Electrochemical Sensing of Pb2+ and Cd2+ Ions with the Use of Electrode Modified with Carbon-Covered Halloysite and Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Choice of Sensor Modification Composite. Preliminary Study
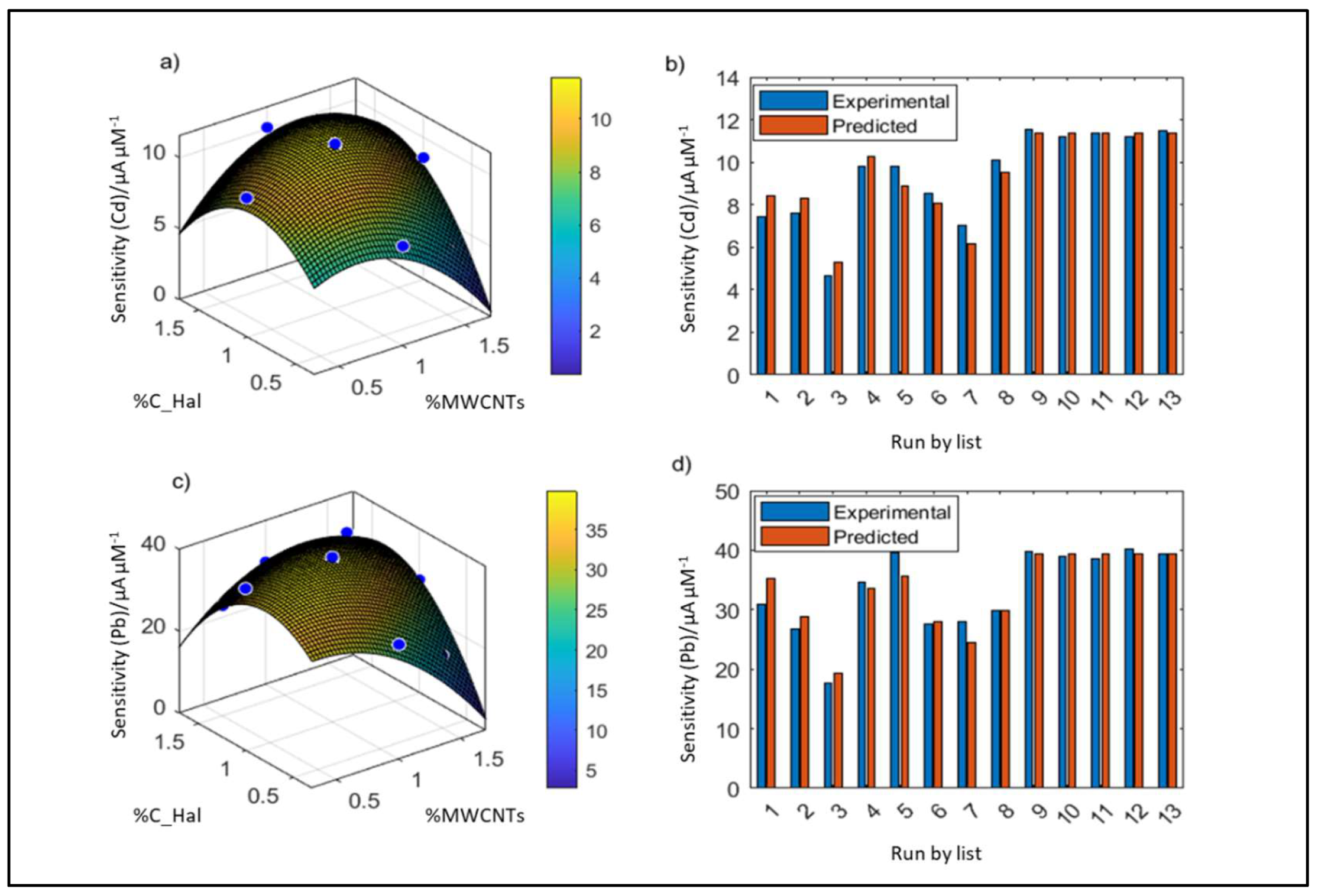
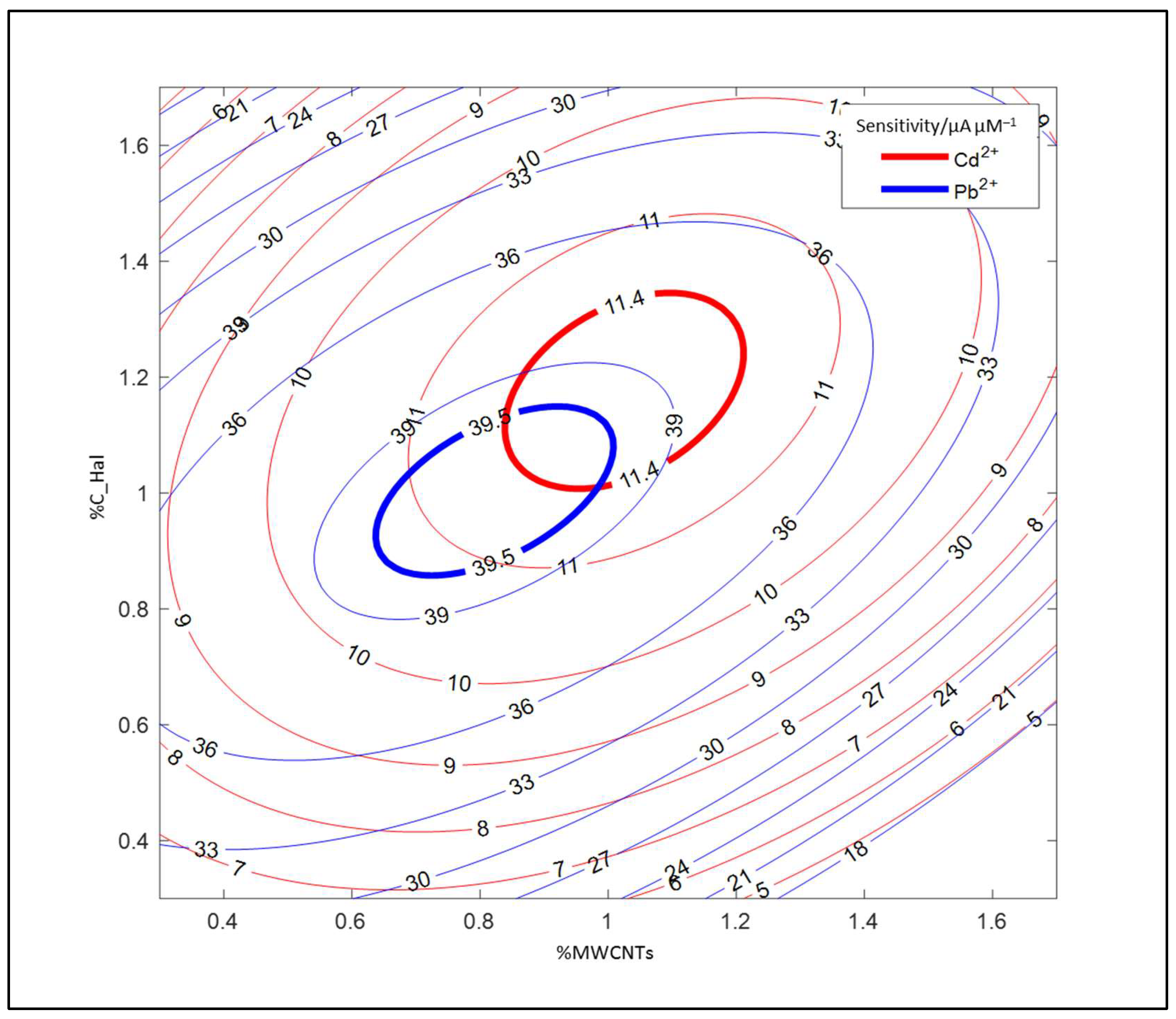
2.2. Application of CCD Method
2.3. Composite Characterization
2.3.1. Structural and Textural Characterization of Halloysite
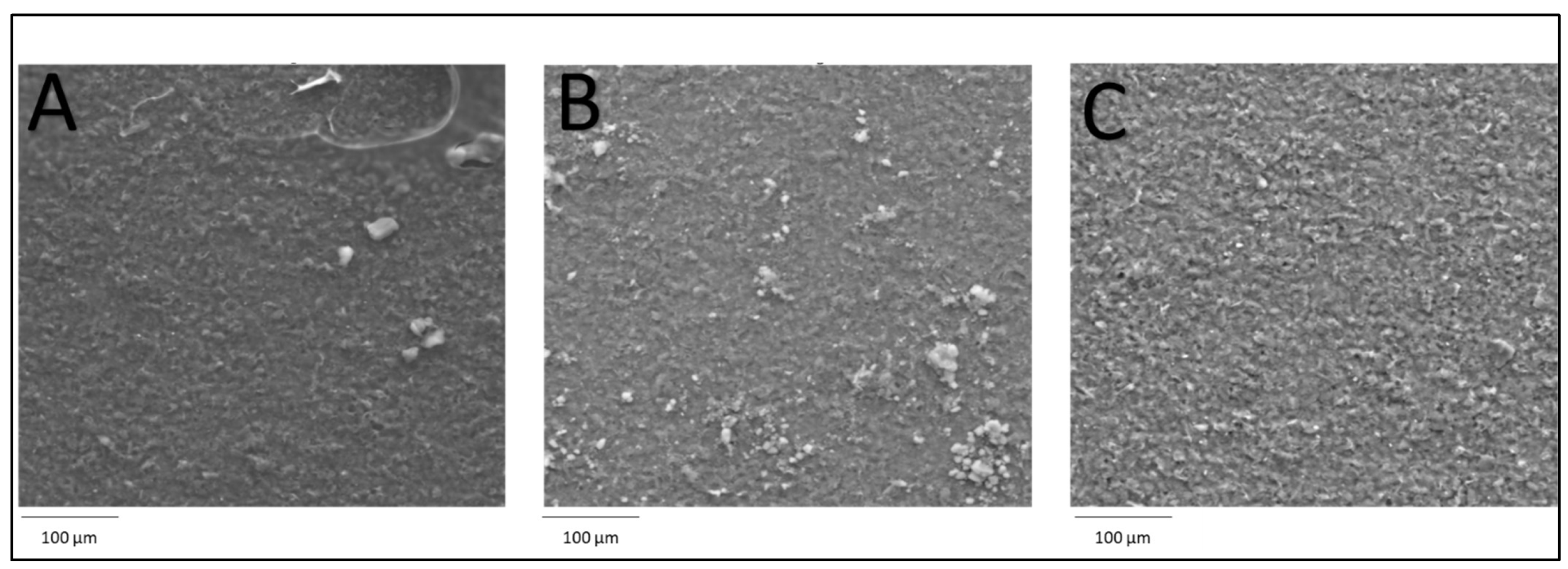
2.3.2. Morphological Characterization of Electrode Nanocomposite
2.3.3. Electrochemical Characterization of MWCNTs/C_Hal/Nafion/SPCE
2.4. Optimization of Experimental Parameters of Cd2+ and Pb2+ Cations Determination
2.4.1. Selection of Supporting Electrolyte
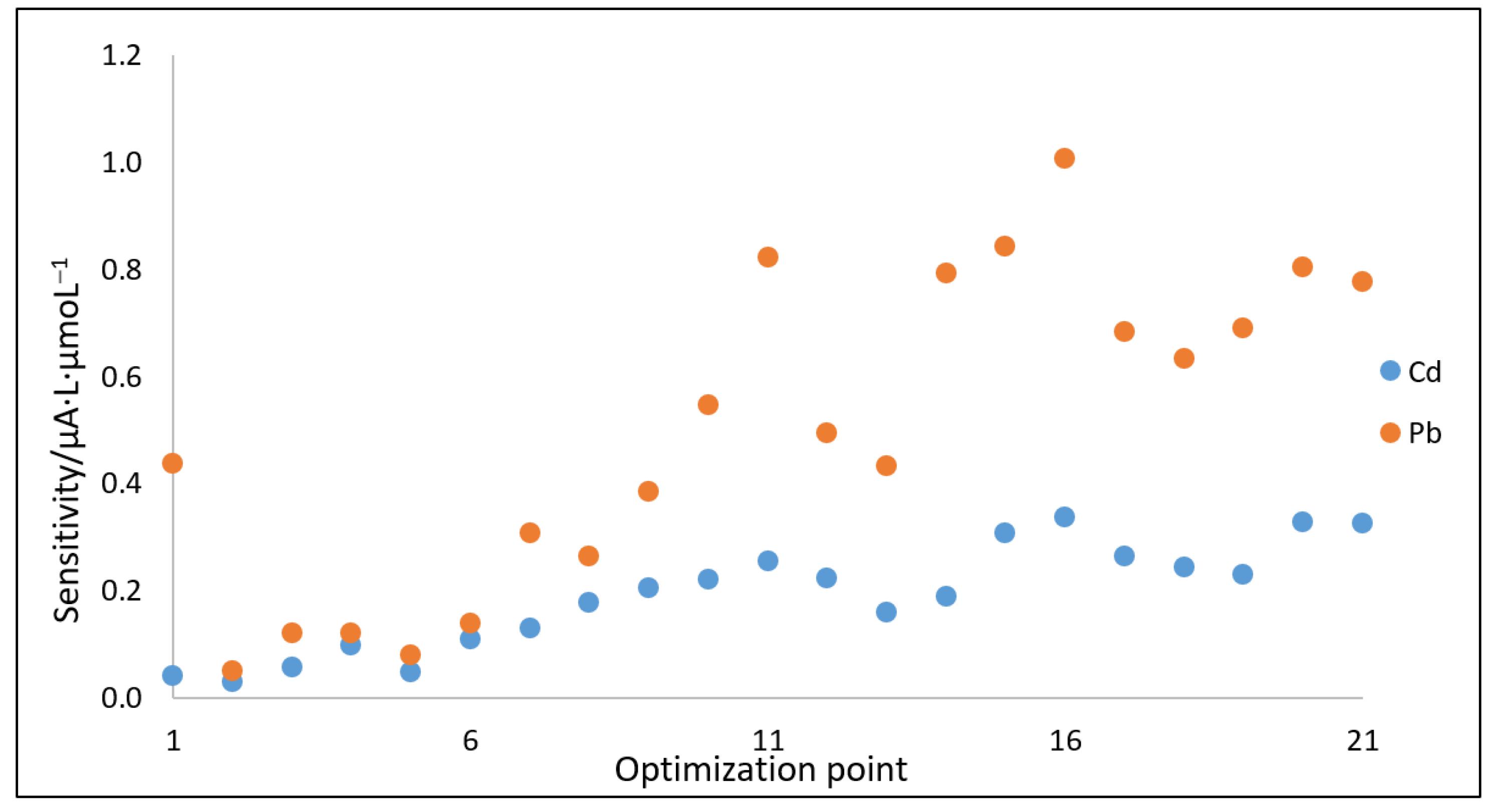
2.4.2. Optimization of DPASV Parameters Using the Nelder–Mead Simplex Method
2.4.3. Study of Deposition Potential and Time (Edep, tdep)
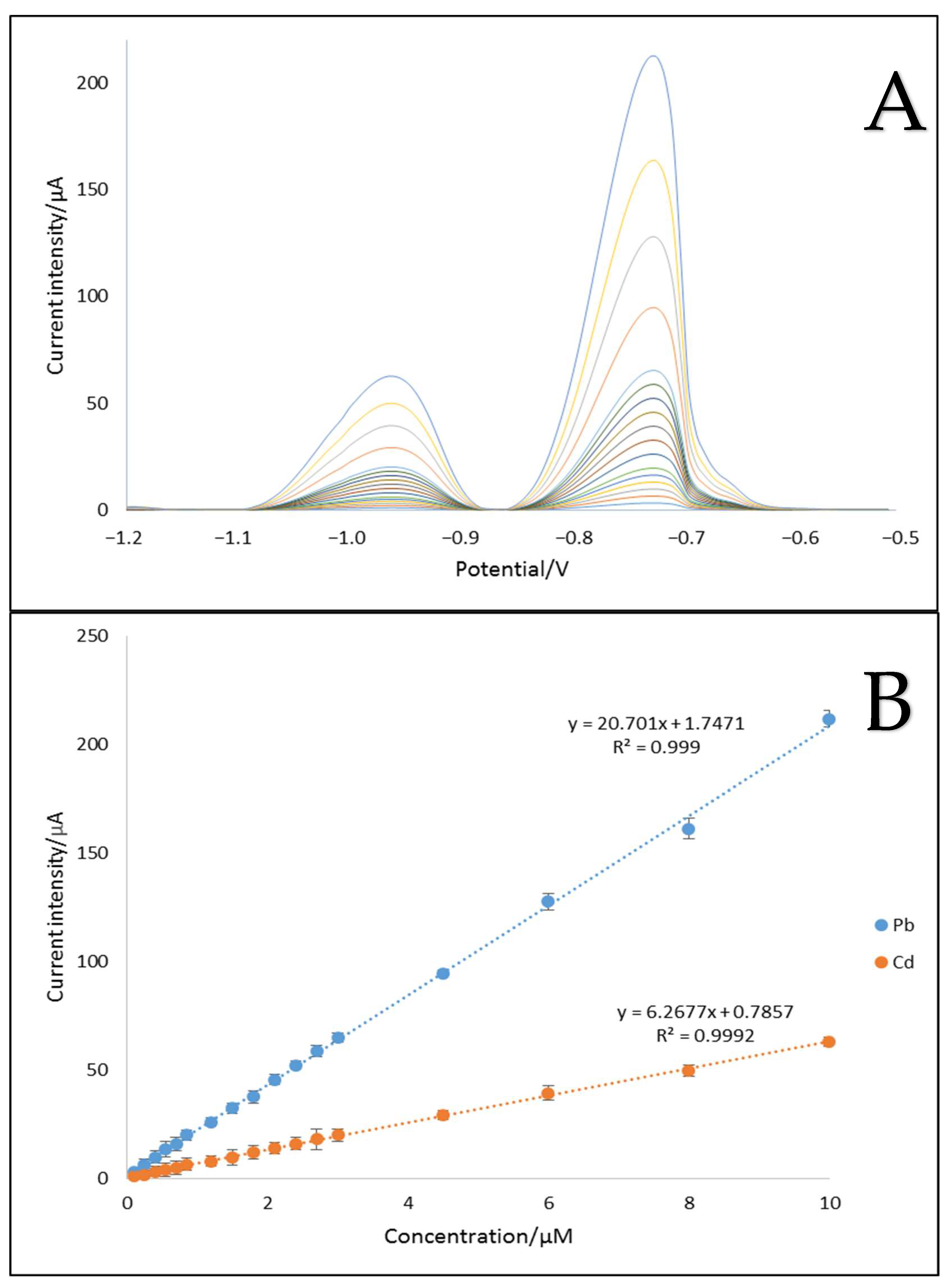
2.4.4. Analytical Characteristics of MWCNTs/C_Hal/Nafion/SPE toward Cd2+ and Pb2+ Cations
2.4.5. Real Sample Analysis
3. Materials and Methods
3.1. Chemical and Materials
3.2. Apparatus and Measurement Procedures
3.3. Halloysite-Based Sensor Preparation
3.4. Samples Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somerset, V.S.; Hernandez, L.H.; Iwuoha, E.I. Stripping voltammetric measurement of trace metal ions using screen-printed carbon and modified carbon paste electrodes on river water from the Eerste-Kuils River System. J. Environ. Sci. Health 2011, 46, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-L.; Zhang, G.-R.; Li, W.; Biesalski, M.; Etzold, B.J.M. Modifier-Free Microfluidic Electrochemical Sensor for Heavy-Metal Detection. ACS Omega 2017, 2, 45934603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Huang, W.; Shen, R.; Shuai, Q. Chitosan/thiol functionalized metal-organic framework composite for the simultaneous determination of lead and cadmium ions in food samples. Food Chem. 2020, 330, 127212–127219. [Google Scholar] [CrossRef] [PubMed]
- Koosha, E.; Shamsipur, M.; Salimi, F.; Ramezani, M. A microextraction method based on precipitation for the simultaneous separation and preconcentration of cadmium and lead before their determination by FAAS: Experimental design methodology. Sep. Sci. Technol. 2020, 56, 1721–1729. [Google Scholar] [CrossRef]
- Rohanifar, A.; Alipourasiabi, N.; Sunder, G.S.S.; Lawrence, J.G.; Kirchoff, J.R. Reversible chelating polymer for determination of heavy metals by dispersive micro solid-phase extraction with ICP-MS. Microchim. Acta 2020, 187, 339. [Google Scholar] [CrossRef]
- Reichstädter, M.; Divis, P.; Abdulbur-Alfakhoury, E.; Gao, Y. Simultaneous determination of mercury, cadmium and lead in fish sauce using Diffusive Gradients in Thin-films technique. Talanta 2020, 217, 121059. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Cerovac, S.; Mudri’c, S.; Guzsvány, V.; Husáková, L.; Urbanová, I.; Vytřas, K. Antimony nanoparticle-multiwalled carbon nanotubes composite immobilized at carbon paste electrode for determination of trace heavy metals. Sens. Actuators B Chem. 2014, 191, 320–325. [Google Scholar] [CrossRef]
- Tan, Z.; Wu, W.; Feng, C.; Wu, H.; Zhang, Z. Simultaneous determination of heavy metals by an electrochemical method based on a nanocomposite consisting of fluorinated graphene and gold nanocage. Microchim. Acta 2020, 187, 414. [Google Scholar] [CrossRef]
- Scandurra, A.; Ruffino, F.; Urso, M.; Grimaldi, M.G.; Mirabella, S. Disposable and Low-Cost Electrode Based on Graphene Paper-Nafion-Bi Nanostructures for Ultra-Trace Determination of Pb (II) and Cd (II). Nanomaterials 2020, 10, 1620. [Google Scholar] [CrossRef]
- Hassan, K.M.; Gaber, S.E.; Altahan, M.F.; Azzem, M.A. Single and simultaneous voltammetric sensing of lead (II), cadmium (II) and zinc (II) using a bimetallic Hg-Bi supported on poly(1,2-diaminoanthraquinone)/glassy carbon electrode. Sens. Bio. Sens. Res. 2020, 29, 100369. [Google Scholar] [CrossRef]
- Duraia, L.; Badhulika, S. Simultaneous sensing of copper, lead, cadmium and mercury traces in human blood serum using orthorhombic phase aluminium ferrite. Mater. Sci. Eng. C 2020, 112, 110865. [Google Scholar] [CrossRef] [PubMed]
- Kadir, H.A.; Abu Bakar, N.H.H.; Sabri, A.A.; Abdullah, F.H.; Abu Bakar, A.; Furuno, H.; Saito, N. Silver nanoparticles incorporated in dicarboxylic/TEPA modified halloysite nanotubes for the degradation of organic contaminants. Appl. Surf. Sci. 2020, 531, 147417. [Google Scholar] [CrossRef]
- Amid, M.; Nabian, N.; Delavar, M. Fabrication of polycarbonate ultrafiltration mixed matrix membranes including modified halloysite nanotubes and graphene oxide nanosheets for olive oil/water emulsion separation. Sep. Purif. Technol. 2020, 251, 117332. [Google Scholar] [CrossRef]
- Preethika, M.; Sundramoorthy, A.K. Humic acid/halloysite nanotube/flavin adenine dinucleotide nanocomposite based selective electrochemical biosensor for hydrogen peroxide. Appl. Surf. Sci. 2020, 488, 503. [Google Scholar] [CrossRef]
- Hatamie, A.; Echresh, A.; Zargar, B.; Nur, O.; Willander, M. Fabrication and characterization of highly-ordered Zinc Oxide nanorods on gold/glass electrode, and its application as a voltammetric sensor. Electrochim. Acta 2015, 174, 1261–1267. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Labuda, J.; Barek, J.; Brett, C.M.A.; Camões, M.F.; Fojta, M.; Hibbert, D.B. Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 641–694. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Martin, A.; Silva, M.F.; Escarpa, A. Screen-printed electrodes modified with carbon nanotubes or graphene for simultaneous determination of melatonin and serotonin. Microchim. Acta 2015, 18, 1925. [Google Scholar] [CrossRef]
- Cavazzuti, M. Optimization Methods: From Theory to Design; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Tarley, C.R.T.; Silveira, G.; Lopes dos Santos, W.N.; Matos, G.D.; Paranhos da Silva, E.G.; Bezerra, M.A.; Miró, M.; Ferreira, S.L.C. Chemometric tools in electroanalytical chemistry: Methods for optimization based on factorial design and response surface methodology. Microchem. J. 2009, 92, 58–67. [Google Scholar] [CrossRef]
- Koçoğlu, İ.O.; Erden, P.E.; Kenar, A.; Kılıç, E. Application of central composite design for the optimization of electrode surface composition for glucose biosensor fabrication. Anal. Bioanal. Chem. 2019, 411, 413–425. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Lotfi, D.; Anissa, M.; Hafsa, B.; Djamila, B.; Ihdene, Z.; Bakos, P.Y.; Boudjema, H. Optimization of Hydrolysis Degradation of Neurotoxic Pesticide Methylparathion Using a Response Surface Methodology (RSM). J. Appl. Chem. 2015, 8, 43–52. [Google Scholar] [CrossRef]
- Siddheswaran, R.; Manikandan, D.; Avila, R.E.; Jeyanthi, C.E.; Mangalaraja, R.V. Formation of Carbon Nanotube Forest over Spin-coated Fe2O3 Reduced Thin-film by Chemical Vapor Deposition. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 392–398. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Saadouni, M.; Galai, M.; Aoufir Yel Skal, S.; Boukhris, S.; Hassikou, A.; Touhami, M.E.; Guenbour, A.; El Khlifi, A.; Oudda, H.; Habbadi, N.; et al. Experimental and quantum chemical and Monte Carlo simulations studies on the corrosion inhibition of mild steel in 1 M HCl by two benzothiazine derivatives. J. Mater. Environ. Sci. 2018, 9, 2493–2504. [Google Scholar] [CrossRef]
- Baluchová, S.; Brycht, M.; Taylor, A.; Mortet, V.; Krůšek, J.; Dittert, I.; Sedláková, S.; Klimša, L.; Kopeček, J.; Schwarzová-Pecková, K. Enhancing electroanalytical performance of porous boron-doped diamond electrodes by increasing thickness for dopamine detection. Anal. Chim. Acta 2021, 1182, 338949. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Merkoci, A.; Alegret, S. Carbon nanotube-epoxy composites for electrochemical sensing. Sens. Actuators B Chem. 2006, 113, 617–622. [Google Scholar] [CrossRef]
- Madej, M.; Matoga, D.; Skaźnik, K.; Porada, R.; Baś, B.; Kochana, J. A voltammetric sensor based on mixed proton-electron conducting composite including metal-organic framework JUK-2 for determination of citalopram. Microchim. Acta 2021, 188, 184. [Google Scholar] [CrossRef] [PubMed]
- Nelder, A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Knihnicki, P.; Wieczorek, M.; Moos, A.; Kościelniak, P.; Wietecha-Posłuszny, R.; Woźniakiewicz, M. Electrochemical sensor for determination of desipramine in biological material. Sens. Actuators B Chem. 2013, 189, 37–42. [Google Scholar] [CrossRef]
- Lee, P.M.; Wang, Z.; Liu, X.; Chena, Z.; Liu, E. Glassy carbon electrode modified by graphene–gold nanocomposite coating for detection of trace lead ions in acetate buffer solution. Thin Solid Film. 2015, 584, 85–89. [Google Scholar] [CrossRef]
- Geiβ, S.; Einax, J.W. Comparison of detection limits in environmental analysis—Is it possible? An approach on quality assurance in the lower working range by verification. Fresenius J. Anal. Chem. 2001, 370, 673–678. [Google Scholar] [CrossRef]
- Han, X.; Meng, Z.; Zhang, H.; Zheng, J. Fullerene-based anodic stripping voltammetry for simultaneous determination of Hg(II), Cu(II), Pb(II) and Cd(II) in foodstuff. Microchim. Acta 2018, 185, 274. [Google Scholar] [CrossRef] [PubMed]
- Palisoc, S.T.; Micah, J.; Michelle, O.B. Determination of trace heavy metals in canned food using Graphene/AuNPs/[Ru(NH3)6]3+/Nafion modified glassy carbon electrodes. J. Food Meas. Charact. 2019, 13, 169–176. [Google Scholar] [CrossRef]
- Kim, H.J.; Son, D.W.; Park, J.M.; Hwang, D.Y.; Mo, C.Y.; Park, S.W.; Kim, G.; Eun, J.B. Application of an in situ bismuth-coated glassy carbon electrode for electroanalytical determination of Cd (II) and Pb (II) in Korean polished rices. Food Sci. Biotechnol. 2010, 19, 1211–1217. [Google Scholar] [CrossRef]
- Kefala, G.; Economou, A. Polymer-coated bismuth film electrodes for the determination of trace metals by sequential-injection analysis/anodic stripping voltammetry. Anal. Chim. Acta 2006, 576, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wu, K.; Dai, X.; Hu, S. Simultaneous determination of lead (II) and cadmium (II) at a diacetyldioxime modified carbon paste electrode by differential pulse stripping voltammetry. Talanta 2003, 60, 17–24. [Google Scholar] [CrossRef]
- NS, A.K.; Ashoka, S.; Malingappa, P. Nano zinc ferrite modified electrode as a novel electrochemical ensing platform in simultaneous measurement of trace level lead and cadmium. J. Environ. Chem. Eng. 2018, 6, 6939–6946. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, G.; Liu, G. A sensitive electrochemical sensor using a mesoporous carbon and nafion composite for the simultaneous detection of cadmium and lead. Int. J. Electrochem. Sci. 2017, 12, 5378–5391. [Google Scholar] [CrossRef]
- Hao, C.; Shen, Y.; Shen, J.; Xu, K.; Wang, X.; Zhao, Y.; Ge, C. A glassy carbon electrode modified with bismuth oxide nanoparticles and chitosan as a sensor for Pb (II) and Cd (II). Microchim. Acta 2016, 183, 1823–1830. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Voltammetric Determination of Lead (II) and Cadmium (II) Using a Bismuth Film Electrode Modified with Mesoporous Silica Nanoparticles. Electrochim. Acta 2014, 132, 223–229. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Bougrini, M.; Florea, A.; Cristea, C.; Sandulescu, R.; Vocanson, F.; Errachid, A.; Bouchikhi, B.; El Bari, N.; Jaffrezic-Renault, N. Development of a novel sensitive molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework for tetracycline detection in honey. Food Control. 2016, 59, 424–429. [Google Scholar] [CrossRef]


| Run | Actual Value of Factor % MWCNTs | Actual Value of Factor % C_Hal | Coded Value of Factor x1 | Coded Value of Factor x2 |
|---|---|---|---|---|
| 1 | 0.5 | 0.5 | −1 | −1 |
| 2 | 0.5 | 1.5 | −1 | 1 |
| 3 | 1.5 | 0.5 | 1 | −1 |
| 4 | 1.5 | 1.5 | 1 | 1 |
| 5 | 0.3 | 1 | −1.414 | 0 |
| 6 | 1.7 | 1 | 1.414 | 0 |
| 7 | 1 | 0.3 | 0 | −1.414 |
| 8 | 1 | 1.7 | 0 | 1.414 |
| 9 | 1 | 1 | 0 | 0 |
| 10 | 1 | 1 | 0 | 0 |
| 11 | 1 | 1 | 0 | 0 |
| 12 | 1 | 1 | 0 | 0 |
| 13 | 1 | 1 | 0 | 0 |
| Sensor | Cdl/µF cm−2 | Rct/kΩ | σ/kΩ s−1/2 | Aeff/mm2 | ks/m s−1 |
|---|---|---|---|---|---|
| SPCE | 20.8 ± 0.1 | 16.3 ± 0.2 | 8.50 ± 0.07 | 3.30 ± 0.05 | (9.9 ± 0.1) × 10−6 |
| MWCNTs/Nafion/SPCE | 8200 ± 50 | 10.80 ± 0.08 | 6.90 ± 0.04 | 4.07 ± 0.07 | (1.21 ± 0.04) × 10−5 |
| MWCNTs/C_Hal/Nafion/SPCE | 9.68 ± 0.05 | 0.057 ± 0.002 | 3.87 ± 0.03 | 7.25 ± 0.04 | (1.27 ± 0.01) × 10−3 |
| Electrode | Technique | Linearity Range /µmol L−1 | LOD /μmol L−1 | Sample | Ref. |
|---|---|---|---|---|---|
| The fullerene C60-chitosan modified GCE | DPASV | Pb: 0.005–6.0 Cd: 0.5–9.0 | Pb: 0.001 Cd: 0.021 | milk, honey | [33] |
| Graphene/(AuNPs)/[Ru(NH3)6]3+/Nafion/(GCE) | ASV | Pb: 0.036–7.19 Cd: 1.5–11.27 | Pb: 0.0012 Cd: 0.0011 | meat, tuna, mushrooms, canned sardines | [34] |
| Bismuth-coated GCE | SWASV | Pb: 0.007–0.965 Cd: 0.013–1.779 | Pb: 0.007 Cd: 0.356 | rices | [35] |
| Polymer-coated BiFEs/GCE | SWASV | Pb: 0.010–0.290 Cd: 0.018–0.534 | Pb: 0.010 Cd: 0.018 | rock, urine | [36] |
| Diacetyldioxime -CPE | AdSV | Pb: 0.10–1.0 Cd: 0.25–2.5 | Pb: 0.01 Cd: 0.04 | water | [37] |
| ZnFe2O4/GCE | DPASV | Pb: 0.048–0.627 Cd: 0.089–1.156 | Pb: 0.005 Cd: 0.022 | wastewater | [38] |
| MC/Nafion/GCE | SWASV | Pb: 0.024–0.338 Cd: 0.044–0.623 | Pb: 0.0006 Cd: 0.0004 | soil | [39] |
| BiONPs-CS-GCE | DPASV | Pb: 0.4–2.8 Cd: 0.8–5.6 | Pb: 0.15 Cd: 0.05 | tap water | [40] |
| NanoSiO2/BiFEs/GCE | SWASV | Pb: 0.010–0.724 Cd: 0.018–1.334 | Pb: 0.001 Cd: 0.005 | water | [41] |
| MWCNTs/C_Hal/Nafion/SPCE | DPASV | Pb/Cd: 0.10–10 | Pb: 0.0051 Cd: 0.0106 | tap water, mineral waters, well water, honey | This work |
| Sample | Amount Added/nmol L−1 | Amount Obtained a/nmol L−1 | Recovery/% | RSD/% |
|---|---|---|---|---|
| Tap water | 250 | 248 | 99.2 ± 0.6 | 3.5 |
| 750 | 751 | 100.1 ± 0.8 | 4.2 | |
| Well water | 250 | 244 | 97.6 ± 0.5 | 2.8 |
| 750 | 742 | 98.9 ± 0.7 | 3.8 | |
| Mineral water 1 | 250 | 247 | 98.8 ± 1.3 | 6.9 |
| 750 | 742 | 98.9 ± 0.8 | 4.5 | |
| Mineral water 2 | 250 | 254 | 101.6 ± 0.7 | 3.9 |
| 750 | 760 | 101.3 ± 0.5 | 2.7 | |
| Honey | 250 | 241 | 96.4 ± 1.6 | 8.5 |
| 750 | 739 | 98.5 ± 1.2 | 6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knihnicki, P.; Skrzypek, A.; Jakubowska, M.; Porada, R.; Rokicińska, A.; Kuśtrowski, P.; Kościelniak, P.; Kochana, J. Electrochemical Sensing of Pb2+ and Cd2+ Ions with the Use of Electrode Modified with Carbon-Covered Halloysite and Carbon Nanotubes. Molecules 2022, 27, 4608. https://doi.org/10.3390/molecules27144608
Knihnicki P, Skrzypek A, Jakubowska M, Porada R, Rokicińska A, Kuśtrowski P, Kościelniak P, Kochana J. Electrochemical Sensing of Pb2+ and Cd2+ Ions with the Use of Electrode Modified with Carbon-Covered Halloysite and Carbon Nanotubes. Molecules. 2022; 27(14):4608. https://doi.org/10.3390/molecules27144608
Chicago/Turabian StyleKnihnicki, Paweł, Aleksandra Skrzypek, Małgorzata Jakubowska, Radosław Porada, Anna Rokicińska, Piotr Kuśtrowski, Paweł Kościelniak, and Jolanta Kochana. 2022. "Electrochemical Sensing of Pb2+ and Cd2+ Ions with the Use of Electrode Modified with Carbon-Covered Halloysite and Carbon Nanotubes" Molecules 27, no. 14: 4608. https://doi.org/10.3390/molecules27144608







