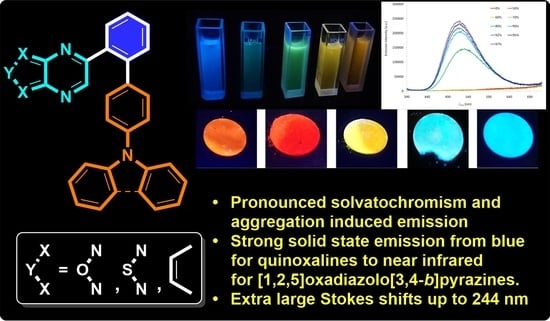
Push–Pull Derivatives Based on 2,4′-Biphenylene Linker with Quinoxaline, [1,2,5]Oxadiazolo[3,4-B]Pyrazine and [1,2,5]Thiadiazolo[3,4-B]Pyrazine Electron Withdrawing Parts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Electrochemical Properties
2.3. Photophysical Properties
2.4. NLO Properties
2.5. Theoretical Calculation
3. Experimental Methods
3.1. General Information
3.2. Electrochemical Characterization
3.3. Photophysical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bureš, F. Fundamental aspects of property tuning in push-pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef] [Green Version]
- Beverina, L.; Pagani, G.A. π-Conjugated zwitterions as paradigm of donor-acceptor building blocks in organic-based materials. Acc. Chem. Res. 2014, 47, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kivala, M.; Diederich, F. Acetylene-derived strong organic acceptors for planar and nonplanar push-pull chromophores. Acc. Chem. Res. 2009, 42, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Diederich, F. Non-planar push-pull chromophores. Chem. Commun. 2010, 46, 1994–2006. [Google Scholar] [CrossRef]
- Meier, H. Conjugated oligomers with terminal donor-acceptor. Angew. Chem. Int. Ed. 2005, 44, 2482–2506. [Google Scholar] [CrossRef]
- Kivala, M.; Diederich, F. Conjugation and optoelectronic properties of acetylenic scaffolds and charge-transfer chromophores. Pure Appl. Chem. 2008, 80, 411–427. [Google Scholar] [CrossRef] [Green Version]
- Ipuy, M.; Billon, C.; Micouin, G.; Samarut, J.; Andraud, C.; Bretonniere, Y. Fluorescent push-pull repsonsive probes for ratiometric detection of intracellular pH. Org. Biomol. Chem. 2014, 2, 3641–3648. [Google Scholar] [CrossRef]
- Lee, S.-C.; Heo, J.; Woo, H.C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.-P. Fluorescent molecular rotors for viscosity. Chem.-Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.-H.; Lee, S.; Lee, Y.; Lee, S.; Yang, K.-H.; Wang, K.-K.; Han, W.-S. Pyridazine-carbazole based fluorescent probes for volatile acid detection. Dyes Pigment. 2021, 194, 109613. [Google Scholar]
- Dal Molin, M.; Verolet, Q.; Soleimanpour, S.; Matile, S. Mechanosensitive membrane probes. Chem.-Eur. J. 2015, 21, 6012–6021. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.M.; Zhou, H.; Kim, H.K. Rational design criterifor D-π-A structured organic and porphyrin sensitizers for highly efficient dyes-sensitized solar cells. J. Mater. Chem. A 2018, 6, 14518–14545. [Google Scholar] [CrossRef]
- Lu, J.; Liu, S.; Wang, M. Push-pull zinc porphyrins as light-harverters for efficient dye sensitized solar cells. Front. Chem. 2018, 6, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yella, A.; Mai, C.-L.; Zakeeruddin, S.M.; Chang, S.N.; Hsieh, C.-H.; Yeh, C.-Y.; Graetzel, M. Molecular engineering of push-pull porphyrin dyes for highly efficient dye-sensitized solar cells: The role of benzene spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Azines as unconventional anchoring groups for dye-sensitized solar cells: The first decade of research advances and future outlook. Dyes Pigment. 2021, 194, 109650. [Google Scholar] [CrossRef]
- Li, W.; Ye, L.; Li, S.; Yao, H.; Ade, H.; Hou, J. A high-efficiency organic solar cell enabled by the strong intramolecular electron push-pull effect of the nonfullerene acceptor. Adv. Mater. 2018, 30, 1707170. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Yao, N.; Liu, S.; Xu, Y.; Cao, J.; Zhuang, W.; Yu, J.; Wang, N.; Yu, D.; Zhang, F.; et al. Nonfullerene acceptors from thieno[3,2-b]thiophene-fused naphthalene donor with six-member-ring connection for efficient organic solar cells. Dyes Pigment. 2021, 185, 108892. [Google Scholar] [CrossRef]
- Simón Marqués, P.; Castán, J.M.A.; Raul, B.A.L.; Londi, G.; Ramirez, I.; Pshenichkov, M.S.; Beljonne, D.; Walzer, K.; Blais, M.; Allain, M.; et al. Triphenylamine/tetracyclobutadiene-based π-conjugated push-pull molecules end-capped with arene platforms: Synthesis, photophysics, and photovoltaic response. Chem.-Eur. J. 2020, 26, 16422–16433. [Google Scholar] [CrossRef] [PubMed]
- Ostroverkhova, O. Organic Optolectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef]
- Payne, A.-J.; Rice, N.A.; McAfee, S.M.; Lo, S.; Josse, P.; Cabanetos, P.; Rishko, C.; Lessard, B.H.; Welch, G.C. Donor or Acceptor? How selection of the rylene imide end cap impacts the polarity of pi-conjugated molecules for organic electronics. ACS Appl. Energy Mater. 2019, 1, 4906–4916. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, C.; He, W.; Cao, A. Progress in the enhancement of electro-optic coefficients and orientation stability for organic second-order nonlinear optical materials. Dyes Pigment. 2020, 181, 108509. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Yruela, C.; Garín, J.; Orduna, J.; Franco, S.; Quintero, E.; López-Navarrete, J.T.; Diosdado, B.E.; Villacampa, B.; Casado, J.; Andreu, R. D-π-A compounds with tunable intramolecular charge transfer achieved by incorporation of bitenolide nitriles as acceptor moieties. J. Org. Chem. 2015, 80, 12115–12128. [Google Scholar] [CrossRef] [Green Version]
- Tydlitát, J.; Fecková, M.; Le Poul, P.; Pytela, O.; Klikar, M.; Rodríguez-López, J.; Robin-le Guen, F.; Achelle, S. Influence of donor-substituents on triphenylamine chromophores bearing pyridine fragments. Eur. J. Org. Chem. 2019, 2019, 1921–1930. [Google Scholar] [CrossRef]
- Klikar, M.; Bureš, F.; Pytela, O.; Mikysek, T.; Padĕlková, Z.; Barsella, A.; Dorkenoo, K.; Achelle, S. N,N′-Dibutylbarbituric acid as an acceptor moiety in push-pull chromophores. New J. Chem. 2013, 37, 4230–4240. [Google Scholar] [CrossRef] [Green Version]
- Stefko, M.; Tzirakis, M.D.; Breiten, B.; Ebert, M.O.; Dumele, O.; Schweizer, W.B.; Gisselbrecht, J.P.; Boudon, C.; Beels, M.T.; Biaggio, I.; et al. Donor-acceptor (D-A)-substituted polyyne chromophores: Modulation of their optoelectronic properties by varying the length of the acetylene spacer. Chem.-Eur. J. 2013, 19, 12693–12704. [Google Scholar] [CrossRef]
- Achelle, S.; Rodríguez-López, J.F.; Robin-Le Guen, F. Photoluminescence properties of Aryl-, Arylvinyl-, and arylethynylpyrimidine derivatives. ChemistrySelect 2018, 3, 1852–1886. [Google Scholar] [CrossRef]
- Meti, P.; Park, H.-H.; Gong, Y.-D. Recent developments in pyrazine functionalized pi-conjugated materials for optoelectronc applications. J. Mater. Chem. C 2020, 8, 352–379. [Google Scholar] [CrossRef]
- Nosova, E.V.; Achelle, S.; Lipunova, G.N.; Charushin, V.N.; Chupakhin, O.N. Functionalized benzazines as luminescent materials and component for optoelectronics. Russ. Chem. Rev. 2019, 88, 1128–1178. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of fluorescent sensors based on azaheterocyclic push-pull systems towards nitroaromatic explosives and related compounds: Review. Dyes Pigment. 2020, 180, 108141. [Google Scholar] [CrossRef]
- Fecková, M.; Le Poul, P.; Bureš, F.; Robin-le Guen, F.; Achelle, S. Nonlinear optical properties of pyrimidine chromophores. Dyes Pigment. 2020, 182, 108659. [Google Scholar] [CrossRef]
- Gupta, S.; Milton, M.D. Design and synthesis of novel V-shaped AIEE active quinoxalines for acidochromic applications. Dyes Pigment. 2019, 165, 474–487. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Zibarev, A.V. Synthesis and Applications of 5-Membered Chalcogen-Nitrogen π-Heterocycles with Three Heteroatoms. Asian J. Org. Chem. 2018, 7, 2397–2416. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A. Chalcogen exchange in chalcogen–nitrogen π-heterocycles. Mendeleev Commun. 2021, 31, 433–441. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Yudin, I.L. Advances in the chemistry of furazano[3,4-b]pyrazines and their analogues. Russ. Chem. Rev. 2003, 72, 87–100. [Google Scholar] [CrossRef]
- Makhova, N.N.; Belen’kii, L.I.; Gazieva, G.A.; Dalinger, I.L.; Konstantinova, L.S.; Kuznetsov, V.V.; Kravchenko, A.N.; Krayushkin, M.M.; Rakitin, O.A.; Starosotnikov, A.M.; et al. Progress in the chemistry of nitrogen-, oxygen- and sulfur-containing heterocyclic systems. Russ. Chem. Rev. 2020, 89, 55–124. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Yakovleva, Y.A.; Khokhlov, K.O.; Rusinov, G.L.; Charushin, V.N. 9-Ethyl-3-{6-(het)aryl-[1,2,5]oxadiazolo[3,4-b]pyrazin-5-yl}-9H-carbazoles: Synthesis and study of sensitivity to nitroaromatic compounds. Russ. Chem. Bull. 2018, 67, 1078–1082. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Khokhlov, K.O.; Chuvashov, R.D.; Yakovleva, Y.A.; Makarova, N.I.; Vetrova, E.V.; Metelitsa, A.V.; Rusinov, G.L.; et al. Novel fluorophores based on imidazopyrazine derivatives: Synthesis and photophysical characterization focusing on solvatochromism and sensitivity towards nitroaromatic compounds. Dyes Pigment. 2019, 168, 248–256. [Google Scholar] [CrossRef]
- Kvashnin, Y.A.; Verbitskiy, E.V.; Eltsov, O.S.; Slepukhin, P.A.; Tameev, A.R.; Nekrasova, N.V.; Rusinov, G.L.; Nunzi, J.-M.; Chupakhin, O.N.; Charushin, V.N. Dibenzo[f,h]furazano[3,4-b]quinoxalines: Synthesis by intramolecular cyclization through direct transition metal-free C–H functionalization and electrochemical, photophysical, and charge mobility characterization. ACS Omega 2020, 5, 8200–8210. [Google Scholar] [CrossRef] [Green Version]
- Verbitskiy, E.V.; Achelle, S.; Bureš, F.; Le Poul, P.; Barsella, A.; Kvashnin, Y.A.; Rusinov, G.L.; Robin-le Guen, F.; Chupakhin, O.N.; Charushin, V.N. Synthesis, photophysical and nonlinear optical properties of [1,2,5]oxadiazolo[3,4-b]pyrazine-based linear push-pull systems. J. Photochem. Photobiol. A Chem. 2021, 404, 112900. [Google Scholar] [CrossRef]
- Kulhánek, J.; Bureš, F.; Opršal, J.; Kuznik, W.; Mikysek, T.; Růžička., A. 1,4-phenylene and 2,5-thinenylene π-linkers in charge-trasnfer chromophores. Asian J. Org. Chem. 2013, 2, 422–431. [Google Scholar] [CrossRef]
- Marco, A.B.; Burrezo, P.M.; Mosteo, L.; Franco, S.; Garín, J.; Orduna, J.; Diosdado, B.E.; Villacampa, B.; Lopez Navarrete, J.T.; Casado, J.; et al. Polarization, second order nonlinear optical properties and electrochromsim in 4H-pyranylidene chromophores with quinoid/aromatic thiophene ring bridge. RSC Adv. 2015, 5, 231–242. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Mathew, S.M.; Cornett, S.D.; Grundy, S.C.; Hartley, C.S. Ortho-phenylene oligomers with terminal push-pull substitution. Org. Biomol. Chem. 2012, 10, 3398–3405. [Google Scholar] [CrossRef]
- Hammer, B.A.G.; Müllen, K. Dimensional evolution of polyphenylenes: Expanding in all directions. Chem. Rev. 2016, 116, 2103–2140. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Lartia, R.; Allain, C.; Bordeau, G.; Schmidt, F.; Fiorini-Desbuischert, C.; Charra, F.; Teulade Fichou, M.-P. Synthetic strategies to derivatizable triphenylamine displaying high two-photon absorption. J. Org. Chem. 2008, 73, 1732–1744. [Google Scholar] [CrossRef]
- Katan, C.; Charlot, M.; Mongin, O.; Le Droumaguet, C.; Juikov, V.; Terenziani, F.; Badaeva, E.; Tretiak, S.; Blanchard-Desce, M. Simultaneous, control of emission localization and two-photon absorption efficiency in dissymetrical chromophores. J. Phys. Chem. B 2010, 114, 3152–3169. [Google Scholar] [CrossRef] [Green Version]
- Merkt, F.K.; Höwedes, S.P.; Gers-Panther, C.F.; Gruber, I.; Jeniak, C.; Müller, T.J.J. Three component activation/alkynylation/cyclocondensation (AACC) synthesis of enhanced emission solvatochromic 3-ethynylquinoxalines. Chem. Eur. J. 2018, 24, 8114–8125. [Google Scholar] [CrossRef]
- Fecková, M.; Le Poul, P.; Robin-le Guen, F.; Roisnel, T.; Pytela, O.; Klikar, M.; Bureš, F.; Achelle, S. 2,4-Distyryl and 2,4,6-tristyrylpyrimidines: Synthesis and photophysical properties. J. Org. Chem. 2018, 83, 11712–11726. [Google Scholar] [CrossRef]
- Achelle, S.; Robin-le Guen, F. Emission properties of diazines chromophores: Structure properties relationship. J. Photochem. Photobiol. A Chem. 2017, 348, 281–286. [Google Scholar] [CrossRef]
- Shen, X.Y.; Yuan, W.Z.; Liu, Y.; Zhao, Q.; Liu, P.; Ma, Y.; Williams, I.D.; Qin, A.; Sun, J.Z.; Tang, B.Z. Fumaronitrile-based fluorogen: Red to near-infrared fluorescence, aggregation-induced emission, solvatochromism, and twisted intramolecular charge transfer. J. Phys. Chem. C 2012, 116, 10541–10547. [Google Scholar] [CrossRef]
- Gupta, S.; Milton, M.D. Novel Y-shaped AIEE-TICT active π-extended quinoxalines-based donor-acceptor molecules displaying acidofluorochromism and temperature depedent emission. J. Photochem. Photobiol. A Chem. 2022, 424, 113630. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Singer, K.D.; Garito, A.F. Measurements of molecular second order optical susceptibilities usin dc induced second harmonic generation. J. Chem. Phys. 1981, 75, 3572. [Google Scholar] [CrossRef]
- Levine, B.F.; Bethea, C.G. Molecular hyperpolarizabilities determined from conjugated and nonconjugated liquids. Appl. Phys. Lett. 1974, 24, 445. [Google Scholar] [CrossRef]
- Ledoux, I.; Zyss, J. Influence of the molecular environment in solution measurements of the second order opticlsusceptibility for ureand derivatives. Chem. Phys. 1982, 73, 203–213. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bird, C.W. The application of new aromaticity index to six-membered ring heterocycles. Tetrahedron 1986, 42, 89–92. [Google Scholar] [CrossRef]
- Bird, C.W. A new aromaticity index and its application to five-membered heterocycles. Tetrahedron 1985, 41, 1409–1414. [Google Scholar] [CrossRef]
- Kvashnin, Y.A.; Verbitskiy, E.V.; Zhilina, E.F.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Synthesis of Heteroannulated Indolopyrazines through Domino N−H Palladium-Catalyzed/Metal-Free Oxidative C−H Bond Activation. ACS Omega 2020, 5, 15681–15690. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of absorption anf fluorescence spectrof >300 common compounds for use in photochem CAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, G.; Barsella, A.; Boeglin, A.; Niu, S.; Ziessel, R. BODIPY-bridged push-pull chromophores for nonlinear optical applications. ChemPhysChem 2014, 15, 2693–2700. [Google Scholar] [CrossRef] [PubMed]













| Compounds | Epa [V] a | E1/2red [V] a | ΔE [V] b | EHOMO [eV] c | ELUMO [eV] c | λmax [nm] d |
|---|---|---|---|---|---|---|
| 9a | 0.79 | −1.24 | 2.04 | −5.59 | −3.56 | 608 |
| 9b | 1.08 | −1.20 | 2.28 | −5.88 | −3.60 | 544 |
| 10a | 0.72 | −1.50 | 2.22 | −5.52 | −3.30 | 569 |
| 10b | 0.87 | −1.55 | 2.42 | −5.67 | −3.25 | 513 |
| 11a | 0.74 | −2.44 | 3.17 | −5.54 | −2.36 | 391 |
| 11b | 1.10 | −2.36 e | 3.46 | −5.90 | −2.44 | 359 |
| Compounds | DCM Solution | KBr Matrix | ||
|---|---|---|---|---|
| λabs [nm] (ε [mM−1 cm−1]) | λem [nm] (Φ a) | Stoke Shift (cm−1) | λem [nm] | |
| 9a | 327 (23.0), 508 (3.3) | - a | - a | 755 |
| 9b | 324 (13.4), 422 (2.3) | - a | - a | 636 |
| 10a | 335 (23.2), 479 (3.1) | - a | - a | 662 |
| 10b | 340 (16.8), 411 (3.2) | - a | - a | 588 |
| 11a | 311 (25.0), 374 (6.3) | 512 (0.03) | 7206 | 478 |
| 11b | 326 (11.6), 340 (10.9) | 584 (0.01) | 12,288 | 464 |
| Compounds | λem [nm] a | |||||
|---|---|---|---|---|---|---|
| n-Heptane ET(30) = 30.9 a | Toluene ET(30) = 33.9 a | 1,4-Dioxane ET(30) = 36.0 a | THF ET(30) = 37.4 a | CHCl3 ET(30) = 39.1 a | DCM ET(30) = 40.7 a | |
| 11a | 456 | 487 | 521 | 534 | 573 | 584 |
| 11b | 422 | 431 | 452 | 484 | 495 | 512 |
| 9a | 9b | 10a | 10b | 11a | 11b | |
|---|---|---|---|---|---|---|
| µβ (10−48 esu) | 80 | 20 | 80 | 50 | 40 | 0 |
| Compounds | EHOMODFT [eV] | ELUMODFT [eV] | ΔEDFT [eV] | μ [D] | λmaxTD-DFT [nm/eV] | β(–2ω,ω,ω)DFT [10−30 esu] |
|---|---|---|---|---|---|---|
| 9a | –5.49 | –3.45 | 2.04 | 8.11 | 447/2.77 | 504 |
| 9b | –5.84 | –3.55 | 2.29 | 7.86 | 377/3.29 | 117 |
| 10a | –5.43 | –3.26 | 2.17 | 4.24 | 420/2.95 | 311 |
| 10b | –5.81 | –3.34 | 2.47 | 4.30 | 357/3.47 | 74 |
| 11a | –5.36 | –2.33 | 3.03 | 0.87 | 335/3.70 | 53 |
| 11b | –5.77 | –2.40 | 3.37 | 2.37 | 301/4.12 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbitskiy, E.V.; le Poul, P.; Bureš, F.; Achelle, S.; Barsella, A.; Kvashnin, Y.A.; Rusinov, G.L.; Charushin, V.N. Push–Pull Derivatives Based on 2,4′-Biphenylene Linker with Quinoxaline, [1,2,5]Oxadiazolo[3,4-B]Pyrazine and [1,2,5]Thiadiazolo[3,4-B]Pyrazine Electron Withdrawing Parts. Molecules 2022, 27, 4250. https://doi.org/10.3390/molecules27134250
Verbitskiy EV, le Poul P, Bureš F, Achelle S, Barsella A, Kvashnin YA, Rusinov GL, Charushin VN. Push–Pull Derivatives Based on 2,4′-Biphenylene Linker with Quinoxaline, [1,2,5]Oxadiazolo[3,4-B]Pyrazine and [1,2,5]Thiadiazolo[3,4-B]Pyrazine Electron Withdrawing Parts. Molecules. 2022; 27(13):4250. https://doi.org/10.3390/molecules27134250
Chicago/Turabian StyleVerbitskiy, Egor V., Pascal le Poul, Filip Bureš, Sylvain Achelle, Alberto Barsella, Yuriy A. Kvashnin, Gennady L. Rusinov, and Valery N. Charushin. 2022. "Push–Pull Derivatives Based on 2,4′-Biphenylene Linker with Quinoxaline, [1,2,5]Oxadiazolo[3,4-B]Pyrazine and [1,2,5]Thiadiazolo[3,4-B]Pyrazine Electron Withdrawing Parts" Molecules 27, no. 13: 4250. https://doi.org/10.3390/molecules27134250