Methanesulfonate Catalyzed Facile Synthesis of 2-Substituted-2,3-dihydro-4(1h)-quinazolinones by Grinding Technique. J. Heterocycl. Chem. 2012, 49, 1250–1253. [Google Scholar] [CrossRef]
- Sharma, R.; Pandey, A.K.; Chauhan, P.M.S. A Greener Protocol for Accessing 2,3-Dihydro/spiroquinazolin-4(1H)-ones: Natural Acid-SDS Catalyzed Three-Component Reaction. Synlett 2012, 23, 2209–2214. [Google Scholar] [CrossRef]
- Wang, M.; Gao, J.-J.; Song, Z.-G.; Wang, L. Synthesis of 2-Substituted-2,3-dihydro-4(1H)-quinazolinones using Sodium Bisulfate as a Catalyst by the Grinding Technique. Org. Prep. Proceed. Int. 2012, 44, 159–163. [Google Scholar] [CrossRef]
- Miklós, F.; Hum, V.; Fülöp, F. Eco-friendly syntheses of 2,2-disubstituted- and 2-Spiroquinazolinones. Arkivoc 2014, vi, 25–37. [Google Scholar] [CrossRef]
- Magyar, T.; Miklós, F.; Lázár, L.; Fülöp, F. Synthesis of 2-(hetero)arylquinazolinones in aqueous media. Arkivoc 2016, vi, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Szőllősi, G. Asymmetric one-pot reactions using heterogeneous chemical catalysis: Recent steps towards sustainable processes. Catal. Sci. Technol. 2018, 8, 389–422. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Kolcsár, V.J.; Szőllősi, G. Mechanochemical, Water-Assisted Asymmetric Transfer Hydrogenation of Ketones Using Ruthenium Catalyst. ChemCatChem 2022, 14, e202101501. [Google Scholar] [CrossRef]
- Kolcsár, V.J.; Szőllősi, G. Ru-catalyzed mechanochemical asymmetric transfer hydrogenations in aqueous media using chitosan as chirality source. Mol. Catal. 2022, 520, 112162. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.C.; Lee, J.S.; Kim, Y.G. Carbonylation of Formaldehyde over Ion Exchange Resin Catalysts. 1. Batch Reactor Studies. Ind. Eng. Chem. Res. 1993, 32, 253–259. [Google Scholar] [CrossRef]
- Park, H.-S.; Ihm, S.-K. Alkylation of benzene with 1-dodecene by macroreticular resin catalysts. Korean J. Chem. Eng. 1985, 2, 69–74. [Google Scholar] [CrossRef]
- Pal, R.; Sarkar, T.; Khasnobis, S. Amberlyst-15 in organic synthesis. Arkivoc 2012, i, 570–609. [Google Scholar] [CrossRef]
- Fraile, J.M.; Saavedra, C.J. Application of Heterogeneous Catalysts in the First Steps of the Oseltamivir Synthesis. Catalysts 2017, 7, 393. [Google Scholar] [CrossRef]
- Kornas, A.; Śliwa, M.; Ruggiero-Mikołajczyk, M.; Samson, K.; Podobiński, J.; Karcz, R.; Duraczyńska, D.; Rutkowska-Zbik, D.; Grabowski, R. Direct hydrogenation of CO2 to dimethyl ether (DME) over hybrid catalysts containing CuO/ZrO2 as a metallic function and heteropolyacids as an acidic function. Reac. Kin. Mech. Catal. 2020, 130, 179–194. [Google Scholar] [CrossRef]
- Habibi, D.; Marvi, O. Montmorillonite KSF clay as an efficient catalyst for the synthesis of 1,4-dioxo-3,4-dihydrophthalazine-2(1H)-carboxamides and -carbothioamides under solvent-free conditions using microwave irradiation. Catal. Commun. 2007, 8, 127–130. [Google Scholar] [CrossRef]



| Entry | Catalyst | Catalyst Amount | T (°C) b | t (h) c | Conv (%) d | S3 (%) e | S4 (%) e |
|---|---|---|---|---|---|---|---|
| 1 | ‒ | ‒ | 60 | 24 | 40 | 83 | 15 |
| 2 | ‒ | ‒ | 90 | 24 | 95 | 73 (60) f | 24 |
| 3 | Silica gel 60 | 125 mg | 90 | 48 | 99 | 48 | 51 |
| 4 | Mont K10 | 125 mg | 90 | 24 | >99 | 60 | 39 |
| 5 | Mont K10 | 125 mg | 90 | 48 | >99 | 35 | 63 |
| 6 | Mont KSF | 125 mg | 90 | 48 | >99 | 40 | 58 |
| 7 | p-TsOH | 5 mol% | 60 | 24 | >99 | 48 | 51 |
| 8 | p-TsOH | 5 mol% | 90 | 24 | >99 | ‒ | 99 (90) f |
| 9 | Deloxan® ASP | 100 mg | 60 | 24 | >99 | 80 (70) f | 19 |
| 10 | Deloxan® ASP | 125 mg | 90 | 24 | >99 | ‒ | 98 |
| 11 | Nafion™ NR50 | 86 mg (2 pcs) | 60 | 24 | 96 | 77 (61) f | 21 |
| 12 | Nafion™ NR50 | 84 mg (2 pcs) | 90 | 24 | >99 | ‒ | 99 (88) f |
| 13 | Amberlyst® XN-1010 | 100 mg | 60 | 24 | >99 | ‒ | 99 (90) f |
| 14 | Amberlyst® 15 | 100 mg | 60 | 24 | >99 | ‒ | 99 (91) f |
| Entry | EL Amount (eq) | MeOH Amount (μL) | Conv (%) b | S3 (%) c | S4 (%) c |
|---|---|---|---|---|---|
| 1 | 1.5 | ‒ | >99 | 35 | 64 |
| 2 | 1.1 | ‒ | >99 | 55 | 42 |
| 3 | 1.1 | 57 | >99 | 1 | 97 (89) f |
| 4 d,e | 1.1 | 57 | ‒ | 96 | 2 |
| 5 e | 1.1 | 57 | ‒ | ‒ | 99 (91) f |
| Entry | Diameters of Balls (mm) | Number of Balls (pcs) | Conv (%) b | S3 (%) c | S4 (%) c |
|---|---|---|---|---|---|
| 1 | 15 | 1 | 98 | 36 | 62 |
| 2 | 12 | 1 | 98 | 54 | 42 |
| 3 | 5 | 25 | 97 | 13 | 85 |
| 4 | 3 | 125 | >99 | 90 (80) d | 8 |
| 5 | 5 | 35 | 98 | 4 | 94 |
| 6 | 5 | 40 | >99 | ‒ | 99 (92) d |
| 7 e | 5 | 35 | 77 | 78 | 20 |
| Entry | Reaction Time (min) | Agitation Frequency (Hz) | Conv (%) b | S3 (%) c | S4 (%) c |
|---|---|---|---|---|---|
| 1 | 60 | 30 | 90 | 58 | 40 |
| 2 | 120 | 30 | 93 | 28 | 70 |
| 3 e | 120 | 30 | 97 | 25 | 73 |
| 4 | 180 | 30 | 98 | ‒ | 99 (88) d |
| 5 e | 180 | 30 | >99 | ‒ | 99 (92) d |
| 6 | 180 | 20 | 99 | 80 | 18 |
| 7 f | 180 | 30 | 99 | 38 | 60 |
| Entry | Catalyst | Catalyst Amount | Conv (%) b | S3 (%) c | S4 (%) c |
|---|---|---|---|---|---|
| 1 | ‒ | ‒ | 5 | 80 | 18 |
| 2 | Amberlyst® 15 | 100 mg | 98 | ‒ | 99 |
| 3 | Amberlyst® XN-1010 | 100 mg | 99 | 14 | 85 |
| 4 | Nafion™ NR50 | 85 mg (2 pcs) | 48 | 63 | 35 |
| 5 | Mont K10 | 125 mg | 52 | 82 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolcsár, V.J.; Szőllősi, G. Synthesis of a Pyrrolo[1,2-a]quinazoline-1,5-dione Derivative by Mechanochemical Double Cyclocondensation Cascade. Molecules 2022, 27, 5671. https://doi.org/10.3390/molecules27175671
Kolcsár VJ, Szőllősi G. Synthesis of a Pyrrolo[1,2-a]quinazoline-1,5-dione Derivative by Mechanochemical Double Cyclocondensation Cascade. Molecules. 2022; 27(17):5671. https://doi.org/10.3390/molecules27175671
Chicago/Turabian StyleKolcsár, Vanessza Judit, and György Szőllősi. 2022. "Synthesis of a Pyrrolo[1,2-a]quinazoline-1,5-dione Derivative by Mechanochemical Double Cyclocondensation Cascade" Molecules 27, no. 17: 5671. https://doi.org/10.3390/molecules27175671