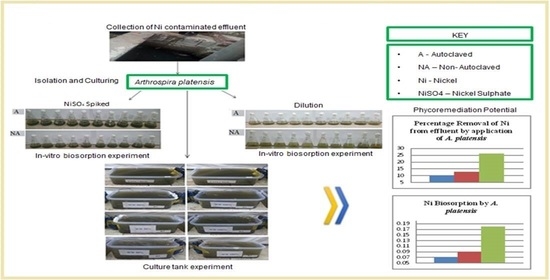
Autochthonous Arthrospira platensis Gomont Driven Nickel (Ni) Phycoremediation from Cooking Oil Industrial Effluent
Abstract
:1. Introduction
2. Results
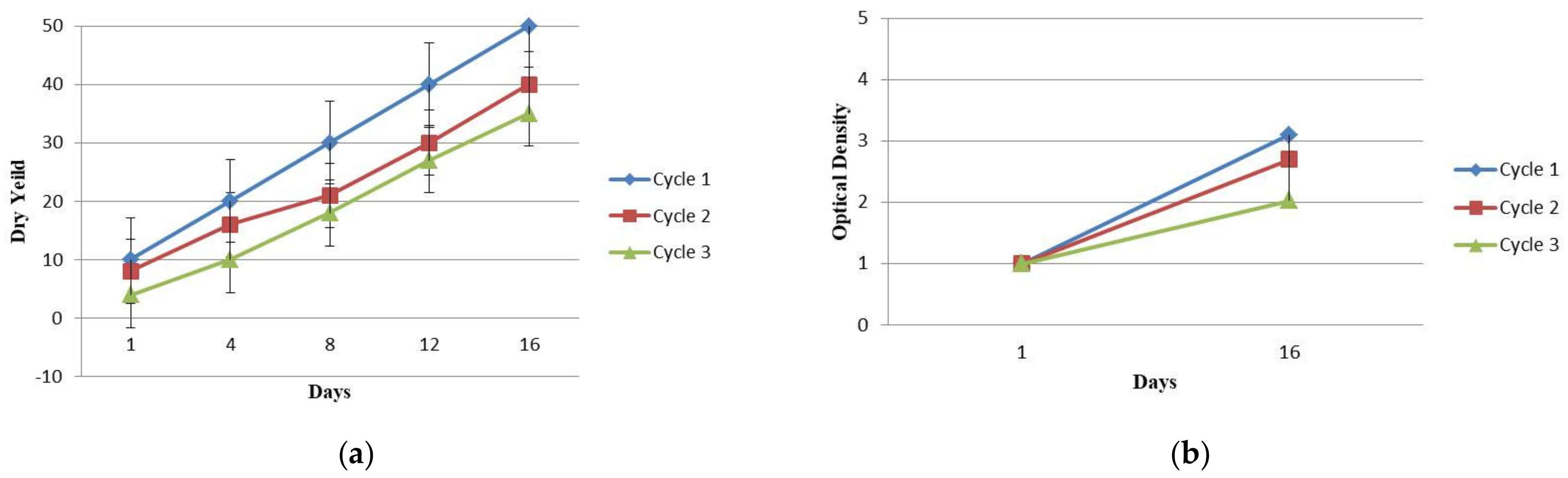
2.1. Biomass Assessment of Arthrospira platensis and Physico-Chemical Characterization of Wastewater
2.2. Ni Removal Efficiency of Arthrospira platensis
2.3. Pollutant Removal Efficiency of Arthrospira platensis
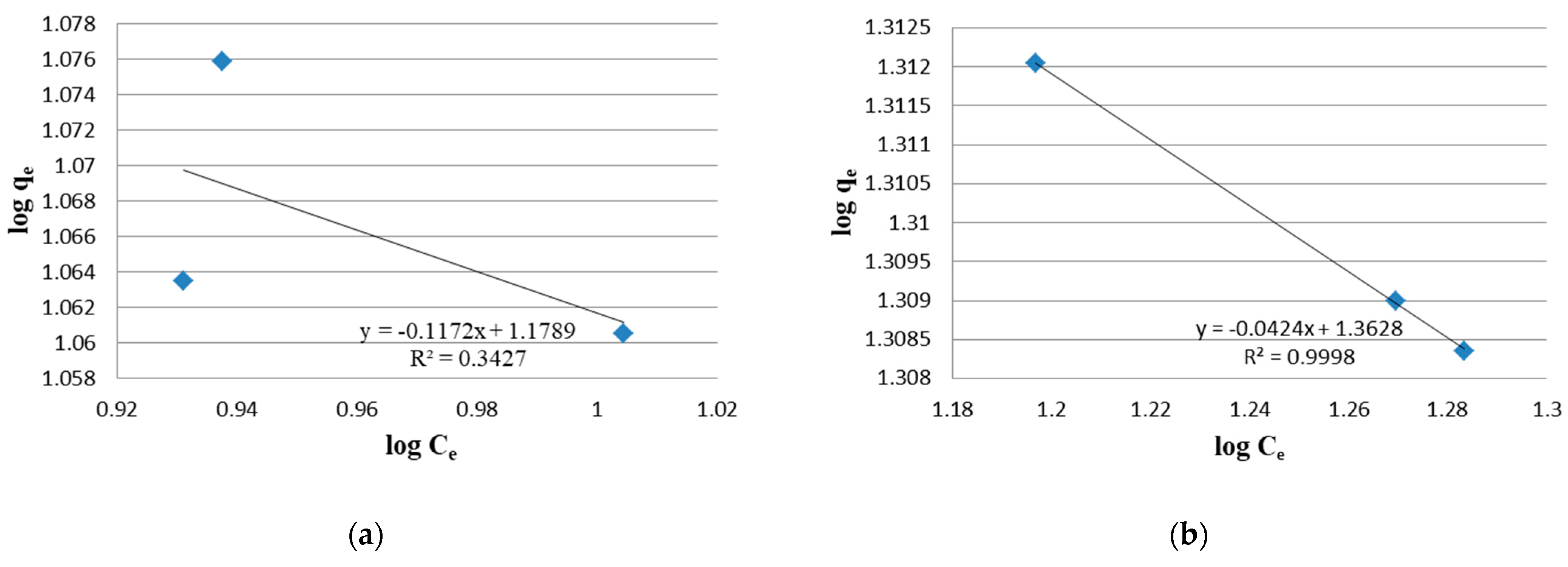
2.4. Study of Adsorption Isotherms
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Pre-Analysis
4.2. Batch Experiment
4.3. Culture Tank Experiment
4.4. Equilibrium Isotherm
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ge, S.; Madill, M.; Champagne, P. Use of freshwater macroalgae Spirogyra sp. for the treatment of municipal wastewaters and biomass production for biofuel applications. Biomass. Bioenerg. 2018, 111, 213–223. [Google Scholar] [CrossRef]
- Elleuch, J.; Amor, F.B.; Chaaben, Z.; Frikha, F.; Michaud, P.; Fendri, I.; Abdelkafi, S. Zinc biosorption by Dunaliella sp. AL-1: Mechanism and effects on cell metabolism. Sci. Total Environ. 2021, 773, 145024. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.H.; Chang, J.S. Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef]
- Gillette, B. Nickel named «Allergen of the Year». ACDS adds to list of substances warranting more attention. Dermatol. Times 2008, 4, 15–16. [Google Scholar]
- Murtaza, G.; Zia, M.H. Wastewater Production, Treatment and Use in Pakistan. In Second Regional Workshop of the Project ‘Safe Use of Wastewater in Agriculture; University of Agriculture: Faisalabad, Pakistan, 2012; pp. 16–18. [Google Scholar]
- Hussain, R.; Ahmad, W.; Nafees, M.; Hussain, A. Optimization of wastewater treatment process in industry “a case study of Hattar Industrial Estate Haripur”. Pak. J. Anal. Environ. Chem. 2014, 15, 7. [Google Scholar]
- El-Gawad, A. Oil and grease removal from industrial wastewater using new utility approach. Adv. Environ. Chem. 2014, 2014, 916878. [Google Scholar] [CrossRef]
- Yang, Q.Z.; Qi, G.J.; Low, H.C.; Song, B. Sustainable recovery of nickel from spent hydrogenation catalyst: Economics, emissions and wastes assessment. J. Clean. Prod. 2011, 19, 365–375. [Google Scholar] [CrossRef]
- Shabir, A. Process Line of Cooking Oil and Vegetable Ghee (Vanaspati) and their Analysis during Processing. Res. Rev. J. Food Dairy Technol. 2016, 4, 1–7. [Google Scholar]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Sili, C.; Torzillo, G.; Vonshak, A. Arthrospira (Spirulina). In Ecology of Cyanobacteria II; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Greshwin, M.E.; Belay, A. Spirulina (Arthrospira): Production and Quality Assurance. Spirulina in Human Nutrition and Health; CRC Press: London, UK, 2008. [Google Scholar]
- Novoa, S.; Wernand, M.; van der Woerd, H.J. The Forel-Ule scale converted to modern tools for participatory water quality monitoring. In Proceedings of the Extended Abstract Ocean Optics Conference XII, Portland, OR, USA, 26–31 October 2014; Volume 20. [Google Scholar]
- Fipps, G. Irrigation Water Quality Standards and Salinity Management; Technical Bulletin B-1664; Texas A&M University, Cooperative Extension: College Station, TX, USA, 2004; Volume 2, pp. 214–227. [Google Scholar]
- AEP. Alberta User Guide for Waste Managers; Alberta Environmental Protection: Edmonton, AB, Canada, 1996. [Google Scholar]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Joo, G.; Lee, W.; Choi, Y. Heavy metal adsorption capacity of powdered Chlorella vulgaris biosorbent: Effect of chemical modification and growth media. Environ. Sci. Pollut. Res. 2011, 28, 25390–25399. [Google Scholar] [CrossRef] [PubMed]
- Weisse, T.; Stadler, P. Effect of pH on growth, cell volume, and production of freshwater ciliates, and implications for their distribution. Limnol. Oceanogr. 2006, 51, 1708–1715. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A. Production of an immobilized hybrid biosorbent for the sorption of Ni (II) from aqueous solution. Process Biochem. 2007, 42, 148–157. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Wu, Y.; Wu, Y. Notice of Retraction: Effect of Nitrogen and Phosphorus Ratio on Algal Growth in Lake Xuanwu. In Proceedings of the 5th International Conference on Bioinformatics and Biomedical Engineering IEEE, Wuhan, China, 10–12 May 2011. [Google Scholar]
- Lawton, R.J.; de Nys, R.; Skinner, S.; Paul, N.A. Isolation and identification of Oedogonium species and strains for biomass applications. PLoS ONE 2014, 9, e90223. [Google Scholar] [CrossRef]
- Rajamani, S.; Siripornadulsil, S.; Falcao, V.; Torres, M.; Colepicolo, P.; Sayre, R. Phycoremediation of heavy metals using transgenic microalgae. In Transgenic Microalgae as Green Cell Factories; Springer: Columbus, OH, USA, 2007. [Google Scholar]
- Balaji, S.; Kalaivani, T.; Shalini, M.; Gopalakrishnan, M.; Rashith, M.M.A.; Rajasekaran, C. Sorption sites of microalgae possess metal binding ability towards Cr (VI) from tannery effluents—A kinetic and characterization study. Desalin. Water Treat. 2016, 57, 14518–14529. [Google Scholar] [CrossRef]
- Tripathi, R.; Gupta, A.; Thakur, I.S. An integrated approach for phycoremediation of wastewater and sustainable biodiesel production by green microalgae, Scenedesmus sp. ISTGA1. Renew. Energy 2019, 135, 617–625. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Gundorina, S.; Demčák, Š.; Frontasyeva, M.; Kamanina, I. Biosorption of nickel from model solutions and electroplating industrial effluent using cyanobacterium Arthrospira platensis. Desalin. Water Treat. 2018, 120, 158–165. [Google Scholar] [CrossRef]
- Gahlout, M.; Prajapati, H.; Chauhan, P.; Savande, L.; Yadav, P. Isolation, screening and identification of cyanobacteria and its uses in bioremediation of industrial effluents and chromium sorption. Int. J. Adv. Res. Biol. Sci. 2017, 4, 138–146. [Google Scholar] [CrossRef]
- Murtaza, G.; Ghafoor, A.; Qadir, M.; Owens, G.; Aziz, M.A.; Zia, M.H. Disposal and use of sewage on agricultural lands in Pakistan: A review. Pedosphere 2010, 20, 23–34. [Google Scholar] [CrossRef]
- Emparan, Q.; Harun, R.; Danquah, M.K. Role of phycoremediation for nutrient removal from wastewaters: A review. Appl. Ecol. Environ. Res. 2019, 17, 889–915. [Google Scholar] [CrossRef]
- Garbowski, T.; Bawiec, A.; Pulikowski, K.; Wiercik, P. Algae proliferation on substrates immersed in biologically treated sewage. J. Ecol. Eng. 2017, 18, 90–98. [Google Scholar] [CrossRef]
- Clesseri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Geitler, L. Cyanophyceae. Kryptogramenflora von Deutschland, Osterreich und der Schweiz; Akademische Verlagsgesellschaft: Leipzig, Germany, 1932; Volume 14, pp. 130–148. [Google Scholar]
- Prasad, R.N.; Sanghamitra, K.; Antonia, G.M.; Juan, G.V.; Benjamin, R.G.; Luis, I.M.J.; Guillermo, V.V. Isolation, identification and germplasm preservation of different native Spirulina species from Western Mexico. Am. J. Plant Sci. 2013, 2013, 65–71. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Moheimani, N.R. Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013; Volume 5. [Google Scholar]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture; John Wiley & Sons: Carlton, VIC, Australia, 2013. [Google Scholar]
- Fourooghifard, H.; Matinfar, A.; Mortazavi, M.S.; Roohani, G.K.; Roohani, G.M. Nitrogen and phosphorous budgets for integrated culture of whiteleg shrimp Litopenaeus vannamei with red seaweed Gracilaria corticata in zero water exchange system. Iran. J. Fish. Sci. 2018, 17, 471–486. [Google Scholar]
- Gupta, S.; Kumar, A. Removal of nickel (II) from aqueous solution by biosorption on A. barbadensis Miller waste leaves powder. Appl. Water Sci. 2019, 9, 96. [Google Scholar] [CrossRef]
- Dębowski, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Romanowska-Duda, Z. Biomass production and nutrient removal by Chlorella vulgaris from anaerobic digestion effluents. Energies 2018, 11, 1654. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A.; Nayak, A. Biosorption of nickel onto treated alga (Oedogonium hatei): Application of isotherm and kinetic models. J. Colloid. Interf. Sci. 2009, 342, 533–539. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Abbad, A.F.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabdullatif, J.A. Lead removal by Spirulina platensis biomass. Int. J. Phytoremediation 2016, 18, 184–189. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Wet weight (mg L−1) | 7972 ± 2.05 |
| Dry weight (mg L−1) | 1123 ± 3.29 |
| Organic dry weight (mg L−1) | 775.00 ± 0.94 |
| Biomass productivity | 0.81 ± 0.04 |
| Chlorophyll content (mg L−1) | 8.00 ± 2.9 |
| Phycocyanin content (mg L−1) | 100.00 ± 2.35 |
| Extraction yield | 11.00 ± 0.47 |
| Optical Density | 3.01 ± 0.004 |
| Total Cell Count | 4.00 ± 0.408 |
| Total Organic Carbon (mg L−1) | 2.50 ± 0.24 |
| Growth yield | 0.03 ± 0.01 |
| Physico-Chemical Parameter | Value | Permissible Limit of NEQS Pakistan (WHO or AEP) |
|---|---|---|
| pH | 8.21 ± 0.012 | 6–10 |
| EC (µS/cm) | 1020.00 ± 2.9 | 1000–2500 (AEP) |
| Temperature (°C) | 17.70 ± 0.12 | 40 |
| TDS (mg L−1) | 211.00 ± 0.47 | 3500 |
| NaCl (%) | 1.10 ± 0.12 | ---- |
| BOD (mg L−1) | 126.90 ± 1.25 | 80 |
| COD (mg L−1) | 371.60 ± 0.24 | 150 |
| DO (mg L−1) | 0.20 ± 0.09 | 5 (WHO) |
| TS (mg L−1) | 252.00 ± 0.94 | ---- |
| TSS (mg L−1) | 41.00 ± 1.24 | 150 |
| TVS (mg L−1) | 19.00 ± 0.81 | ---- |
| CO3 (mg L−1) | ND | ---- |
| HCO3 (mg L−1) | 2.00 ± 0.4 | ---- |
| Cl (mg L−1) | 43.78 ± 0.16 | 1000 |
| S (mg L−1) | 53.40 ± 0.32 | 600 |
| Ca (mg L−1) | 13.00 ± 0.23 | 75 |
| Mg (mg L−1) | 3.40 ± 0.05 | 150 |
| Hardness (mg L−1) | 46.46 ± 0.008 | 200 |
| TKN (mg L−1) | 10.65 ± 0.03 | 50 (WHO) |
| TP (mg L−1) | 1.32 ± 0.01 | 5 (WHO) |
| Na (mg L−1) | 28.00 ± 1.24 | ---- |
| K (mg L−1) | 13.00 ± 1.63 | ---- |
| Oils (mg L−1) | 8.00 ± 0.81 | 10 |
| Ni (mg L−1) | 21.30 ± 0.16 | 1.0 |
| Wastewater Dilution | Concentration of Ni (mg L−1) | Percentage Reduction | |||
|---|---|---|---|---|---|
| (Real Effluent) | Batch 1 (without Microbes) | Batch 2 (with Indigenous Microbes) | Batch 1 (without Microbes) | Batch 2 (with Indigenous Microbes) | |
| 10% | 3.21 | 2.34 ± 0.22 | 1.47 ± 0.17 | 27.04 | 54.04 |
| 20% | 5.60 | 4.27 ± 0.31 | 3.71 ± 0.26 | 23.71 | 33.73 |
| 30% | 7.13 | 5.40 ± 0.42 | 3.60 ± 0.36 | 24.17 | 49.5 |
| 40% | 9.08 | 5.88 ± 0.41 | 3.84 ± 0.48 | 35.24 | 57.63 |
| 50% | 11.52 | 6.26 ± 0.58 | 5.21 ± 0.27 | 45.64 | 54.75 |
| 60% | 13.74 | 7.51 ± 0.36 | 5.52 ± 0.61 | 45.29 | 59.81 |
| 70% | 15.95 | 7.44 ± 0.48 | 6.65 ± 0.39 | 53.32 | 58.28 |
| 80% | 17.60 | 8.91 ± 0.21 | 6.98 ± 0.53 | 49.36 | 60.3 |
| 90% | 19.84 | 11.20 ± 0.54 | 9.46 ± 0.44 | 43.53 | 52.27 |
| 100% | 21.30 | 18.40 ± 0.72 | 15.84 ± 0.56 | 13.61 | 25.6 |
| LSD | 10.09 | 9.13 | - | - | |
| Concentration of Synthetic Solution (mg L−1) | Concentration of Ni (mg L−1) | Percentage Reduction | ||
|---|---|---|---|---|
| Batch 1 (without Microbes) | Batch 2 (with Indigenous Microbes) | Batch 1 (without Microbes) | Batch 2 (with Indigenous Microbes) | |
| 2 | 1.79 ± 0.54 | 1.39 ± 0.53 | 10.15 | 30.30 |
| 4 | 3.96 ± 0.47 | 3.19 ± 0.39 | 1.00 | 20.07 |
| 6 | 5.81 ± 0.33 | 4.40 ± 0.19 | 3.03 | 26.66 |
| 8 | 7.13 ± 0.26 | 6.69 ± 0.74 | 10.86 | 16.30 |
| 10 | 9.71 ± 0.68 | 9.42 ± 0.12 | 2.87 | 5.76 |
| 12 | 11.62 ± 0.61 | 11.12 ± 0.83 | 3.166 | 7.33 |
| 14 | 12.05 ± 0.19 | 12.67 ± 0.47 | 13.89 | 9.43 |
| 16 | 15.63 ± 0.77 | 14.12 ± 0.41 | 2.30 | 11.72 |
| 18 | 15.34 ± 0.39 | 14.16 ± 0.28 | 14.76 | 21.31 |
| 20 | 17.62 ± 0.25 | 17.23 ± 0.55 | 11.88 | 13.82 |
| LSD | 12.03 | 12.04 | 12.16 | 18.60 |
| Parameter | Days of Treatment | Percentage Removal after Treatment Days | |||||
|---|---|---|---|---|---|---|---|
| 0 | 16 | 33 | 49 | 16 | 33 | 49 | |
| EC (µS cm−1) | 780.00 | 241.66 ± 1.24 | 241.00 ± 1.63 | 241.66 ± 2.05 | 69.01 | 69.10 | 69.01 |
| NaCl (%) | 0.40 | BDL | BDL | BDL | 100 | 100 | 100 |
| BOD (mg L−1) | 118.00 | 70.33 ± 1.24 | 69.00 ± 0.81 | 70.00 ± 0.81 | 40.39 | 41.52 | 40.67 |
| COD (mg L−1) | 502.00 | 218.00 ± 1.63 | 205.66 ± 1.24 | 215.66 ± 1.24 | 56.57 | 59.03 | 57.04 |
| TDS (mg L−1) | 129.00 | 61.00 ± 0.81 | 62.00 ± 0.81 | 60.00 ± 0.81 | 52.71 | 51.93 | 53.48 |
| TS (mg L−1) | 117.00 | 59.00 ± 1.63 | 59.00 ± 0.81 | 57.66 ± 1.24 | 49.57 | 49.57 | 50.71 |
| TSS (mg L−1) | 12.00 | 2.66 ± 0.47 | 3.33 ± 0.47 | 2.66 ± 0.47 | 77.77 | 72.22 | 77.77 |
| TVS (mg L−1) | 4.00 | 0.70 ± 0.244 | 0.86 ± 0.32 | 0.93 ± 0.20 | 82.50 | 78.33 | 76.66 |
| CO3 (mg L−1) | BDL | BDL | BDL | BDL | --- | --- | --- |
| HCO3 (mg L−1) | BDL | BDL | BDL | BDL | --- | --- | --- |
| Cl (mg L−1) | 20.20 | 6.50 ± 0.40 | 6.50 ± 1.08 | 5.66 ± 1.24 | 67.82 | 67.82 | 71.94 |
| S (mg L−1) | 31.00 | 12.66 ± 1.69 | 11.33 ± 1.24 | 11.00 ± 0.40 | 59.13 | 63.44 | 64.51 |
| Ca (mg L−1) | 9.00 | 3.00 ± 0.81 | 3.33 ± 0.47 | 4.00 ± 0.81 | 66.66 | 62.96 | 55.55 |
| Mg (mg L−1) | 2.10 | BDL | 0.433 ± 0.12 | 0.33 ± 0.12 | 100 | 79.365 | 84.12 |
| Hardness (mg L−1) | 35.17 | 11.56 ± 0.04 | 11.41 ± 0.17 | 11.27 ± 0.03 | 67.11 | 67.55 | 67.93 |
| TKN (mg L−1) | 5.07 | 0.11 ± 0.04 | 0.12 ± 0.01 | 0.19 ± 0.02 | 97.83 | 97.63 | 96.25 |
| TP (mg L−1) | 0.20 | BDL | BDL | BDL | 100 | 100 | 100 |
| Na (mg L−1) | 12.00 | 3.81 ± 0.41 | 4.03 ± 0.19 | 4.16 ± 0.32 | 68.25 | 66.41 | 65.33 |
| K (mg L−1) | 6.00 | 0.52 ± 0.07 | 0.54 ± 0.03 | 0.59 ± 0.06 | 91.33 | 91 | 90.16 |
| Oils (mg L−1) | 5.00 | 2.93 ± 0.73 | 2.93 ± 0.68 | 2.93 ± 0.71 | 41.40 | 41.40 | 41.40 |
| Ni (mg L−1) | 12.00 | 8.53 ± 2.16 | 8.66 ± 1.03 | 10.1 ± 0.86 | 28.88 | 27.77 | 15.83 |
| Parameter | Days of Treatment | Percentage Removal after Treatment Days | |||||
|---|---|---|---|---|---|---|---|
| 0 | 16 | 33 | 49 | 16 | 33 | 49 | |
| EC (µS cm−1) | 1020.00 | 586.66 ± 2.05 | 565.00 ± 1.41 | 569.00 ± 0.81 | 42.48 | 44.60 | 44.21 |
| NaCl (%) | 1.10 | 0.80 ± 1.11 | 0.80 ± 1.11 | 0.80 ± 1.11 | 27.27 | 27.27 | 27.27 |
| BOD (mg L−1) | 162.90 | 125.46 ± 2.73 | 132.00 ± 1.20 | 128.73 ± 0.83 | 22.97 | 18.96 | 20.97 |
| COD (mg L−1) | 751.60 | 337.66 ± 1.24 | 372.66 ± 2.05 | 380.33 ± 1.24 | 55.07 | 50.42 | 49.40 |
| TDS (mg L−1) | 211.00 | 134.00 ± 3.26 | 122.33 ± 2.05 | 119.66 ± 1.24 | 36.49 | 42.02 | 43.28 |
| TS (mg L−1) | 252.00 | 194.66 ± 1.24 | 186.00 ± 2.16 | 183.66 ± 1.24 | 22.75 | 26.19 | 27.11 |
| TSS (mg L−1) | 41.00 | 31.33 ± 1.24 | 28.00 ± 1.63 | 26.33 ± 1.24 | 23.57 | 31.70 | 35.77 |
| TVS (mg L−1) | 19.00 | 11.66 ± 1.24 | 10.00 ± 0.81 | 9.33 ± 1.24 | 38.59 | 47.36 | 50.87 |
| CO3 (mg L−1) | 0 | --- | --- | --- | --- | --- | --- |
| HCO3(mg L−1) | 2.00 | BDL | BDL | BDL | 100 | 100 | 100 |
| Cl (mg L−1) | 43.78 | 15.00 ± 0.81 | 16.66 ± 0.94 | 15.66 ± 1.24 | 65.73 | 61.93 | 64.21 |
| S (mg L−1) | 53.40 | 22.66 ± 1.69 | 20.00 ± 0.81 | 18.76 ± 1.11 | 57.55 | 62.54 | 64.85 |
| Ca (mg L−1) | 13.00 | 8.60 ± 0.43 | 8.00 ± 0.81 | 9.00 ± 0.81 | 33.84 | 38.46 | 30.76 |
| Mg (mg L−1) | 3.40 | BDL | 0.11 ± 0.03 | 0.24 ± 0.06 | 100 | 96.76 | 92.94 |
| Hardness (mg L−1) | 46.46 | 26.36 ± 0.45 | 24.03 ± 0.82 | 25.23 ± 1.06 | 43.25 | 48.26 | 45.69 |
| TKN (mg L−1) | 10.65 | 1.42 ± 0.26 | 1.64 ± 0.31 | 2.10 ± 0.22 | 86.66 | 84.60 | 80.28 |
| TP (mg L−1) | 1.32 | BDL | BDL | BDL | 100 | 100 | 100 |
| Na (mg L−1) | 28.00 | 7.24 ± 0.62 | 7.64 ± 0.18 | 8.10 ± 0.28 | 74.14 | 72.71 | 64.78 |
| K (mg L−1) | 13.00 | 4.12 ± 0.75 | 4.46 ± 0.81 | 4.99 ± 0.79 | 68.30 | 65.69 | 61.61 |
| Oils (mg L−1) | 8.00 | 3.00 ± 0.81 | 5.33 ± 0.47 | 4.33 ± 1.24 | 62.5 | 33.33 | 45.83 |
| Ni (mg L−1) | 21.30 | 15.73 ± 0.46 | 18.60 ± 0.74 | 19.20 ± 0.24 | 26.13 | 12.67 | 9.85 |
| Parameter Observed | Value |
|---|---|
| Specific Growth rate (mg L−1) | 293.00 ± 136.35 |
| Biomass productivity (mg L−1) | 133.78 ± 41.98 |
| Mineral uptake | 14.24 ± 4.47 |
| Nickel percentage removal | 16.22 ± 7.11 |
| Bioconcentration factor | 0.11 ± 0.04 |
| Desorption ratio | 36.35 ± 10.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakoor, I.; Nazir, A.; Chaudhry, S.; Qurat-ul-Ain; Firdaus-e-Bareen; Capareda, S.C. Autochthonous Arthrospira platensis Gomont Driven Nickel (Ni) Phycoremediation from Cooking Oil Industrial Effluent. Molecules 2022, 27, 5353. https://doi.org/10.3390/molecules27165353
Shakoor I, Nazir A, Chaudhry S, Qurat-ul-Ain, Firdaus-e-Bareen, Capareda SC. Autochthonous Arthrospira platensis Gomont Driven Nickel (Ni) Phycoremediation from Cooking Oil Industrial Effluent. Molecules. 2022; 27(16):5353. https://doi.org/10.3390/molecules27165353
Chicago/Turabian StyleShakoor, Isha, Aisha Nazir, Sonal Chaudhry, Qurat-ul-Ain, Firdaus-e-Bareen, and Sergio C. Capareda. 2022. "Autochthonous Arthrospira platensis Gomont Driven Nickel (Ni) Phycoremediation from Cooking Oil Industrial Effluent" Molecules 27, no. 16: 5353. https://doi.org/10.3390/molecules27165353