Deproteinization of Chitin Extracted with the Help of Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of IL-Extracted Chitin (IL-Chitin) and Determination of the Amount of Proteins
2.2. Treatment of IL-Extracted Chitin (IL-Chitin) with Aqueous K3PO4
2.2.1. Washing IL-Extracted Chitin with K3PO4aq in a Dry State
2.2.2. Washing IL-Extracted Chitin with K3PO4aq in a Swollen State
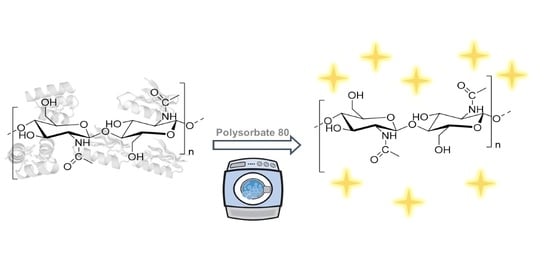
2.3. Treatment of IL-Extracted Chitin (IL-Chitin) with Aqueous Tween® 80
2.3.1. Washing IL-Extracted Chitin with Aqueous Tween® 80 in Dry State
2.3.2. Washing IL-Extracted Chitin with Aqueous Polysorbate-80 (Tween® 80) in Swollen State
3. Materials and Methods
3.1. Materials for Chitin Extraction and Coagulation
3.2. Materials for Biochemical Studies
3.3. Laboratory Processing of Dry Shrimp Shells
3.4. Extraction of Chitin Using Microwave Irradiation
3.5. Washing Chitin Using K3PO4aq
3.5.1. Washing Dry Chitin
3.5.2. Washing Swollen Chitin
3.5.3. Coagulating IL-Chitin Solution in K3PO4aq
3.6. Washing Chitin Using Tween® 80
3.6.1. Washing Dry Chitin
3.6.2. Washing Swollen Chitin with Tween® 80
3.6.3. Coagulating IL-Chitin Solution in Tween® 80
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
3.8. Powder X-ray Diffraction (pXRD)
3.9. Spectrophotometer/Optical Density Readings
3.10. Tropomyosin Quantification
3.11. Quantification of Total Protein Content Using APA
3.12. Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Foster, A.B.; Webber, J.M. Chitin. In Advances in Carbohydrate Chemistry; Melville, L., Wolfrom, R., Tipson, S., Eds.; Academic Press: Oxford, UK, 1961; pp. 371–393. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Alagui, A.; Vottero, P. Investigation of different natural sources of chitin: Influence of the source and deacetylation process on the physicochemical characteristics of chitosan. Polym. Int. 2000, 49, 337–344. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Chitin as a resource for eco-friendly bioplastics. In Green Biomass Conversion Using Ionic Liquids; Itoh, T., Ed.; Series of Encyclopedia of Ionic Liquids; Springer/Nature: New York, NY, USA, 2019; in press. [Google Scholar]
- Costantino, P.D.; Friedman, C.D.; Lane, A. Synthetic biomaterials in facial plastic and reconstructive surgery. Facial Plast. Surg. 1993, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.; Zavgorodyna, O.; Rogers, R. Advances in processing chitin as a promising biomaterial from ionic liquids. Adv. Biochem. 2018, 168, 177–198. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968–971. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Advances in processing biomaterials from ionic liquids. In Advances in Biochemical Engineering/Biotechnology Series; Koo, Y.-M., Itoh, T., Eds.; Springer International Publishing AG: New York, NY, USA, 2018. [Google Scholar]
- Shamshina, J.L. Chitin in ionic liquids: Historical insights on the polymer’s dissolution and isolation. A review. Green Chem. 2019, 21, 3974–3993. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in functional chitin materials: A review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Khor, E. (Ed.) Chapter 7—Chitin and Chitosan: Making the Grade, In Chitin: Fulfilling a Biomaterials Promise; Elsevier: Oxford, UK, 2001. [Google Scholar] [CrossRef]
- Unites States Pharmacopeia, Biological Reactivity Tests, In Vivo. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c88.html (accessed on 30 August 2019).
- Rahman, A.; Helleur, R.; Jeebhay, M.; Lopata, A. Characterization of seafood proteins causing allergic diseases. In Allergic Diseases—Highlights in the Clinic, Mechanisms and Treatment; Celso, P., Ed.; Intech: Rijecka, Croatia, 2012. [Google Scholar]
- Sicherer, S.H.; Munoz-Furlong, A.; Sampson, H.A. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 2004, 114, 159–165. [Google Scholar] [CrossRef]
- Palm, T.; Greenfield, N.; Hitchcock-DeGregori, S. Tropomyosin ends determine the stability and functionality of overlap and Troponin T complexes. Biophys. J. 2003, 84, 3181–3189. [Google Scholar] [CrossRef] [Green Version]
- King, C.; Stein, R.S.; Shamshina, J.L.; Rogers, R.D. MultiCP 13C NMR as a clean, quantitative method for measuring the purity of chitin. ACS Sustain. Chem. Eng. 2017, 5, 8011–8016. [Google Scholar] [CrossRef]
- Black, M.; Schwartz, H.M. The estimation of chitin and chitin nitrogen in crawfish waste and derived products. Analyst 1950, 75, 185–189. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef]
- Rasweefali, M.K.; Sabu, S.; Muhammed Azad, K.S.; Raseel Rahman, M.K.; Sunooj, K.V.; Sasidharan, A.; Anoop, K.K. Influence of deproteinization and demineralization process sequences on the physicochemical and structural characteristics of chitin isolated from Deep-sea mud shrimp (Solenocera hextii). Adv. Biomarker Sci. Tech. 2022, 4, 12–27. [Google Scholar] [CrossRef]
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Tech. Appl. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Valdez-Peña, A.U.; Espinoza-Perez, J.D.; Sandoval-Fabian, G.C.; Balagurusamy, N.; Hernandez-Rivera, A.; De-la-Garza-Rodriguez, I.M.; Contreras-Esquivel, J.C. Screening of industrial enzymes for deproteinization of shrimp head for chitin recovery. Food Sci. Biotechnol. 2010, 19, 553–557. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Duan, S.; Li, L.; Zhuang, Z.; Wu, W.; Hong, S.; Zhou, J. Improved production of chitin from shrimp waste by fermentation with epiphytic lactic acid bacteria. Carbohydr. Polym. 2012, 89, 1283–1288. [Google Scholar] [CrossRef]
- Kageyama, M.; Namiki, H.; Fukushima, H.; Ito, Y.; Shibata, N.; Takada, K. In vivo effects of Cyclosporin A and Ketoconazole on the pharmacokinetics of representative substrates for P-Glycoprotein and Cytochrome P450 (CYP) 3A in rats. Biol. Pharm. Bull. 2005, 28, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavgorodnya, O.; Shamshina, J.L.; Rogers, R.D. Coagulation of Chitin from Ionic Liquid Solutions Using Kosmotropic Salts. US Patent Application 62/443,019, 23 February 2021. [Google Scholar]
- The United States Pharmacopeial Convention. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/excipients/polysorbate_80.pdf (accessed on 30 August 2019).
- Polysorbate 80. The European Pharmacopoeia. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/usp-nf-notices/pf35-4-polysorbate-80.pdf (accessed on 30 August 2019).
- Polysorbate 80. The European Pharmacopoeia. Available online: https://www.pharmacopoeia.com/ban-2012/ban-2017.pdf (accessed on 30 August 2019).
- Polysorbate 80. The European Pharmacopoeia. Available online: http://www.newdruginfo.com/pharmacopeia/ep50/Polysorbate%2080.pdf (accessed on 18 June 2022).
- Shah, R.; Kolanos, R.; DiNovi, M.J.; Mattia, A.; Kaneko, K.J. Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety. Food Addit. Contam. Part A 2017, 34, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Gurau, G.; Block, L.E.; Hansen, L.K.; Dingee, C.; Walters, A.; Rogers, R.D. Chitincalcium alginate composite fibers for wound care dressings spun from ionic liquid solution. J. Mater. Chem. B 2014, 2, 3924–3936. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13 C cross polarization/mass angle spinning NMR. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nge, T.T.; Takemura, A.; Hori, N.; Ono, H. Characterization of Uniaxially Aligned Chitin Film by 2D FT-IR Spectroscopy. Biomacromolecules 2005, 6, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Darmon, S.; Rudall, K. Infra-red and X-ray studies of chitin. Disc. Faraday Soc. 1950, 9, 251–260. [Google Scholar] [CrossRef]
- Shanti, K.N.; Martin, B.M.; Nagpal, S.; Metcalfe, D.D.; Subba Rao, P.V. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J. Immunol. 1993, 151, 5354–5363. [Google Scholar]
- Akpan, I.; Gbenebor, O.P.; Adeosun, S.O. Synthesis and characterisation of chitin from periwinkle (Tympanotonus fusatus (L.)) and snail (Lissachatina fulica (Bowdich)) shells. Int. J. Biol. Macromol. 2018, 106, 1080–1088. [Google Scholar] [CrossRef]
- Dooleweerdt, K.; Birkedal, H.; Ruhland, T.; Skrydstrup, T. Irregularities in the Effect of Potassium Phosphate in Ynamide Synthesis. J. Org. Chem. 2008, 73, 9447–9450. [Google Scholar] [CrossRef]
- Sciavolino, F.; Mathias, G. Mineral Amino-Acid Complexes of Active Agents. WO 2015/195491 Al, 6 December 2015. [Google Scholar]
- Nesvizhskii, A.; Keller, A.; Kolker, E.; Ruedi, A. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]



| Type of Chitin | Treatment | Protein Content Determined through APA, µg/g (%) |
|---|---|---|
| IL-chitin | 1. Extraction with [C2mim][OAc]; 2. coagulation in DI water; 3. washing with DI water; 4. drying | 15,882 ± 3422 (1.59%) |
| PG-chitin | Commercial, no additional chemical treatment employed | <1 |
| IL-chitindry/K3PO4 | 1. Extraction with [C2mim][OAc]; 2. coagulation in DI water; 3. washing with DI water; 4. drying; 5. washing dry chitin with 40 wt% K3PO4aq; 6. washing with DI water; 7. drying | 6990 ± 453 (0.70%) |
| IL-chitinswollen/K3PO4 | 1. Extraction with [C2mim][OAc]; 2. coagulation in DI water; 3. washing with DI water; 4. washing (x2) swollen gel chitin with 25 wt% K3PO4aq; 5. washing with DI water; 6. drying | 1398 ± 256 (0.14%) |
| Type of Chitin | Treatment | Protein Content Determined through APA, µg/g (%) |
|---|---|---|
| IL-chitin | 1.Extraction with [C2mim][OAc]; 2. coagulation in DI water; 3. washing with DI water; 4. drying | 15,882 ± 3422 (1.59%) |
| IL-chitinswollen/Polysorbate 80 | 1.Extraction with [C2mim][OAc]; 2. coagulation in water; 3. washing with Polysorbate 80; 4. washing with DI water, 5. drying | 1110 ± 256 (0.11%) |
| IL-chitincoagulated/Polysorbate 80 | 1.Extraction with [C2mim][OAc]; 2. coagulation in Polysorbate 80; 3. washing with DI water, 4. drying | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyon, D.R., Jr.; Smith, B.R.; Abidi, N.; Shamshina, J.L. Deproteinization of Chitin Extracted with the Help of Ionic Liquids. Molecules 2022, 27, 3983. https://doi.org/10.3390/molecules27133983
Lyon DR Jr., Smith BR, Abidi N, Shamshina JL. Deproteinization of Chitin Extracted with the Help of Ionic Liquids. Molecules. 2022; 27(13):3983. https://doi.org/10.3390/molecules27133983
Chicago/Turabian StyleLyon, Douglas R., Jr., Bryan R. Smith, Noureddine Abidi, and Julia L. Shamshina. 2022. "Deproteinization of Chitin Extracted with the Help of Ionic Liquids" Molecules 27, no. 13: 3983. https://doi.org/10.3390/molecules27133983