A Comparative Loading and Release Study of Vancomycin from a Green Mesoporous Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Mesoporous Silica Synthesis
2.2.2. Drug Loading Process
2.2.3. Physico-Chemical Characterization of the Materials
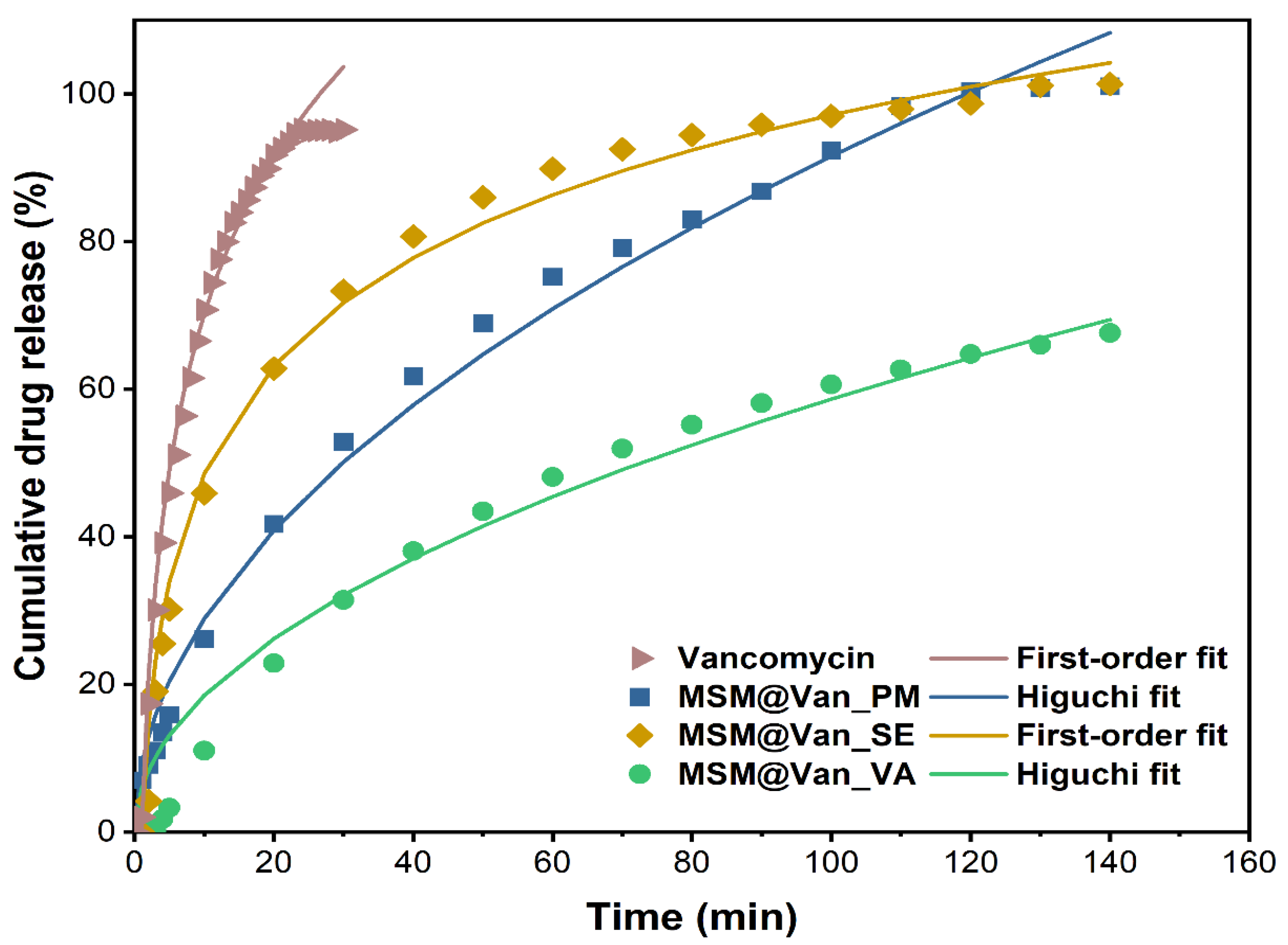
2.2.4. Drug Release and Dissolution Kinetic
| Release Kinetic | Equation | Plot Representation |
|---|---|---|
| Zero-order | cumulative % drug released vs. time | |
| First order | log cumulative %drug remaining vs. time | |
| Higuchi model | cumulative % drug released vs. square root of time |
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, H.-S.; Wang, Y.-H.; Ding, Y. Development of biological metal-organic frameworks designed for biomedical applications: From bio-sensing/bio-imaging to disease treatment. Nanoscale Adv. 2020, 2, 3788–3797. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Zhao, S.; Bi, F.; Song, L.; Liu, N.; Xu, J.; Wang, Y.; Zhang, X. Stable Black Phosphorus Encapsulation in Porous Mesh-like UiO-66 Promoted Charge Transfer for Photocatalytic Oxidation of Toluene and o-Dichlorobenzene: Performance, Degradation Pathway, and Mechanism. ACS Catal. 2022, 12, 8069–8081. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, F.; Zhao, Z.; Yang, Y.; Li, Y.; Song, L.; Liu, N.; Xu, J.; Cui, L. Boosting toluene oxidation by the regulation of Pd species on UiO-66: Synergistic effect of Pd species. J. Catal. 2022, 413, 59–75. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Jin, E.; Palanikumar, L.; Lee, J.H.; Kim, J.C.; Nam, J.S.; Jana, B.; Kwon, T.-H.; Kwak, S.K.; et al. MOF × Biopolymer: Collaborative Combination of Metal–Organic Framework and Biopolymer for Advanced Anticancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 27512–27520. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, D.; Dougherty, C.A.; Lu, W.; Wu, H.; He, X.; Cai, T.; Van Dort, M.E.; Ross, B.D.; Hong, H. In Vivo Targeting and Positron Emission Tomography Imaging of Tumor with Intrinsically Radioactive Metal–Organic Frameworks Nanomaterials. ACS Nano 2017, 11, 4315–4327. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Singh, N.; Qutub, S.; Khashab, N.M. Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef]
- Ailawar, S.; Hunoor, A.; Basu, D.; Rudzinski, B.; Burel, L.; Millet, J.-M.M.; Miller, J.T.; Edmiston, P.L.; Ozkan, U.S. Aqueous phase hydrodechlorination of trichloroethylene using Pd supported on swellable organically modified silica (SOMS): Effect of support derivatization. J. Catal. 2022, 411, 15–30. [Google Scholar] [CrossRef]
- Yu, K.; Kong, W.; Zhao, Z.; Duan, A.; Kong, L.; Wang, X. Hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene over NiMo supported on yolk-shell silica catalysts with adjustable shell thickness and yolk size. J. Catal. 2022, 410, 128–143. [Google Scholar] [CrossRef]
- Bensacia, N.; Fechete, I.; Boutemak, K.; Kettab, A. Mesoporous Materials for Adsorption of Heavy Metals from Wastewater; Springer: Singapore, 2022; pp. 169–186. [Google Scholar]
- Al-Shehri, B.M.; Khder, A.E.R.S.; Ashour, S.S.; Hamdy, M.S. A review: The utilization of mesoporous materials in wastewater treatment. Mater. Res. Express 2019, 6, 122002. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Patescu, R.-E.; Dragne, M.; Stanica, N.; Oprea, O. o-Vanillin functionalized mesoporous silica—Coated magnetite nanoparticles for efficient removal of Pb(II) from water. J. Solid State Chem. 2016, 238, 311–320. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-Ouda, A.; Bak-Sypien, I.I.; Potrzebowski, M.J. Mesoporous Silica Particles as Drug Delivery Systems—The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pîrjol, T.; Muntean, D.; Danciu, C. Properties of Salvia officinalis L. and Thymus serpyllum L. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Ficai, D.; Motelica, L.; Trusca, R.D.; Bîrcă, A.C.; Vasile, B.S.; Voicu, G.; Oprea, O.C.; Semenescu, A.; Ficai, A.; et al. Mesoporous Silica Materials Loaded with Gallic Acid with Antimicrobial Potential. Nanomaterials 2022, 12, 1648. [Google Scholar] [CrossRef]
- Fathi Vavsari, V.; Ziarani, G.; Badiei, A. The role of SBA-15 in drug delivery. RSC Adv. 2015, 5, 91686–91707. [Google Scholar] [CrossRef]
- Alazzawi, H.F.; Salih, I.K.; Albayati, T.M. Drug delivery of amoxicillin molecule as a suggested treatment for covid-19 implementing functionalized mesoporous SBA-15 with aminopropyl groups. Drug Deliv. 2021, 28, 856–864. [Google Scholar] [CrossRef]
- Martens, J.A.; Jammaer, J.; Bajpe, S.; Aerts, A.; Lorgouilloux, Y.; Kirschhock, C.E.A. Simple synthesis recipes of porous materials. Microporous Mesoporous Mater. 2011, 140, 2–8. [Google Scholar] [CrossRef]
- Telalović, S.; Ramanathan, A.; Mul, G.; Hanefeld, U. TUD-1: Synthesis and Application of a Versatile Catalyst, Carrier, Material. New J. Chem. = Nouv. J. De Chim. 2010, 20, 642–658. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Heikkilä, T.; Salonen, J.; Tuura, J.; Hamdy, M.S.; Mul, G.; Kumar, N.; Salmi, T.; Murzin, D.Y.; Laitinen, L.; Kaukonen, A.M.; et al. Mesoporous silica material TUD-1 as a drug delivery system. Int. J. Pharm. 2007, 331, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Salonen, J.; Tuura, J.; Kumar, N.; Salmi, T.; Hamdy, M.; Mul, G.; Laitinen, L.; Tötterman, A.; Hirvonen, J.; et al. Evaluation of mesoporous TCPSi, MCM-41, SBA-15, and TUD-1 materials as API carriers for oral drug delivery. Drug Deliv. 2007, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Jabariyan, S.; Zanjanchi, M.A. A simple and fast sonication procedure to remove surfactant templates from mesoporous MCM-41. Ultrason. Sonochem. 2012, 19, 1087–1093. [Google Scholar] [CrossRef]
- Brigante, M.; Avena, M. Biotemplated synthesis of mesoporous silica for doxycycline removal. Effect of pH, temperature, ionic strength and Ca2+ concentration on the adsorption behaviour. Microporous Mesoporous Mater. 2016, 225, 534–542. [Google Scholar] [CrossRef]
- Ghaedi, H.; Zhao, M. Review on Template Removal Techniques for Synthesis of Mesoporous Silica Materials. Energy Fuels 2022, 36, 2424–2446. [Google Scholar] [CrossRef]
- Kondoh, E.; Segawa, K.; Watanabe, M.; Jin, L.H.; Zhang, L.P.; Baklanov, M.R. Removal of organic template of mesoporous organosilicate thin films using supercritical carbon dioxide fluids. Jpn. J. Appl. Phys. 2017, 56, 07KF02. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, L.; Li, J.H.; Kawi, S.; Goh, A.H. Organic template removal from hexagonal mesoporous silica by means of methanol-enhanced CO2 extraction: Effect of temperature, pressure and flow rate. Sep. Purif. Technol. 2011, 77, 112–119. [Google Scholar] [CrossRef]
- Joshi, H.; Jalalpoor, D.; Ochoa-Hernandez, C.; Schmidt, W.; Schuth, F. Ozone Treatment: A Versatile Tool for the Postsynthesis Modification of Porous Silica-Based Materials. Chem. Mater. 2018, 30, 8905–8914. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, S.; Wang, R.W.; Zhang, Z.T.; Qiu, S.L. A Novel, Efficient and Facile Method for the Template Removal from Mesoporous Materials. Chem. Res. Chin. Univ. 2014, 30, 894–899. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.T. Template Removal from SBA-15 by Ionic Liquid for Amine Grafting: Applications to CO2 Capture and Natural Gas Desulfurization. ACS Sustain. Chem. Eng. 2020, 8, 8295–8304. [Google Scholar] [CrossRef]
- Avila, S.; Cides, L.C.; Fantini, M.; Matos, J. Optimisation of SBA-15 properties using Soxhlet solvent extraction for template removal. Microporous Mesoporous Mater. 2016, 234, 277–286. [Google Scholar] [CrossRef]
- Ferreira, T.M.; Bernin, D.; Topgaard, D. Chapter Three—NMR Studies of Nonionic Surfactants. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 79, pp. 73–127. [Google Scholar]
- Wan, Y.; Shi, Y.; Zhao, D. Designed Synthesis of Mesoporous Solids via Nonionic-Surfactant-Templating Approach. Chem. Commun. 2007, 38, 897–926. [Google Scholar] [CrossRef] [PubMed]
- Smarsly, B.; Polarz, S.; Antonietti, M. Preparation of Porous Silica Materials via Sol−Gel Nanocasting of Nonionic Surfactants: A Mechanistic Study on the Self-Aggregation of Amphiphiles for the Precise Prediction of the Mesopore Size. J. Phys. Chem. B 2001, 105, 10473–10483. [Google Scholar] [CrossRef]
- Ravetti Duran, R.; Blin, J.-L.; Stébé, M.-J.; Castel, C.; Pasc, A. Tuning the morphology and the structure of hierarchical meso–macroporous silica by dual templating with micelles and solid lipid nanoparticles (SLN). J. Mater. Chem. 2012, 22, 21540–21548. [Google Scholar] [CrossRef]
- Zan, G.; Wu, Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Mater. 2016, 28, 2099–2147. [Google Scholar] [CrossRef]
- Ulfa, M.; Aristia, K.; Prasetyoko, D. Synthesis of mesoporous silica materials via dual templating method from starch of waste rice and their application for drug delivery system. In Proceedings of the 3rd International Seminar on Chemistry: Green Chemistry and Its Role for Sustainability, Surabaya, Indonesia, 18–19 July 2018; Volume 2049, p. 020002. [Google Scholar]
- Matmin, J.; Affendi, I.; Endud, S. Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template. Nanomaterials 2018, 8, 514. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Wang, J.; Qu, Y.; Liu, G. In vivo drug release and antibacterial properties of vancomycin loaded hydroxyapatite/chitosan composite. Drug Deliv. 2012, 19, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Cao, Y.; Kumeria, T.; Blaskovich, M.; Falconer, J.; Popat, A. Engineering Mesoporous Silica Nanoparticles for Improved Oral Delivery of Vancomycin. J. Mater. Chem. B 2021, 9, 7145–7166. [Google Scholar] [CrossRef]
- Qi, G.; Li, L.; Yu, F.; Wang, H. Vancomycin-Modified Mesoporous Silica Nanoparticles for Selective Recognition and Killing of Pathogenic Gram-Positive Bacteria Over Macrophage-Like Cells. ACS Appl. Mater. Interfaces 2013, 5, 10874–10881. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Laracuente, M.-L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Christoper, G.V.P. Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2019, 8, 12–20. [Google Scholar] [CrossRef]
- Park, I.; Pinnavaia, T.J. Large-pore mesoporous silica with three-dimensional wormhole framework structures. Microporous Mesoporous Mater. 2009, 118, 239–244. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Gao, C.-G.; Li, Y.-X.; Zhang, T.-M. Wormhole-like mesoporous silica templated by double-chained cationic surfactant. Microporous Mesoporous Mater. 2009, 124, 42–44. [Google Scholar] [CrossRef]
- Maqbool, Q.; Chanchal, A.; Srivastava, A. Tween 20-Assisted Synthesis of Uniform Mesoporous Silica Nanospheres with Wormhole Porosity for Efficient Intracellular Curcumin Delivery. ChemistrySelect 2018, 3, 3324–3329. [Google Scholar] [CrossRef]
- Venkatathri, N. Synthesis of Mesoporous silica microsphere by dual surfactant. Mater. Res. 2008, 11, 443. [Google Scholar] [CrossRef]
- Morante-Zarcero, S.; Endrino, A.; Casado, N.; Pérez-Quintanilla, D.; Sierra, I. Evaluation of mesostructured silica materials with different structures and morphologies as carriers for quercetin and naringin encapsulation. J. Porous Mater. 2022, 29, 33–48. [Google Scholar] [CrossRef]
- Alahmadi, S.M.; Mohamad, S.; Maah, M.J. Synthesis and Characterization of Mesoporous Silica Functionalized with Calix[4]arene Derivatives. Int. J. Mol. Sci. 2012, 13, 13726–13736. [Google Scholar] [CrossRef]
- Jin, X.; Ge, J.; Zhang, L.; Wu, Z.; Zhu, L.; Xiong, M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics 2022, 10, 87. [Google Scholar] [CrossRef]
- Shawky, S.; Aboalhassan, A.; Lill, H.; Bald, D.; El-Khamisy, S.; Ebeid, E.-Z. Efficient Loading and Encapsulation of Anti-Tuberculosis Drugs using Multifunctional Mesoporous Silicate Nanoparticles Running Title: Mesoporous Silicate Nanoparticles as Smart Drug Delivery System. J. Nanosci. Curr. Res. 2016, 1, 1000103. [Google Scholar] [CrossRef]
- Sun, M.; Cheng, L.; Xu, Z.; Chen, L.; Liu, Y.; Xu, Y.; Zhou, D.; Zhang, X.; Zhou, Q.; Sun, J. Preparation and Characterization of Vancomycin Hydrochloride-Loaded Mesoporous Silica Composite Hydrogels. Front. Bioeng. Biotechnol. 2022, 10, 826971. [Google Scholar] [CrossRef] [PubMed]
- Alothman, Z. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Kozlova, S.A.; Kirik, S.D. Post-synthetic activation of silanol covering in the mesostructured silicate materials MCM-41 and SBA-15. Microporous Mesoporous Mater. 2010, 133, 124–133. [Google Scholar] [CrossRef]
- Žid, L.; Zeleňák, V.; Almáši, M.; Zeleňáková, A.; Szücsová, J.; Bednarčík, J.; Šuleková, M.; Hudák, A.; Váhovská, L. Mesoporous Silica as a Drug Delivery System for Naproxen: Influence of Surface Functionalization. Molecules 2020, 25, 4722. [Google Scholar] [CrossRef]
- Doadrio, A.L.; Doadrio, J.C.; Sánchez-Montero, J.M.; Salinas, A.J.; Vallet-Regí, M. A rational explanation of the vancomycin release from SBA-15 and its derivative by molecular modelling. Microporous Mesoporous Mater. 2010, 132, 559–566. [Google Scholar] [CrossRef]
- Kurczewska, J.; Sawicka, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Vancomycin-modified silica: Synthesis, controlled release and biological activity of the drug. Int. J. Pharm. 2015, 486, 226–231. [Google Scholar] [CrossRef]
- Zhou, X.; Weng, W.; Chen, B.; Feng, W.; Wang, W.; Nie, W.; Chen, L.; Mo, X.-M.; Su, J.-C.; He, C. Mesoporous silica nanoparticles/gelatin porous composite scaffolds with localized and sustained release of vancomycin for treatment of infected bone defects. J. Mater. Chem. B 2018, 6, 740–752. [Google Scholar] [CrossRef]
- Al-Kady, A.S.; Gaber, M.; Hussein, M.M.; Ebeid, E.-Z.M. Nanostructure-loaded mesoporous silica for controlled release of coumarin derivatives: A novel testing of the hyperthermia effect. Eur. J. Pharm. Biopharm. 2011, 77, 66–74. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, J.; Shen, W.; Chen, H.; Dong, X.; Ruan, M. Preparation of novel hollow mesoporous silica spheres and their sustained-release property. Nanotechnology 2005, 16, 2633–2638. [Google Scholar] [CrossRef]
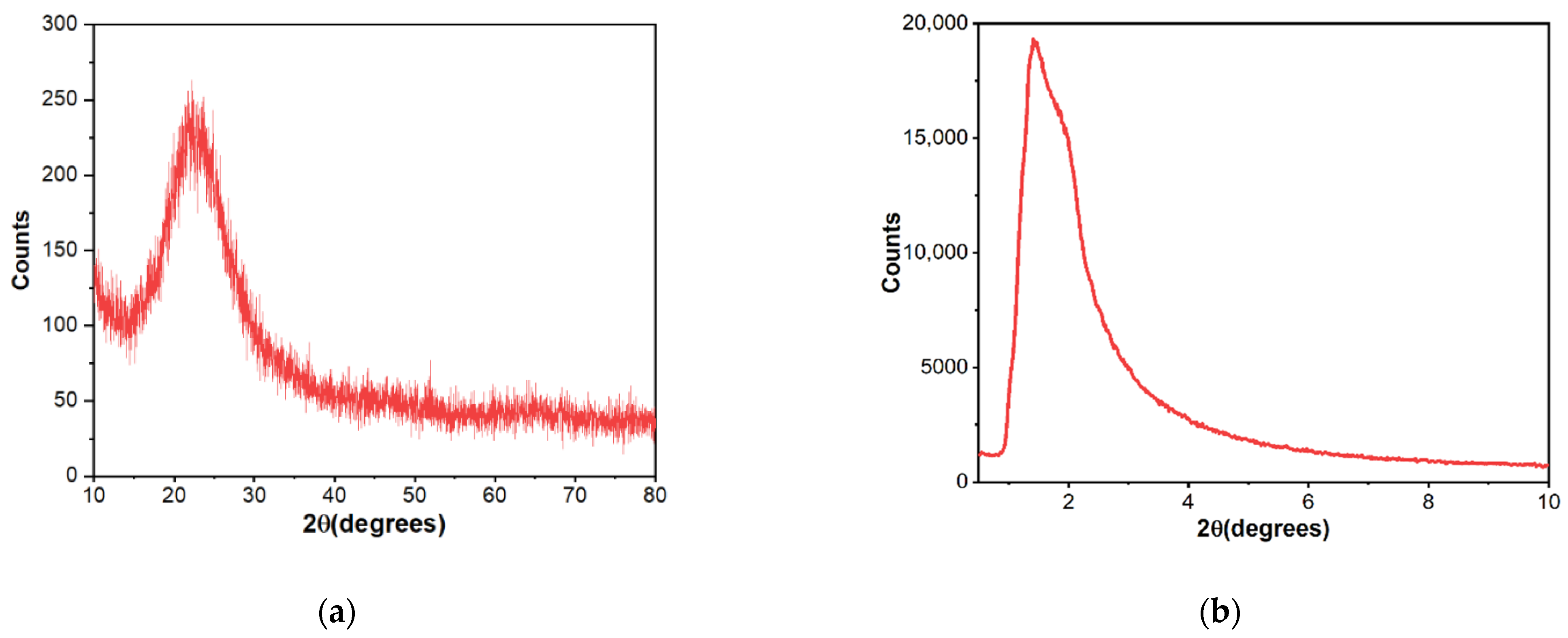
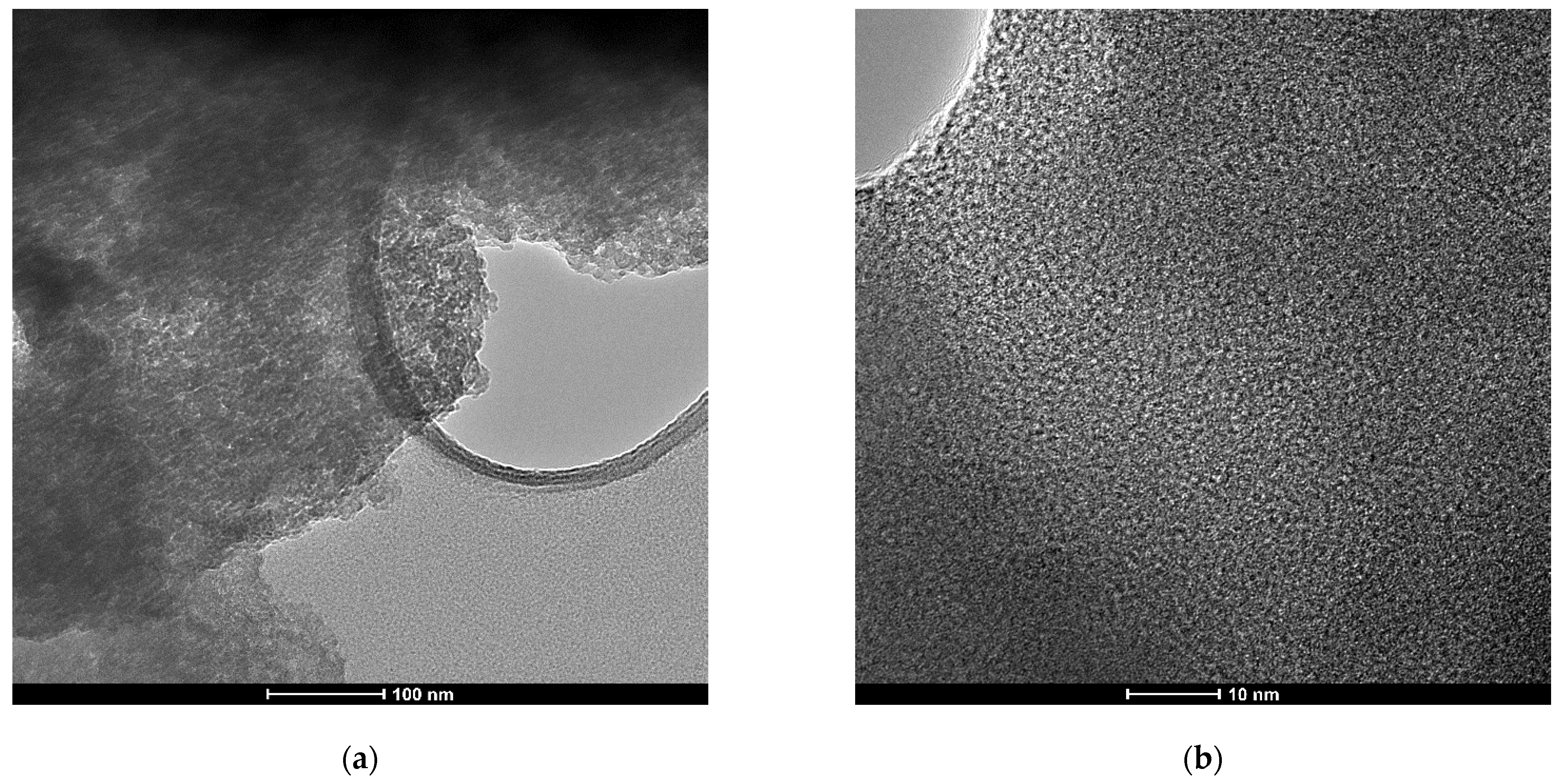

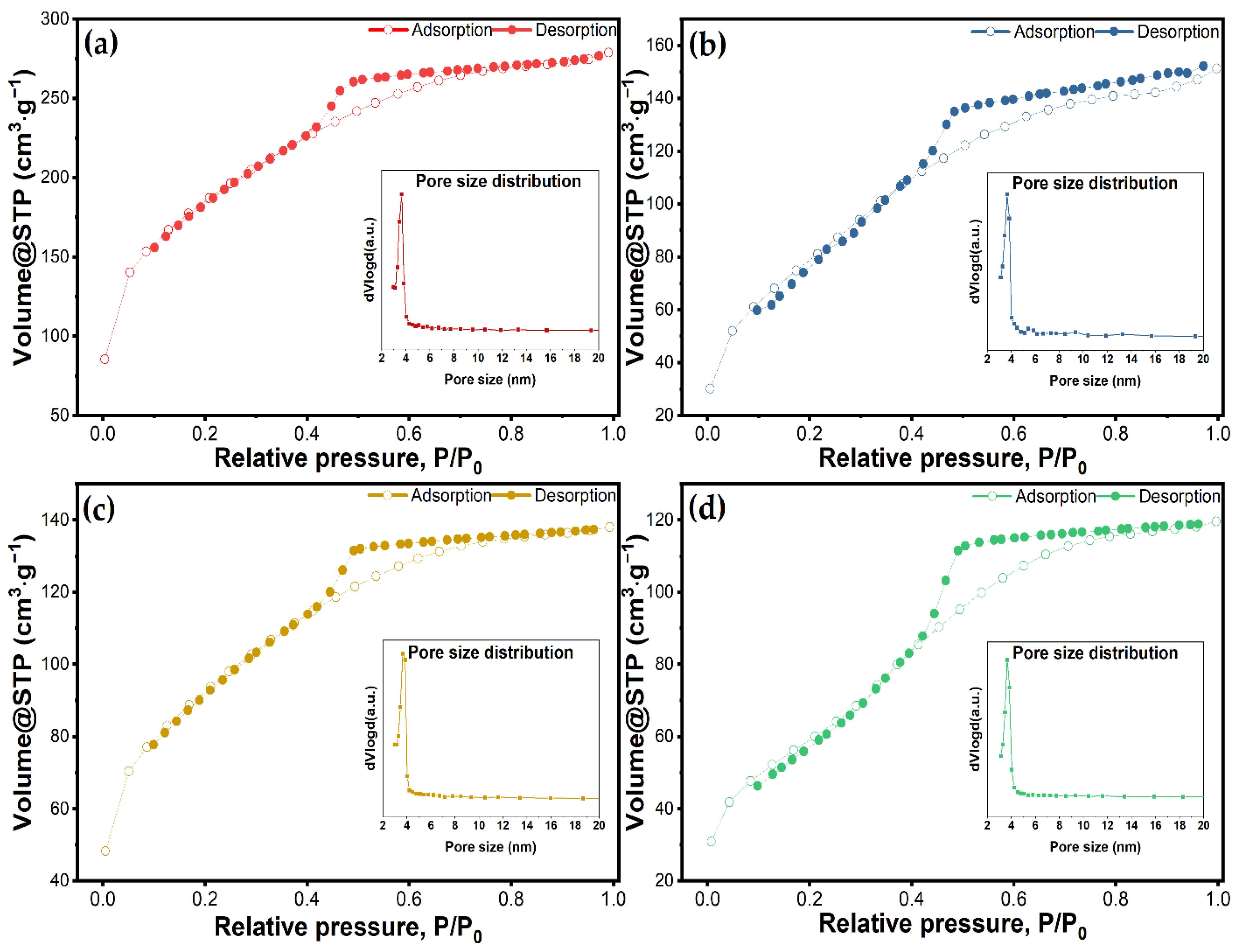

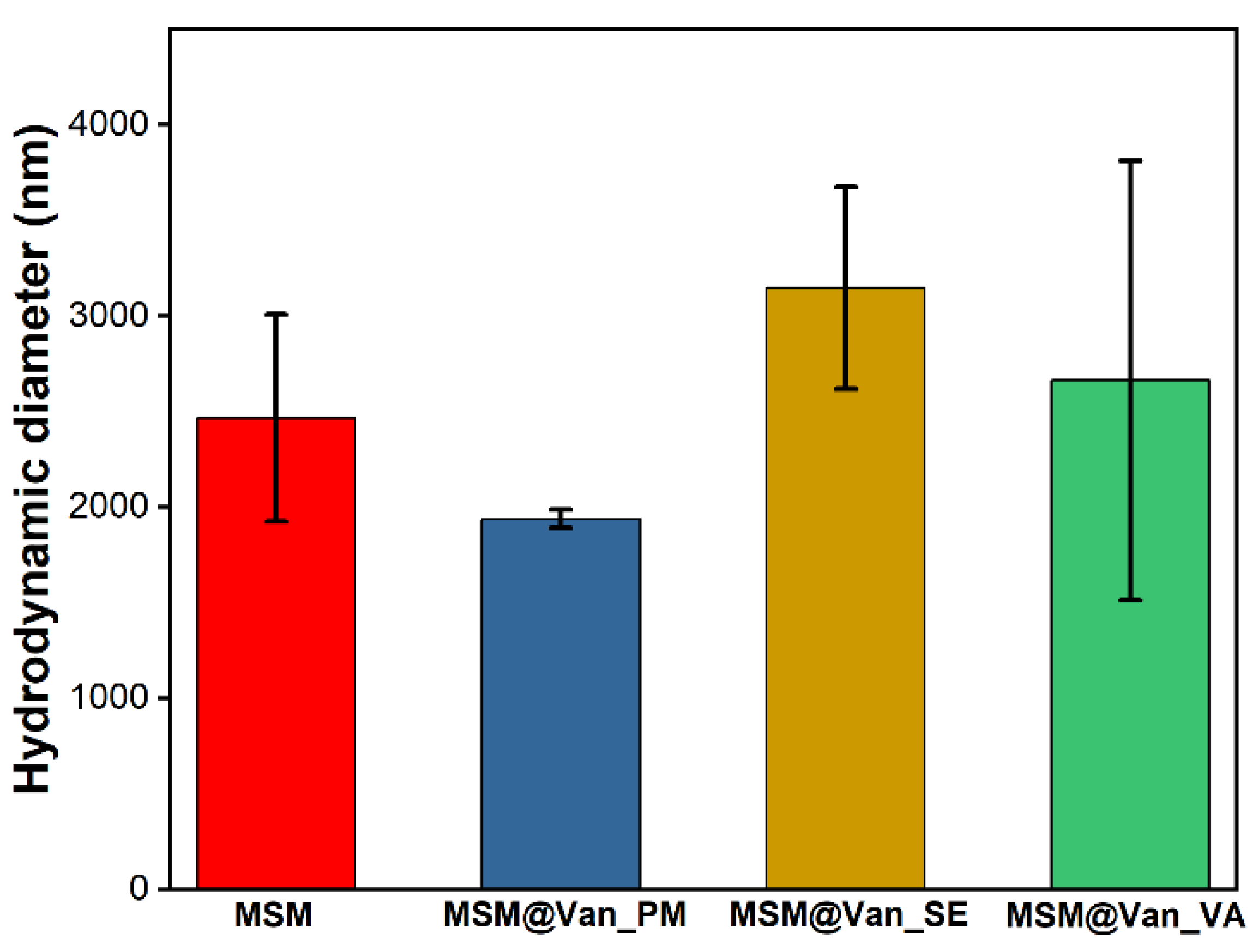

| Sample Name | Surface Area (m2∙g−1) | Pore Volume (cm3∙g−1) | Pore Diameter (nm) |
|---|---|---|---|
| MSM | 642.8 | 0.4313 | 3.66 |
| MSM@Van_PM | 327.6 | 0.2648 | 3.66 |
| MSM@Van_SE | 322.1 | 0.2136 | 3.65 |
| MSM@Van_VA | 218.3 | 0.1850 | 3.46 |
| Sample | Mass Loss (%) | nH2O | nOH | NH2O | NOH | ||
|---|---|---|---|---|---|---|---|
| RT-150 °C | 150–900 °C | Residual Mass | (mmol/g) | (Groups/nm2) | |||
| MSM | 5.16 | 7.41 | 87.42 | 2.87 | 8.23 | 1.46 | 4.21 |
| Sample | Mass Loss (%) | Thermal Effects (°C) | Calculated Vancomycin (%) | |||
|---|---|---|---|---|---|---|
| 20–150 °C | 150–600 °C | Residual Mass | Endothermic | Exothermic | ||
| MSM | 5.16 | 6.73 | 87.42 | 81.3 | 311.9 407.0 | - |
| VAN | 7.43 | 86.69 | 5.76 | 85.8 | 584.4 644.4 | - |
| MSM@Van_PM | 5.61 | 29.62 | 64.06 | 80.7 | 350.6 492.1 | 28.61 |
| MSM@Van_SE | 7.27 | 29.76 | 62.18 | 87.8 | 344.1 479.6 | 30.91 |
| MSM@Van_VA | 8.28 | 23.88 | 67.16 | 85.9 | 341.3 498.0 | 24.81 |
| Unit | Van | MSM@Van_PM | MSM@Van_SE | MSM@Van_VA | |
|---|---|---|---|---|---|
| First order | - min−1 | R2 = 0.9984 K1 = 0.1246 | R2 = 0.9281 K1 = 0.0085 | R2 = 0.9941 K1 = 0.0337 | R2 = 0.9715 K1 = 0.0331 |
| Higuchi model | - min−1/2 | R2 = 0.9735 KH = 0.0381 | R2 = 0.9910 KH = 0.1430 | R2 = 0.9044 KH = 0.1004 | R2 = 0.9884 KH = 0.1050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolete, G.; Purcăreanu, B.; Mihaiescu, D.E.; Ficai, D.; Oprea, O.-C.; Bîrcă, A.C.; Chircov, C.; Vasile, B.Ș.; Vasilievici, G.; Ficai, A.; et al. A Comparative Loading and Release Study of Vancomycin from a Green Mesoporous Silica. Molecules 2022, 27, 5589. https://doi.org/10.3390/molecules27175589
Dolete G, Purcăreanu B, Mihaiescu DE, Ficai D, Oprea O-C, Bîrcă AC, Chircov C, Vasile BȘ, Vasilievici G, Ficai A, et al. A Comparative Loading and Release Study of Vancomycin from a Green Mesoporous Silica. Molecules. 2022; 27(17):5589. https://doi.org/10.3390/molecules27175589
Chicago/Turabian StyleDolete, Georgiana, Bogdan Purcăreanu, Dan Eduard Mihaiescu, Denisa Ficai, Ovidiu-Cristian Oprea, Alexandra Cătălina Bîrcă, Cristina Chircov, Bogdan Ștefan Vasile, Gabriel Vasilievici, Anton Ficai, and et al. 2022. "A Comparative Loading and Release Study of Vancomycin from a Green Mesoporous Silica" Molecules 27, no. 17: 5589. https://doi.org/10.3390/molecules27175589








