HCnH− Anion Chains with n ≤ 8 Are Nonlinear and Their Permanent Dipole Makes Them Potential Candidates for Astronomical Observation
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Enthalpies of Formation: HCnH versus HCnN
3.2. Stable HCnH− Anion Chains with Astrochemically Interesting Sizes Are Nonlinear
3.3. Relevant Properties of Cis and Trans Anions
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

| n | HCn+1H | HCnN |
|---|---|---|
| 0 | 128.668 | |
| 1 | 479.383 | 389.195 |
| 2 | 370.720 | 228.807 |
| 3 | 659.255 | 544.779 |
| 4 | 597.481 | 457.818 |
| 5 | 863.361 | 729.829 |
| 6 | 822.640 | 679.914 |
| 7 | 1080.580 | 938.613 |
| 8 | 1043.330 | 905.162 |
| 9 | 1298.220 | 1153.130 |
| 10 | 1266.560 | 1124.610 |
| 11 | 1525.320 | 1375.680 |
| 12 | 1347.420 |
Appendix B
| HC3H− | cis | Trans | ||||
|---|---|---|---|---|---|---|
| 0.7692 | 0.7692 | |||||
| 0.7502 | 0.7502 | |||||
| Atom | X | Y | Z | X | Y | Z |
| H | 0.000000 | 0.566638 | −2.029360 | 0.000000 | −0.431664 | −2.096631 |
| C | 0.000000 | −0.226564 | −1.293825 | 0.000000 | 0.267660 | −1.271822 |
| C | 0.000000 | −0.080148 | −0.000000 | 0.000000 | −0.000000 | 0.000000 |
| C | 0.000000 | −0.226564 | 1.293825 | 0.000000 | −0.267660 | 1.271822 |
| H | 0.000000 | 0.566638 | 2.029360 | 0.000000 | 0.431664 | 2.096631 |
| HC5H− | cis | Trans | ||||
|---|---|---|---|---|---|---|
| 0.7791 | 0.7794 | |||||
| 0.7505 | 0.7505 | |||||
| Atom | X | Y | Z | X | Y | Z |
| H | 0.000000 | 0.325138 | −3.463683 | 0.000000 | −0.389640 | −3.424168 |
| C | 0.000000 | −0.217856 | −2.538309 | 0.000000 | 0.241108 | −2.553977 |
| C | 0.000000 | 0.034756 | −1.295056 | 0.000000 | 0.047397 | −1.297268 |
| C | 0.000000 | 0.154378 | 0.000000 | 0.000000 | −0.000000 | −0.000000 |
| C | 0.000000 | 0.034756 | 1.295056 | 0.000000 | −0.047397 | 1.297268 |
| C | 0.000000 | −0.217856 | 2.538309 | 0.000000 | −0.241108 | 2.553977 |
| H | 0.000000 | 0.325138 | 3.463683 | 0.000000 | 0.389640 | 3.424168 |
| HC6H− | cis | Trans | ||||
|---|---|---|---|---|---|---|
| 0.7660 | 0.7660 | |||||
| 0.7502 | 0.7501 | |||||
| Atom | X | Y | Z | X | Y | Z |
| H | 0.000000 | 0.509362 | −4.068471 | 0.000000 | −0.429635 | −4.080898 |
| C | 0.000000 | −0.122855 | −3.197356 | 0.000000 | 0.179751 | −3.193660 |
| C | 0.000000 | 0.007350 | −1.948075 | 0.000000 | 0.017416 | −1.948151 |
| C | 0.000000 | −0.019817 | −0.627139 | 0.000000 | 0.011855 | −0.626980 |
| C | 0.000000 | −0.019817 | 0.627139 | 0.000000 | −0.011855 | 0.626980 |
| C | 0.000000 | 0.007350 | 1.948075 | 0.000000 | −0.017416 | 1.948151 |
| C | 0.000000 | −0.122855 | 3.197356 | 0.000000 | −0.179751 | 3.193660 |
| H | 0.000000 | 0.509362 | 4.068471 | 0.000000 | 0.429635 | 4.080898 |
| HC7H− | cis | Trans | ||||
|---|---|---|---|---|---|---|
| 0.7870 | 0.7871 | |||||
| 0.7509 | 0.7509 | |||||
| Atom | X | Y | Z | X | Y | Z |
| H | 0.431660 | 0.000000 | −0.921860 | 0.000000 | −0.330843 | −4.787382 |
| C | −0.017204 | 0.000000 | 0.047244 | 0.000000 | 0.144397 | −3.832244 |
| C | 0.160726 | 0.000000 | 1.283517 | 0.000000 | 0.020735 | −2.589456 |
| C | 0.226367 | 0.000000 | 2.596912 | 0.000000 | 0.016697 | −1.275568 |
| C | 0.291294 | 0.000000 | 3.870346 | 0.000000 | −0.000000 | −0.000000 |
| C | 0.367671 | 0.000000 | 5.146221 | 0.000000 | −0.016697 | 1.275568 |
| C | 0.445956 | 0.000000 | 6.455698 | 0.000000 | −0.020735 | 2.589456 |
| C | 0.395178 | 0.000000 | 7.708239 | 0.000000 | −0.144397 | 3.832244 |
| H | 0.997605 | 0.000000 | 8.593355 | 0.000000 | 0.330843 | 4.787382 |
| HC8H− | cis | trans | ||||
|---|---|---|---|---|---|---|
| 0.7695 | 0.7695 | |||||
| 0.7502 | 0.7502 | |||||
| Atom | X | Y | Z | X | Y | Z |
| H | 0.000000 | 0.395448 | −5.421548 | −0.570041 | 0.000000 | −0.903775 |
| C | 0.000000 | −0.095135 | −4.472275 | 0.000000 | 0.000000 | 0.000000 |
| C | 0.000000 | 0.012485 | −3.233941 | 0.000000 | 0.000000 | 1.242922 |
| C | 0.000000 | −0.007051 | −1.908770 | 0.134072 | 0.000000 | 2.561466 |
| C | 0.000000 | −0.010887 | −0.656694 | 0.247551 | 0.000000 | 3.808382 |
| C | 0.000000 | −0.010887 | 0.656694 | 0.364166 | 0.000000 | 5.116584 |
| C | 0.000000 | −0.007051 | 1.908770 | 0.477517 | 0.000000 | 6.363543 |
| C | 0.000000 | 0.012485 | 3.233941 | 0.611671 | 0.000000 | 7.681980 |
| C | 0.000000 | −0.095135 | 4.472275 | 0.611324 | 0.000000 | 8.925066 |
| H | 0.000000 | 0.395448 | 5.421548 | 1.183595 | 0.000000 | 9.827551 |
Appendix C

References
- Herbst, E.; van Dishoeck, E.F. Complex Organic Interstellar Molecules. Annu. Rev. Astron. Astrophys. 2009, 47, 427–480. [Google Scholar] [CrossRef]
- McGuire, B.A. 2018 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. Astrophys. J. Suppl. Ser. 2018, 239, 17. [Google Scholar] [CrossRef]
- Snyder, L.E.; Buhl, D. Observations of Radio Emission from Interstellar Hydrogen Cyanide. Astrophys. J. Lett. 1971, 163, L47. [Google Scholar] [CrossRef]
- Guélin, M.; Cernicharo, J. Astronomical Detection of the HCCN Radical—Toward a New Family of Carbon-Chain Molecules? Astron. Astrophys. 1991, 244, L21–L24. [Google Scholar]
- Turner, B.E. Detection of Interstellar Cyanoacetylene. Astrophys. J. Lett. 1971, 163, L35. [Google Scholar] [CrossRef]
- Cernicharo, J.; Guélin, M.; Pardo, J.R. Detection of the Linear Radical HC4N in IRC +10216. Astrophys. J. Lett. 2004, 615, L145. [Google Scholar] [CrossRef] [Green Version]
- Avery, L.W.; Broten, N.W.; MacLeod, J.M.; Oka, T.; Kroto, H.W. Detection of the Heavy Interstellar Molecule Cyanodiacetylene. Astrophys. J. Lett. 1976, 205, L173–L175. [Google Scholar] [CrossRef]
- Kroto, H.W.; Kirby, C.; Walton, D.R.M.; Avery, L.W.; Broten, N.W.; MacLeod, J.M.; Oka, T. The Detection of Cyanohexatriyne, H(C≡ C)3CN, in Heile’s Cloud 2. Astrophys. J. Lett. 1978, 219, L133–L137. [Google Scholar] [CrossRef]
- Broten, N.W.; Oka, T.; Avery, L.W.; MacLeod, J.M.; Kroto, H.W. The Detection of HC9N in Interstellar Space. Astrophys. J. Lett. 1978, 223, L105–L107. [Google Scholar] [CrossRef]
- Fan, Q.; Pfeiffer, G.V. Theoretical Study of Linear Cn (n = 6–10) and HCnH (n = 2–10) Molecules. Chem. Phys. Lett. 1989, 162, 472–478. [Google Scholar] [CrossRef]
- Fulara, J.; Freivogel, P.; Forney, D.; Maier, J.P. Electronic Absorption Spectra of Linear Carbon Chains in Neon Matrices. III. HC2n+1H. J. Chem. Phys. 1995, 103, 8805–8810. [Google Scholar] [CrossRef]
- Seburg, R.A.; DePinto, J.T.; Patterson, E.V.; McMahon, R.J. Structure of Triplet Propynylidene. J. Am. Chem. Soc. 1995, 117, 835–836. [Google Scholar] [CrossRef]
- Maier, J.P. Electronic Spectroscopy of Carbon Chains. J. Phys. Chem. A 1998, 102, 3462–3469. [Google Scholar] [CrossRef]
- Pino, T.; Ding, H.; Güthe, F.; Maier, J.P. Electronic Spectra of the Chains HC2nH (n = 8–13) in the Gas Phase. J. Chem. Phys. 2001, 114, 2208–2212. [Google Scholar] [CrossRef]
- Ding, H.; Schmidt, T.W.; Pino, T.; Boguslavskiy, A.E.; Güthe, F.; Maier, J.P. Gas Phase Electronic Spectra of the Linear Carbon Chains HC2n+1H (n = 3–6,9). J. Chem. Phys. 2003, 119, 814–819. [Google Scholar] [CrossRef] [Green Version]
- Woods, P.M.; Millar, T.J.; Zijlstra, A.A.; Herbst, E. The Chemistry of CRL 618. In Planetary Nebulae: Their Evolution and Role in the Universe, Proceedings of the 209th Symposium of the International Astronomical Union, Canberra, Australia, 19–23 November 2001; Kwok, S., Dopita, M., Sutherland, R., Eds.; Astronomical Society of the Pacific: San Francisco, CA, USA, 2003; Volume 209, p. 279. [Google Scholar]
- Ridgway, S.T.; Hall, D.N.B.; Kleinmann, S.G.; Weinberger, D.A.; Wojslaw, R.S. Circumstellar acetylene in the infrared spectrum of IRC+10 216. Nature 1976, 264, 345–346. [Google Scholar] [CrossRef]
- Lacy, J.H.; Evans, N.J.; Achtermann, J.M.; Bruce, D.E.; Arens, J.F.; Carr, J.S. Discovery of Interstellar Acetylene. Astrophys. J. Lett. 1989, 342, L43. [Google Scholar] [CrossRef]
- Cernicharo, J.; Heras, A.M.; Tielens, A.G.G.M.; Pardo, J.R.; Herpin, F.; Guélin, M.; Waters, L.B.F.M. Infrared Space Observatory’s Discovery of C4H2, C6H2, and Benzene in CRL 618. Astrophys. J. Lett. 2001, 546, L123–L126. [Google Scholar] [CrossRef] [Green Version]
- Bâldea, I. Alternation of Singlet and Triplet States in Carbon-Based Chain Molecules and Its Astrochemical Implications: Results of an Extensive Theoretical Study. Adv. Theor. Simul. 2019, 2, 1900084. [Google Scholar] [CrossRef]
- Kaiser, R.I.; Sun, B.J.; Lin, H.M.; Chang, A.H.H.; Mebel, A.M.; Kostko, O.; Ahmed, M. An Experimental and Theoretical Study on the Ionization Energies of Polyynes H–(C≡C)n–H; n = 1–9). Astrophys. J. 2010, 719, 1884. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.S.; Lau, K.C. Thermochemical Trends in Carbon Chain Molecules HC2kH/HC2k-1H (k = 1–6) Studied by Explicitly Correlated CCSD(T)-F12b Composite Methods. J. Phys. Chem. A 2021, 125, 5385–5396. [Google Scholar] [CrossRef] [PubMed]
- Carelli, F.; Satta, M.; Grassi, T.; Gianturco, F.A. Carbon-Rich Molecular Chains in Protoplanetary and Planetary Atmospheres: Quantum Mechanisms and Electron Attachment Rates for Anion Formation. Astrophys. J. 2013, 774, 97. [Google Scholar] [CrossRef] [Green Version]
- Horny, L.; Petraco, N.D.K.; Pak, C.; Schaefer, H.F. What Is the Nature of Polyacetylene Neutral and Anionic Chains HC2nH and HC2nH− (n = 6–12) That Have Recently Been Observed? J. Am. Chem. Soc. 2002, 124, 5861–5864. [Google Scholar] [CrossRef] [PubMed]
- Horny, L.; Petraco, N.D.K.; Schaefer, H.F. Odd Carbon Long Linear Chains HC2n+1H (n = 4–11): Properties of the Neutrals and Radical Anions. J. Am. Chem. Soc. 2002, 124, 14716–14720. [Google Scholar] [CrossRef] [PubMed]
- Bâldea, I. Long Carbon-Based Chains of Interstellar Medium Can Have a Triplet Ground State. Why Is This Important for Astrochemistry? ACS Earth Space Chem. 2019, 3, 863–872. [Google Scholar] [CrossRef]
- Bâldea, I. Profiling C4N Radicals of Astrophysical Interest. Mon. Not. R. Astron. Soc. 2020, 493, 2506–2510. [Google Scholar] [CrossRef]
- Bâldea, I. Extensive Quantum Chemistry Study of Neutral and Charged C4N Chains: An Attempt to Aid Astronomical Observations. ACS Earth Space Chem. 2020, 4, 434–448. [Google Scholar] [CrossRef]
- Bâldea, I. Profiling Astrophysically Relevant MgC4H Chains. An Attempt to Aid Astronomical Observations. Mon. Not. R. Astron. Soc. 2020, 498, 4316–4326. [Google Scholar] [CrossRef]
- Bâldea, I. Alternation of Singlet and Triplet States in Carbon-Based Chain Molecules and Its Astrochemical Implications: Results of an Extensive Theoretical Study. Adv. Theor. Simul. 2022, 5, 2100480. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Gottlieb, C.A.; Gupta, H.; Thaddeus, P. Laboratory and Astronomical Identification of the Negative Molecular Ion C6H−. Astrophys. J. Lett. 2006, 652, L141. [Google Scholar] [CrossRef]
- Vogelhuber, K.M.; Wren, S.W.; Shaffer, C.J.; McMahon, R.J.; McCoy, A.B.; Lineberger, W.C. Photoelectron Spectroscopy of HC4N−. J. Chem. Phys. 2011, 135, 204307. [Google Scholar] [CrossRef] [PubMed]
- Millar, T.J.; Walsh, C.; Field, T.A. Negative Ions in Space. Chem. Rev. 2017, 117, 1765–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- BwForCluster JUSTUS 2. Available online: https://wiki.bwhpc.de/e/Category:BwForClusterJUSTUS2 (accessed on 12 April 2022).
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 Theory. J. Chem. Phys. 2007, 126, 084108. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 Theory Using Reduced Order Perturbation Theory. J. Chem. Phys. 2007, 127, 124105. [Google Scholar] [CrossRef]
- Ochterski, J.W. Thermochemistry in Gaussian; Gaussian Inc.: Pittsburg, PA, USA, 2000; Available online: https://gaussian.com/wp-content/uploads/dl/thermo.pdf (accessed on 12 April 2022).
- Bartmess, J.E. Thermodynamics of the Electron and the Proton. J. Phys. Chem. 1994, 98, 6420–6424. [Google Scholar] [CrossRef]
- Kelly, C.P.; Cramer, C.J.; Truhlar, D.G. Single-Ion Solvation Free Energies and the Normal Hydrogen Electrode Potential in Methanol, Acetonitrile, and Dimethyl Sulfoxide. J. Phys. Chem. B 2007, 111, 408–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bâldea, I. Extending the Newns-Anderson Model to Allow Nanotransport Studies Through Molecules with Floppy Degrees of Freedom. Europhys. Lett. 2012, 99, 47002. [Google Scholar] [CrossRef] [Green Version]
- Bâldea, I.; Köppel, H.; Wenzel, W. (4,4’)-Bipyridine in Vacuo and in Solvents: A Quantum Chemical Study of a Prototypical Floppy Molecule From a Molecular Transport Perspective. Phys. Chem. Chem. Phys. 2013, 15, 1918–1928. [Google Scholar] [CrossRef] [Green Version]
- Bâldea, I. A Quantum Chemical Study from a Molecular Transport Perspective: Ionization and Electron Attachment Energies for Species Often Used to Fabricate Single-Molecule Junctions. Faraday Discuss. 2014, 174, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Bâldea, I.; Frisbie, C.D. Why One Can Expect Large Rectification in Molecular Junctions Based on Alkane Monothiols and Why Rectification Is So Modest. Chem. Sci. 2018, 9, 4456–4467. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Bâldea, I.; Haugstad, G.; Frisbie, C.D. Mechanical Deformation Distinguishes Tunneling Pathways in Molecular Junctions. J. Am. Chem. Soc. 2019, 141, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bâldea, I.; Frisbie, C.D. Energy Level Alignment in Molecular Tunnel Junctions by Transport and Spectroscopy: Self-Consistency for the Case of Alkyl Thiols and Dithiols on Ag, Au, and Pt Electrodes. J. Am. Chem. Soc. 2019, 141, 18182–18192. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.D.; Gauss, J.; Bartlett, R.J. Coupled-Cluster Methods with Noniterative Triple Excitations for Restricted Open-Shell Hartree-Fock and Other General Single Determinant Reference Functions. Energies and Analytical Gradients. J. Chem. Phys. 1993, 98, 8718–8733. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A New Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, J.F.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A Complete Basis Set Model Chemistry. I. The Total Energies of Closed-Shell Atoms and Hydrides of the First-Row Elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A Complete Basis Set Model Chemistry. II. Open-Shell Systems and the Total Energies of the First-Row Atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Dua, S.; Bowie, J.H.; Schröder, D.; Schwarz, H. Gas-Phase Syntheses of Three Isomeric C5H2 Radical Anions and Their Elusive Neutrals. A Joint Experimental and Theoretical Study. J. Phys. Chem. A 1998, 102, 9949–9956. [Google Scholar] [CrossRef]
- Dua, S.; Blanksby, S.J.; Bowie, J.H. Formation of Neutral C7H2 Isomers from Four Isomeric C7H2 Radical Anion Precursors in the Gas Phase. J. Phys. Chem. A 2000, 104, 77–85. [Google Scholar] [CrossRef]
- Arié, E.; Johns, J. The bending energy levels of C4H2. J. Mol. Spectrosc. 1992, 155, 195–204. [Google Scholar] [CrossRef]
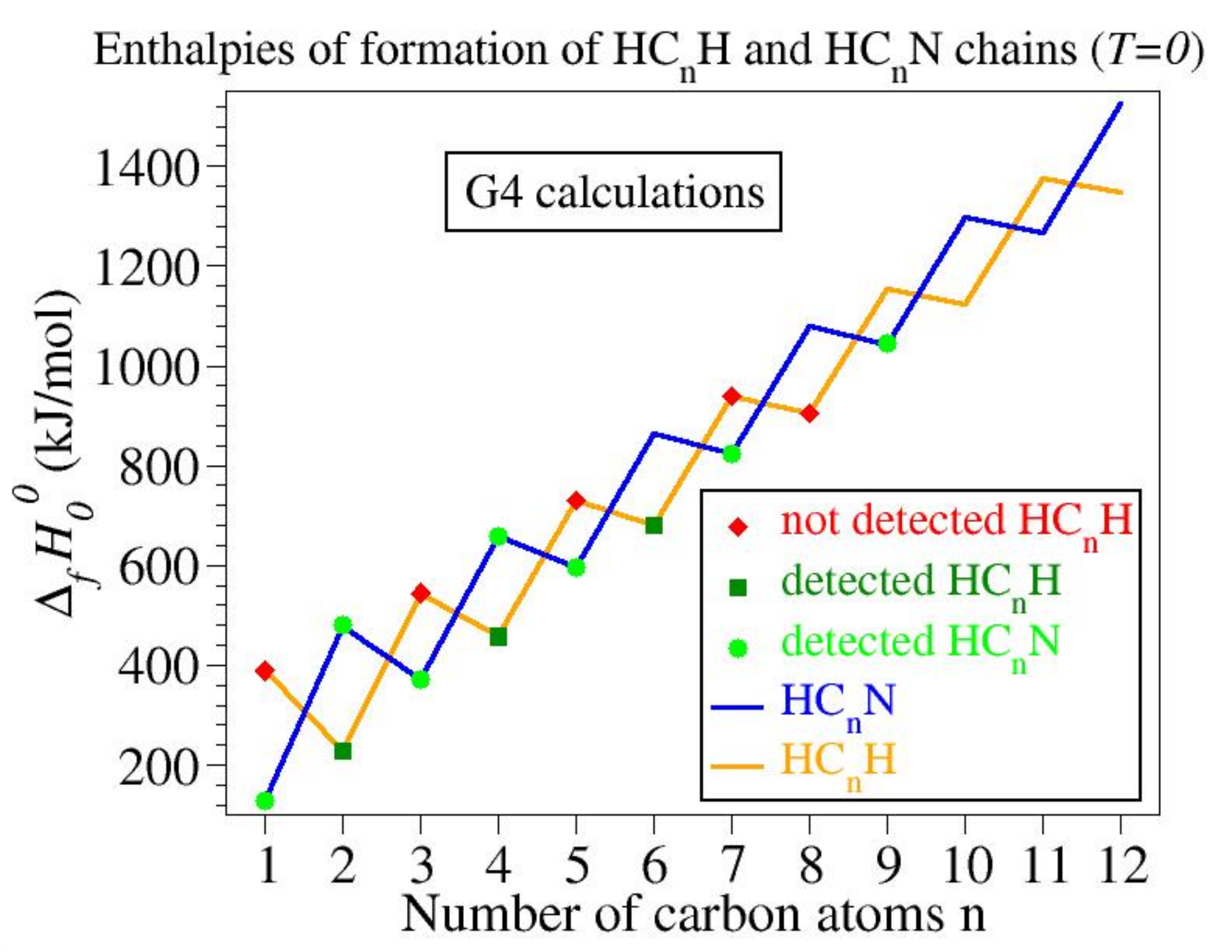



| Number of Carbon Atoms | HCnH | HCnN |
|---|---|---|
| 1 | 128.668 | 389.195 |
| 2 | 479.383 | 228.807 |
| 3 | 370.720 | 544.779 |
| 4 | 659.255 | 457.818 |
| 5 | 597.481 | 729.829 |
| 6 | 863.361 | 679.914 |
| 7 | 822.640 | 938.613 |
| 8 | 1080.580 | 905.162 |
| 9 | 1043.330 | 1153.130 |
| 10 | 1298.220 | 1124.610 |
| 11 | 1266.560 | 1375.680 |
| 12 | 1525.320 | 1347.420 |
| Molecule | G4 | ROCCSD(T) |
|---|---|---|
| HC3H | 1.185 | 1.047 |
| HC4H | −0.355 | −0.736 |
| HC5H 1 | 1.531 | 1.420 |
| HC6H | 0.298 | 0.195 |
| HC7H 2 | 1.935 | 2.029 |
| HC8H | 0.805 | 0.667 |
| Anion | kcal/mol | meV |
|---|---|---|
| HC3H− | 0.505 | 21.9 |
| HC4H− | 0.178 | 7.7 |
| HC5H− | −0.260 | −11.3 |
| HC6H− | 0.188 | 8.2 |
| HC7H− | 0.494 | 21.4 |
| HC8H− | −0.217 | −9.4 |
| HC9H− | −0.668 | 29.0 |
| Cis Anion | Dipole Moment (Debye) |
|---|---|
| HC3H− | 3.061 |
| HC4H− | 2.385 |
| HC5H− | 2.313 |
| HC6H− | 2.310 |
| HC7H− | 1.801 1 |
| HC8H− | 1.752 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bâldea, I. HCnH− Anion Chains with n ≤ 8 Are Nonlinear and Their Permanent Dipole Makes Them Potential Candidates for Astronomical Observation. Molecules 2022, 27, 3100. https://doi.org/10.3390/molecules27103100
Bâldea I. HCnH− Anion Chains with n ≤ 8 Are Nonlinear and Their Permanent Dipole Makes Them Potential Candidates for Astronomical Observation. Molecules. 2022; 27(10):3100. https://doi.org/10.3390/molecules27103100
Chicago/Turabian StyleBâldea, Ioan. 2022. "HCnH− Anion Chains with n ≤ 8 Are Nonlinear and Their Permanent Dipole Makes Them Potential Candidates for Astronomical Observation" Molecules 27, no. 10: 3100. https://doi.org/10.3390/molecules27103100







