Effects of Impregnated Amidophosphonate Ligand Concentration on the Uranium Extraction Behavior of Mesoporous Silica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
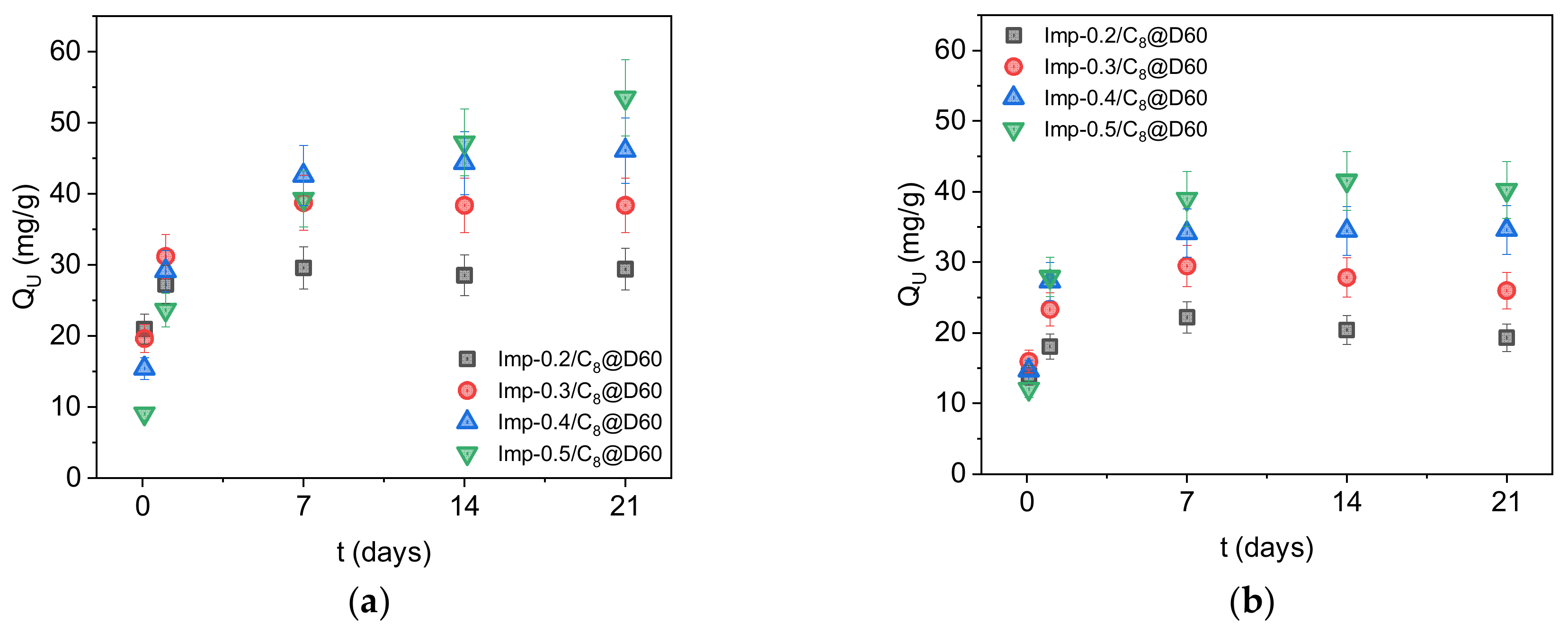
2.2. Extraction of Uranium from Sulfuric Acid Solutions
3. Materials and Methods
3.1. Chemicals
3.2. Materials Synthesis
3.3. Uranium-Containing Solutions
3.4. Characterization of the Organic Ligand
3.5. Characterization of the Materials
3.6. Uranium Extraction Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lunt, D.; Boshoff, P.; Boylett, M.; El-Ansary, Z. Uranium extraction: The key process drivers. J. S. Afr. Inst. Min. Metall. 2007, 107, 419–426. [Google Scholar]
- Edwards, C.R.; Oliver, A.J. Uranium processing: A review of current methods and technology. JOM 2000, 52, 12–20. [Google Scholar] [CrossRef]
- Sole, K.C.; Cole, P.M.; Feather, A.M.; Kotze, M.H. Solvent Extraction and Ion Exchange Applications in Africa’s Resurging Uranium Industry: A Review. Solvent Extr. Ion Exch. 2011, 29, 868–899. [Google Scholar] [CrossRef]
- Dolatyari, L.; Yaftian, M.R.; Rostamnia, S. Removal of uranium(VI) ions from aqueous solutions using Schiff base functionalized SBA-15 mesoporous silica materials. J. Environ. Manag. 2016, 169, 8–17. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J.; Jolliffe, K.A.; Hanley, T.L. Selective Sorption of Actinides by Titania Nanoparticles Covalently Functionalized with Simple Organic Ligands. ACS Appl. Mater. Interfaces 2013, 5, 11984–11994. [Google Scholar] [CrossRef]
- Yang, S.; Qian, J.; Kuang, L.; Hua, D. Ion-Imprinted Mesoporous Silica for Selective Removal of Uranium from Highly Acidic and Radioactive Effluent. ACS Appl. Mater. Interfaces 2017, 9, 29337–29344. [Google Scholar] [CrossRef]
- Lebed, P.J.; Savoie, J.-D.; Florek, J.; Bilodeau, F.; Larivière, D.; Kleitz, F. Large Pore Mesostructured Organosilica-Phosphonate Hybrids as Highly Efficient and Regenerable Sorbents for Uranium Sequestration. Chem. Mater. 2012, 24, 4166–4176. [Google Scholar] [CrossRef]
- Carboni, M.; Abney, C.; Taylor-Pashow, K.; Vivero-Escoto, J.L.; Lin, W. Uranium Sorption with Functionalized Mesoporous Carbon Materials. Ind. Eng. Chem. Res. 2013, 52, 15187–15197. [Google Scholar] [CrossRef]
- Guo, H.; Mei, P.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Carbon materials for extraction of uranium from seawater. Chemosphere 2021, 278, 130411. [Google Scholar] [CrossRef]
- Mohamud, H.; Ivanov, P.; Russell, B.C.; Regan, P.H.; Ward, N.I. Selective sorption of uranium from aqueous solution by graphene oxide-modified materials. J. Radioanal. Nucl. Chem. Artic. 2018, 316, 839–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Bai, Z.; Zhu, L.; Zhang, L.; Cai, Y.; Li, Y.; Liu, W.; Wang, Y.; Chen, L.; Diwu, J.; et al. Ultrafast and Efficient Extraction of Uranium from Seawater Using an Amidoxime Appended Metal–Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 32446–32451. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Chen, C.; Luo, F.; Huang, S.Y.; Wang, L.L.; Zhang, N. Isoreticular MOFs functionalized in the pore wall by different organic groups for high-performance removal of uranyl ions. J. Radioanal. Nucl. Chem. Artic. 2016, 310, 317–327. [Google Scholar] [CrossRef]
- Cheng, Y.; He, P.; Dong, F.; Nie, X.; Ding, C.; Wang, S.; Zhang, Y.; Liu, H.; Zhou, S. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem. Eng. J. 2019, 367, 198–207. [Google Scholar] [CrossRef]
- Wang, D.; Song, J.; Wen, J.; Yuan, Y.; Liu, Z.; Lin, S.; Wang, H.; Wang, H.; Zhao, S.; Zhao, X.; et al. Significantly Enhanced Uranium Extraction from Seawater with Mass Produced Fully Amidoximated Nanofiber Adsorbent. Adv. Energy Mater. 2018, 8, 1802607. [Google Scholar] [CrossRef]
- Karve, M.; Rajgor, R.V. Amberlite XAD-2 impregnated organophosphinic acid extractant for separation of uranium(VI) from rare earth elements. Desalination 2008, 232, 191–197. [Google Scholar] [CrossRef]
- Merdivan, M.; Düz, M.; Hamamci, C. Sorption behaviour of uranium(VI) with N,N-dibutyl-N′-benzoylthiourea Impregnated in Amberlite XAD-16. Talanta 2001, 55, 639–645. [Google Scholar] [CrossRef]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2017, 4, 110–128. [Google Scholar] [CrossRef]
- Fontanals, N.; Marcé, R.M.; Borrull, F. Materials for Solid-Phase Extraction of Organic Compounds. Separations 2019, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Charlot, A.; Cuer, F.; Grandjean, A. The effect of pore diameter in the arrangement of chelating species grafted onto silica surfaces with application to uranium extraction. New J. Chem. 2016, 41, 503–511. [Google Scholar] [CrossRef]
- Charlot, A.; Dumas, T.; Solari, P.L.; Cuer, F.; Grandjean, A. A Spectroscopic Study of Uranium and Molybdenum Complexation within the Pore Channels of Hybrid Mesoporous Silica. Eur. J. Inorg. Chem. 2016, 2017, 563–573. [Google Scholar] [CrossRef]
- Charlot, A.; Mourabit, S.E.; Goettmann, F.; Arrachart, G.; Turgis, R.; Grandjean, A. From phosphate rocks to uranium raw materials: Hybrid materials designed for selective separation of uranium from phosphoric acid. RSC Adv. 2014, 4, 64138–64141. [Google Scholar] [CrossRef]
- Le Nedelec, T.; Charlot, A.; Calard, F.; Cuer, F.; Leydier, A.; Grandjean, A. Uranium adsorption from sulfuric acid media using silica materials functionalised with amide and phosphorous ligands. New J. Chem. 2018, 42, 14300–14307. [Google Scholar] [CrossRef]
- Dressler, A.; Le Nedelec, T.; Leydier, A.; Cuer, F.; Dumas, T.; Grandjean, A. The effects of amidophosphonate ligand immobilization method on the uranium extraction efficiency of functionalized silica. Chem. Eng. J. Adv. 2022, 9, 100225. [Google Scholar] [CrossRef]
- Singh, D.K.; Mondal, S.; Chakravartty, J.K. Recovery of Uranium from Phosphoric Acid: A Review. Solvent Extr. Ion Exch. 2016, 34, 201–225. [Google Scholar] [CrossRef]
- Turgis, R.; Leydier, A.; Arrachart, G.; Burdet, F.; Dourdain, S.; Bernier, G.; Miguirditchian, M.; Pellet-Rostaing, S. Carbamoylalkylphosphonates Type Ligand for Uranium Extraction from Phosphates Ores. Procedia Eng. 2016, 138, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Baron, P.; Bernier, G.; Hartmann, D.; Laluc, C.; Marbet, M. Use of compounds comprising amide and phosphonate functions for extracting uranium(VI) from aqueous solutions of sulphuric acid, resulting in particular from sulphuric acid leaching of uranium ores. WIPO Patent WO2014139869, 18 September 2014. Available online: https://worldwide.espacenet.com/patent/search/family/048613868/publication/WO2014139869A1?q=WO2014139869A1 (accessed on 25 May 2022).
- Song, Y.; Du, Y.; Lv, D.; Ye, G.; Wang, J. Macrocyclic receptors immobilized to monodisperse porous polymer particles by chemical grafting and physical impregnation for strontium capture: A comparative study. J. Hazard. Mater. 2014, 274, 221–228. [Google Scholar] [CrossRef]
- Rao, N.; Wang, M.; Shang, Z.; Hou, Y.; Fan, G.; Li, J. CO2 Adsorption by Amine-Functionalized MCM-41: A Comparison between Impregnation and Grafting Modification Methods. Energy Fuels 2018, 32, 670–677. [Google Scholar] [CrossRef]
- Cecilia, J.; Vilarrasa-García, E.; García-Sancho, C.; Saboya, R.; Azevedo, D.; Cavalcante, C.; Rodríguez-Castellón, E. Functionalization of hollow silica microspheres by impregnation or grafted of amine groups for the CO 2 capture. Int. J. Greenh. Gas Control 2016, 52, 344–356. [Google Scholar] [CrossRef]
- Builes, S.; Vega, L. Effect of Immobilized Amines on the Sorption Properties of Solid Materials: Impregnation versus Grafting. Langmuir 2013, 29, 199–206. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andrésen, J.M.; Miller, B.G.; Scaroni, A.W. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 2003, 62, 29–45. [Google Scholar] [CrossRef]
- Son, W.-J.; Choi, J.-S.; Ahn, W.-S. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous Mesoporous Mater. 2008, 113, 31–40. [Google Scholar] [CrossRef]
- Whitty-Léveillé, L.; Tremblay-Cantin, J.-C.; Picard-Lafond, A.; Boudreau, D.; Reynier, N.; Larivière, D. Core-shell nanoparticles bearing Schiff base ligand for the selective extraction of uranium from REE leach liquors. Hydrometallurgy 2022, 208, 105780. [Google Scholar] [CrossRef]
- Magi, M.; Lippmaa, E.; Samoson, A.; Engelhardt, G.; Grimmer, A.R. Solid-state high-resolution silicon-29 chemical shifts in silicates. J. Phys. Chem. 1984, 88, 1518–1522. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Turgis, R.; Leydier, A.; Arrachart, G.; Burdet, F.; Dourdain, S.; Bernier, G.; Miguirditchian, M.; Pellet-Rostaing, S. Carbamoylalkylphosphonates for Dramatic Enhancement of Uranium Extraction from Phosphates Ores. Solvent Extr. Ion Exch. 2014, 32, 685–702. [Google Scholar] [CrossRef]
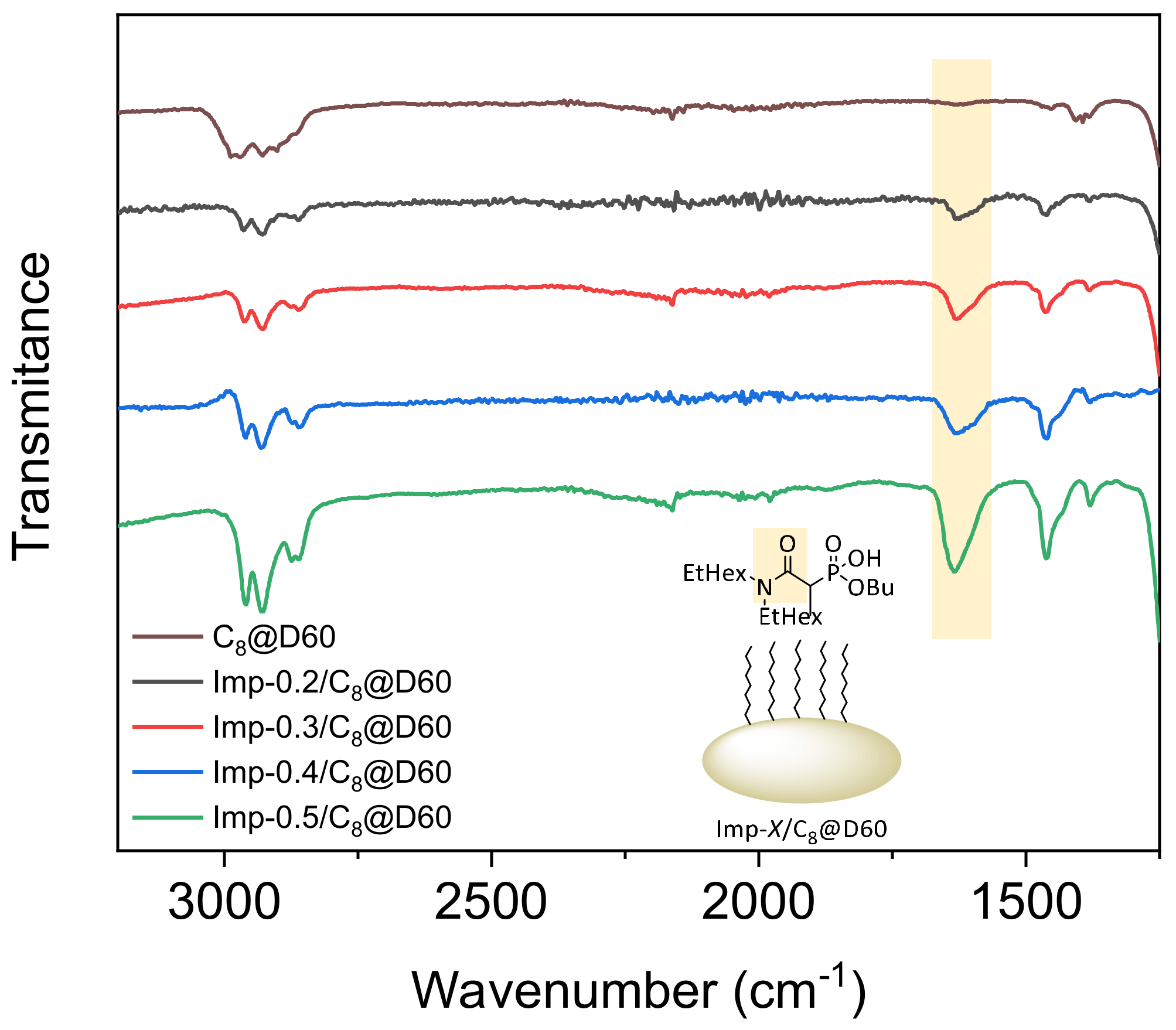

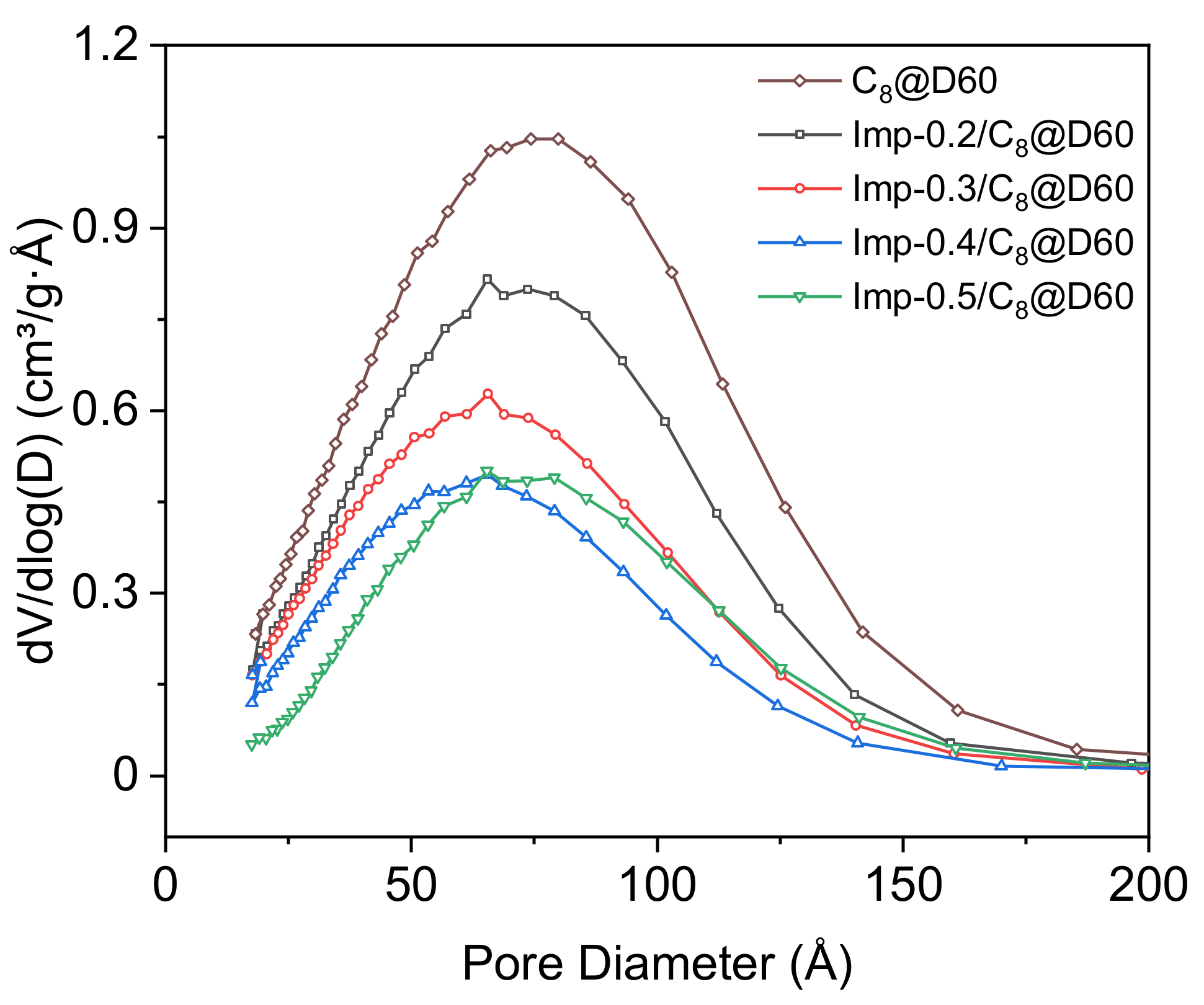
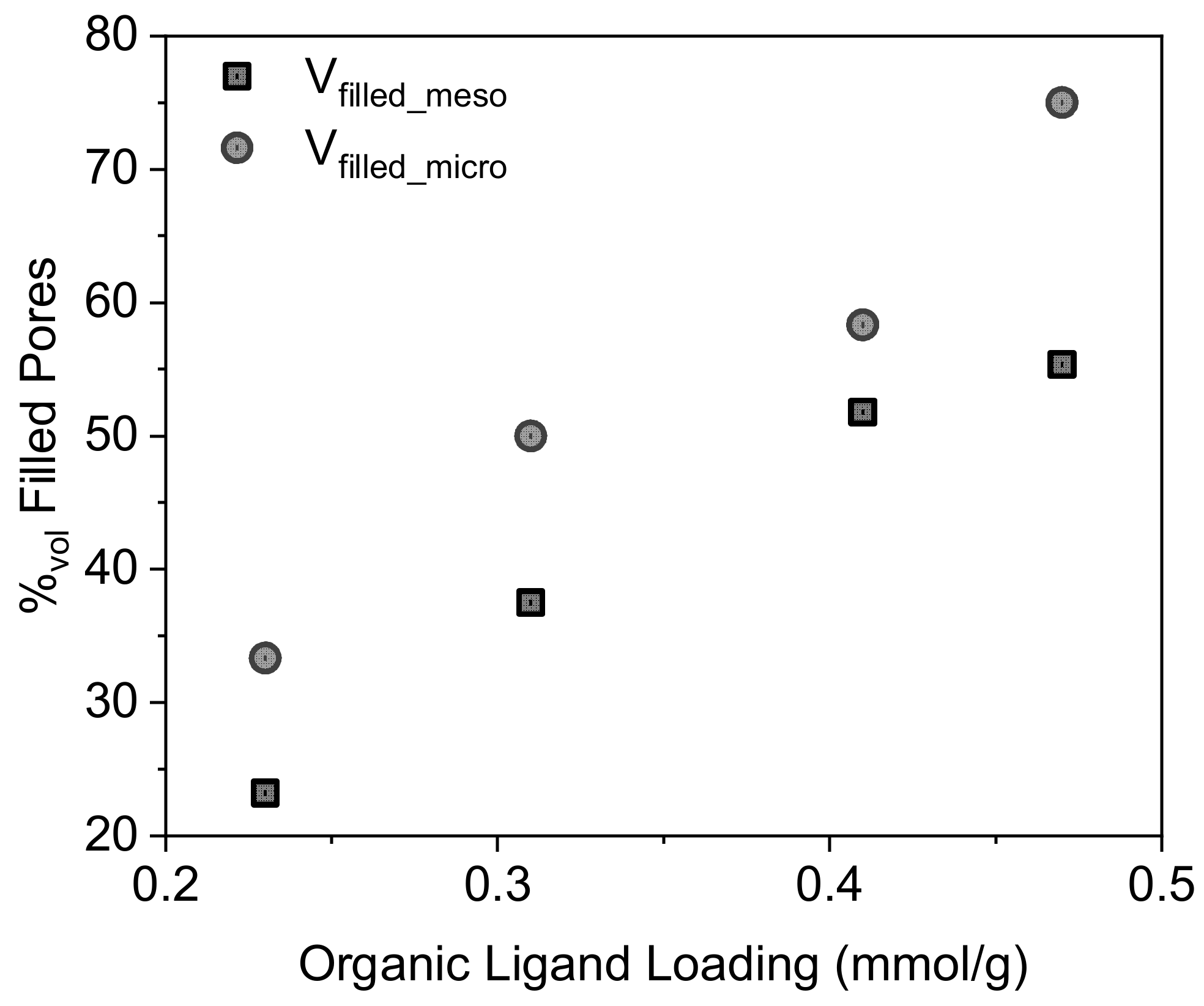
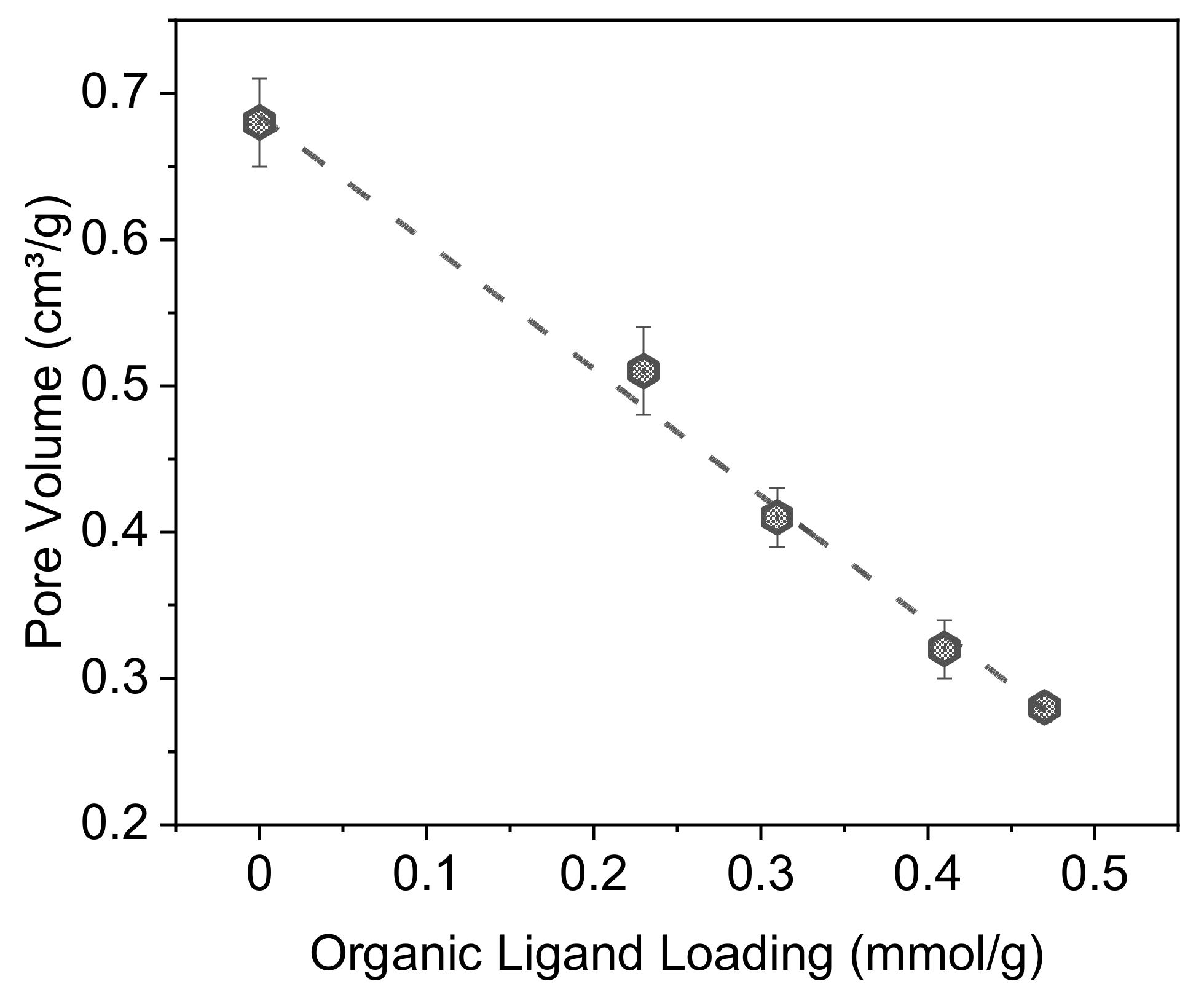


| Material | τL a | %L b | SBET c | Vpores d | Vmicropores e | VL f | dSLigand g |
|---|---|---|---|---|---|---|---|
| C8@D60 | 447 (±22) | 0.68 (±0.03) | 0.12 | ||||
| Imp-0.2/C8@D60 | 0.23 (±0.02) | 9.9 (±0.9) | 337 (±17) | 0.51 (±0.03) | 0.08 | 1.23 | 0.31 |
| Imp-0.3/C8@D60 | 0.31 (±0.02) | 13.7 (±0.9) | 294 (±15) | 0.41 (±0.02) | 0.06 | 1.45 | 0.42 |
| Imp-0.4/C8@D60 | 0.41 (±0.02) | 17.6 (±0.9) | 222 (±11) | 0.32 (±0.02) | 0.05 | 1.46 | 0.55 |
| Imp-0.5/C8@D60 | 0.47 (±0.02) | 20.4 (±0.9) | 149 (±7) | 0.28 (±0.01) | 0.03 | 1.41 | 0.63 |
| Elements Present | U (mg/L) | Fe (mg/L) | Mo (mg/L) | (SO4)2− (g/L) | pH |
|---|---|---|---|---|---|
| U only | 400 | - | - | 146 | 1 |
| U + competing ions | 400 | 5000 | 57 | 146 | 1 |
| Material | Elements Present in the Solution | |||||
|---|---|---|---|---|---|---|
| U | U, Fe, Mo | |||||
| QUmax a | L/U b | t c | QUmax a | L/U b | t c | |
| Imp-0.2/C8@D60 | 30 (±3) | 2.0 (± 0.2) | 0–1 | 22 (±2) | 2.5 (±0.3) | 0–1 |
| Imp-0.3/C8@D60 | 39 (±4) | 1.9 (± 0.2) | 1–7 | 29 (±2) | 2.5 (±0.3) | 1–7 |
| Imp-0.4/C8@D60 | 46 (±5) | 2.1 (± 0.2) | 1–7 | 35 (±3) | 2.8 (±0.3) | 1–7 |
| Imp-0.5/C8@D60 | 54 (±5) | 2.1 (± 0.2) | 14–21 | 42 (±4) | 2.7 (±0.3) | 1–7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dressler, A.; Leydier, A.; Grandjean, A. Effects of Impregnated Amidophosphonate Ligand Concentration on the Uranium Extraction Behavior of Mesoporous Silica. Molecules 2022, 27, 4342. https://doi.org/10.3390/molecules27144342
Dressler A, Leydier A, Grandjean A. Effects of Impregnated Amidophosphonate Ligand Concentration on the Uranium Extraction Behavior of Mesoporous Silica. Molecules. 2022; 27(14):4342. https://doi.org/10.3390/molecules27144342
Chicago/Turabian StyleDressler, Aline, Antoine Leydier, and Agnès Grandjean. 2022. "Effects of Impregnated Amidophosphonate Ligand Concentration on the Uranium Extraction Behavior of Mesoporous Silica" Molecules 27, no. 14: 4342. https://doi.org/10.3390/molecules27144342






