Hyaluronic Acid-Conjugated Carbon Nanomaterials for Enhanced Tumour Targeting Ability
Abstract
:1. Introduction
1.1. Hyaluronic Acid
1.2. Carbon Nanomaterials and Interactions with Hyaluronic Acid
2. Carbon Nanomaterial Conjugated with Hyaluronic Acid for Drug Delivery
2.1. Carbon Nanotubes
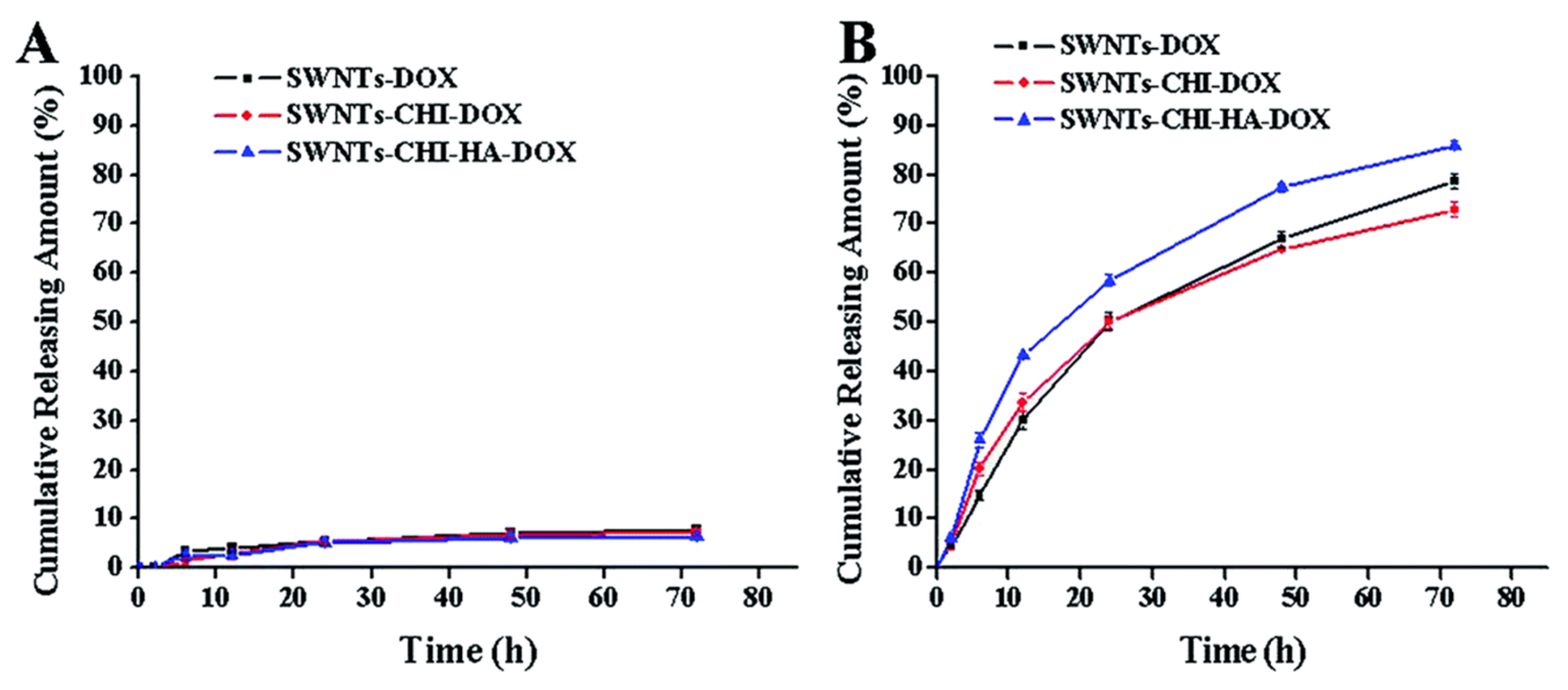
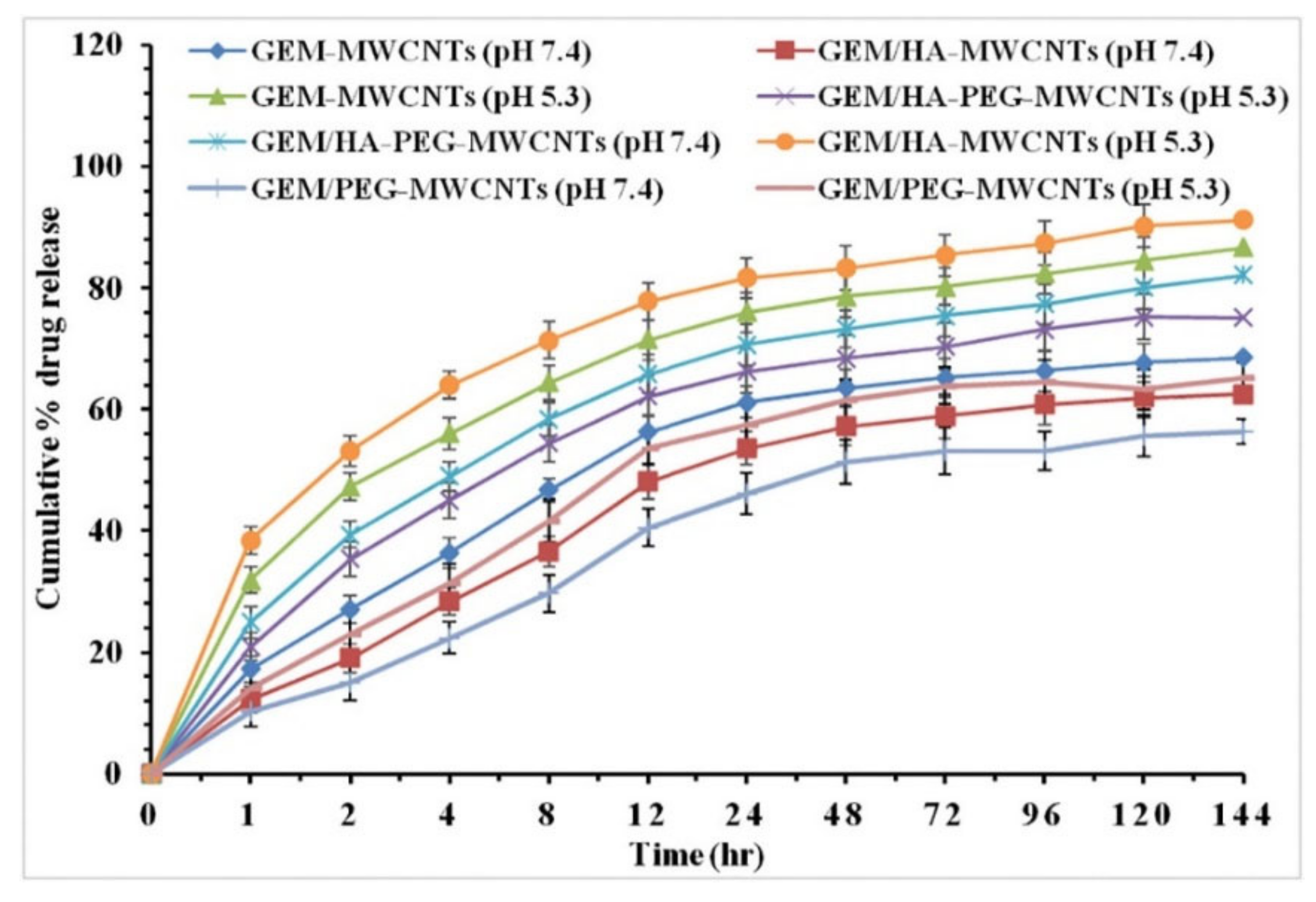
2.1.1. Cumulative Release Profiles of HA-Conjugated CNTs Loaded with Anticancer Drugs
2.1.2. Relative Tumour Volume Reduction
2.2. Graphene, Graphene Oxide and Graphene Quantum Dots
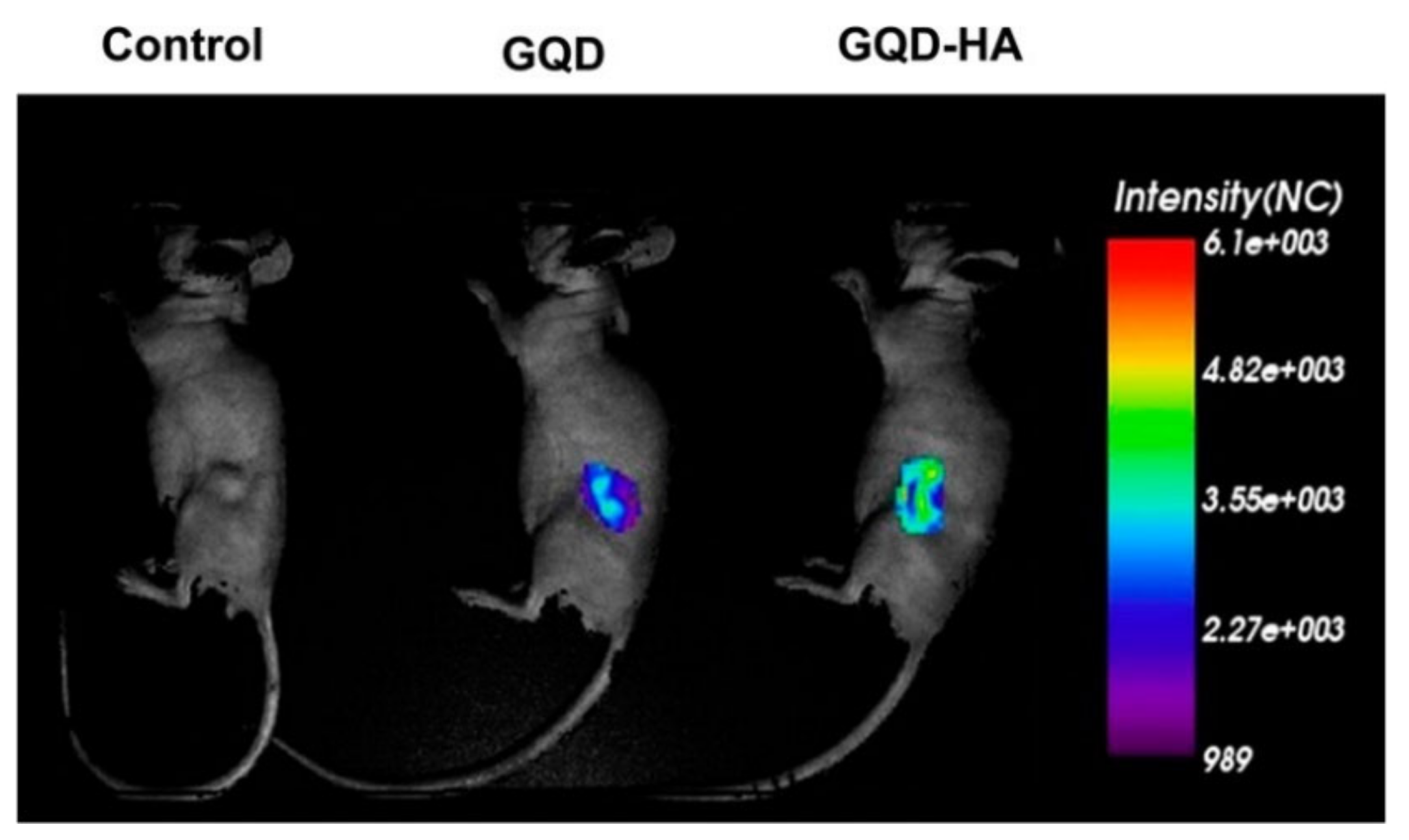
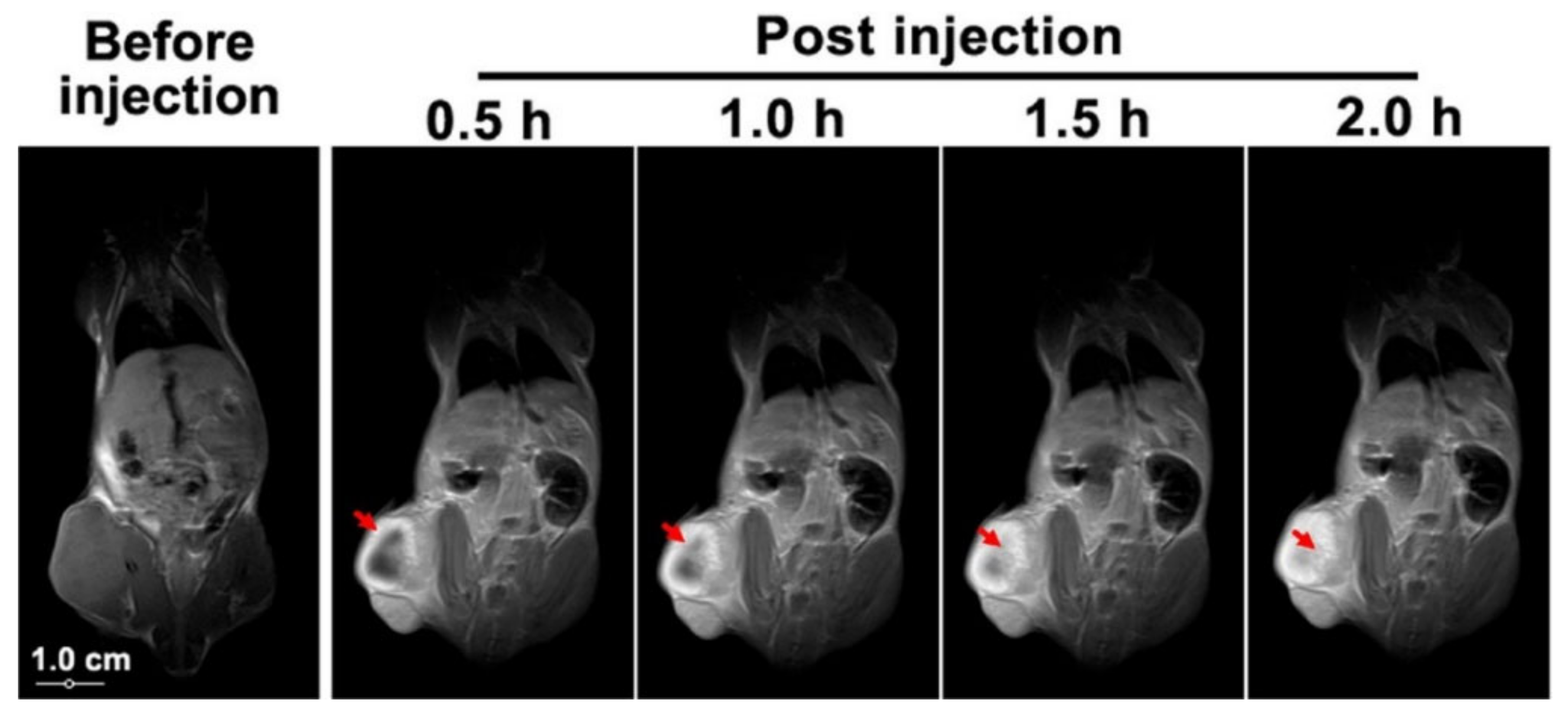
2.2.1. Chemotherapy Using HA-Conjugated Graphene, Graphene Oxide, and Graphene Quantum Dots Delivery Systems
2.2.2. Photoablation Therapy Using HA-Conjugated Graphene, Graphene Oxide, and Graphene Quantum Dots Delivery Systems
2.2.3. Combined Chemo-Photothermal Therapy
2.2.4. Dual Receptor Targeting
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, C.; Liu, X.-R.; Chen, Q.-B.; Li, Y.; Zhou, J.-L.; Zhou, L.-Y.; Zou, T. Hyaluronic Acid and Albumin Based Nanoparticles for Drug Delivery. J. Control. Release 2021, 331, 416–433. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Chapter Nine—Beneficial Effects of Hyaluronic Acid. In Advances in Food and Nutrition Research; Marine Carbohydrates: Fundamentals and Applications, Part A; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 72, pp. 137–176. [Google Scholar]
- Garantziotis, S.; Savani, R.C. Hyaluronan Biology: A Complex Balancing Act of Structure, Function, Location and Context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Rostami, S.; Parsian, H. Hyaluronic Acid: From Biochemical Characteristics to Its Clinical Translation in Assessment of Liver Fibrosis. Hepat. Mon. 2013, 13, e13787. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, A.D.D.; Santana, M.H.A. Structural and Surface Properties Control the Recovery and Purity of Bio- Hyaluronic Acid upon Precipitation with Isopropyl Alcohol. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 112–118. [Google Scholar] [CrossRef]
- Yang, M.-H.; Jong, S.-B.; Lu, C.-Y.; Lin, Y.-F.; Chiang, P.-W.; Tyan, Y.-C.; Chung, T.-W. Assessing the Responses of Cellular Proteins Induced by Hyaluronic Acid-Modified Surfaces Utilizing a Mass Spectrometry-Based Profiling System: Over-Expression of CD36, CD44, CDK9, and PP2A. Analyst 2012, 137, 4921–4933. [Google Scholar] [CrossRef] [PubMed]
- Datir, S.R.; Das, M.; Singh, R.P.; Jain, S. Hyaluronate Tethered, “Smart” Multiwalled Carbon Nanotubes for Tumor-Targeted Delivery of Doxorubicin. Bioconjug. Chem. 2012, 23, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Wang, H.; Liu, J.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. Controlled Release and Targeted Delivery to Cancer Cells of Doxorubicin from Polysaccharide-Functionalised Single-Walled Carbon Nanotubes. J. Mater. Chem. B 2015, 3, 1846–1855. [Google Scholar] [CrossRef]
- Vahedi, N.; Tabandeh, F.; Mahmoudifard, M. Hyaluronic Acid–Graphene Quantum Dot Nanocomposite: Potential Target Drug Delivery and Cancer Cell Imaging. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Pardo, J.; Peng, Z.; Leblanc, R.M. Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules 2018, 23, 378. [Google Scholar] [CrossRef] [Green Version]
- Quéré, R.; Andradottir, S.; Brun, A.C.M.; Zubarev, R.A.; Karlsson, G.; Olsson, K.; Magnusson, M.; Cammenga, J.; Karlsson, S. High Levels of the Adhesion Molecule CD44 on Leukemic Cells Generate Acute Myeloid Leukemia Relapse after Withdrawal of the Initial Transforming Event. Leukemia 2011, 25, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Liu, J.; Zhao, Z.; Zhao, Y.; Dong, L.; Yang, M.; Zhou, J.; Huo, M. Redox Sensitive Hyaluronic Acid-Decorated Graphene Oxide for Photothermally Controlled Tumor-Cytoplasm-Selective Rapid Drug Delivery. Adv. Funct. Mater. 2017, 27, 1604620. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Juhlin, L. Hyaluronan in Skin. J. Intern. Med. 1997, 242, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Akima, K.; Ito, H.; Iwata, Y.; Matsuo, K.; Watari, N.; Yanagi, M.; Hagi, H.; Oshima, K.; Yagita, A.; Atomi, Y.; et al. Evaluation of Antitumor Activities of Hyaluronate Binding Antitumor Drugs: Synthesis, Characterization and Antitumor Activity. J. Drug Target. 2008, 4, 1–8. [Google Scholar] [CrossRef]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic Acid for Anticancer Drug and Nucleic Acid Delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Al-malky, H.S.; Al Harthi, S.E.; Osman, A.-M.M. Major Obstacles to Doxorubicin Therapy: Cardiotoxicity and Drug Resistance. J. Oncol. Pharm. Pr. 2020, 26, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Lee, J.-E.; Lim, J.; Jo, D.-E.; Park, S.-A.; Suh, P.-G.; Kang, B.H. Combination Treatment with Doxorubicin and Gamitrinib Synergistically Augments Anticancer Activity through Enhanced Activation of Bim. BMC Cancer 2014, 14, 431. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, J. The Role of Gemcitabine in the Treatment of Other Tumours. Br. J. Cancer 1998, 78, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Gemcitabine. Available online: https://go.drugbank.com/drugs/DB00441 (accessed on 9 August 2021).
- Jung, H.S.; Lee, M.-Y.; Kong, W.H.; Do, I.H.; Hahn, S.K. Nano Graphene Oxide–Hyaluronic Acid Conjugate for Target Specific Cancer Drug Delivery. RSC Adv. 2014, 4, 14197–14200. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Feng, Q.; Wang, Y.; Yang, X.; Ren, J.; Shi, Y.; Shan, X.; Yuan, Y.; Wang, Y.; Zhang, Z. Multifunctional Hyaluronic Acid Modified Graphene Oxide Loaded with Mitoxantrone for Overcoming Drug Resistance in Cancer. Nanotechnology 2015, 27, 015701. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, X.; Pu, Y.; Yi, Y.; Zhang, T.; Wang, B. PH-Sensitive and Biocompatible Quercetin-Loaded GO–PEA–HA Carrier Improved Antitumour Efficiency and Specificity. Artif. Cells Nanomed. Biotechnol. 2018, 46, S28–S37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of Low Dose Quercetin: Cancer Cell-Specific Inhibition of Cell Cycle Progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-M.; Cao, Y.; Yang, Y.; Chen, J.-T.; Liu, Y. A Small-Sized Graphene Oxide Supramolecular Assembly for Targeted Delivery of Camptothecin. Chem. Commun. 2014, 50, 13066–13069. [Google Scholar] [CrossRef]
- Salas-Treviño, D.; Saucedo-Cárdenas, O.; Loera-Arias, M.d.J.; Rodríguez-Rocha, H.; García-García, A.; Montes-de-Oca-Luna, R.; Piña-Mendoza, E.I.; Contreras-Torres, F.F.; García-Rivas, G.; Soto-Domínguez, A. Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Filled with Carboplatin as a Novel Drug Nanocarrier against Murine Lung Cancer Cells. Nanomaterials 2019, 9, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-Network-Structured Graphene Oxide-Containing Nanogels as Photothermal Agents for the Treatment of Colorectal Cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Upadhyay, P.; Ghosh, A.; Bose, A.; Gupta, P.; Chattopadhyay, S.; Chattopadhyay, D.; Adhikary, A. Hyaluronic Acid Engrafted Metformin Loaded Graphene Oxide Nanoparticle as CD44 Targeted Anti-Cancer Therapy for Triple Negative Breast Cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mo, F.; Jin, X.; Chen, L.; Xu, L.J.; Guo, L.; Fu, F. Tumor-Targeting Photothermal Heating-Responsive Nanoplatform Based on Reduced Graphene Oxide/Mesoporous Silica/Hyaluronic Acid Nanocomposite for Enhanced Photodynamic Therapy. Adv. Mater. Interfaces 2017, 4, 1700425. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, X.; Wang, F.; Feng, L.; Wang, Y.; Zheng, Y.; Guo, R. Targeted Delivery of SNX-2112 by Polysaccharide-Modified Graphene Oxide Nanocomposites for Treatment of Lung Cancer. Carbohydr. Polym. 2018, 185, 85–95. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Sun, L.; Liu, Y. The Effect of Hyaluronic Acid Functionalized Carbon Nanotubes Loaded with Salinomycin on Gastric Cancer Stem Cells. Biomaterials 2014, 35, 9208–9223. [Google Scholar] [CrossRef]
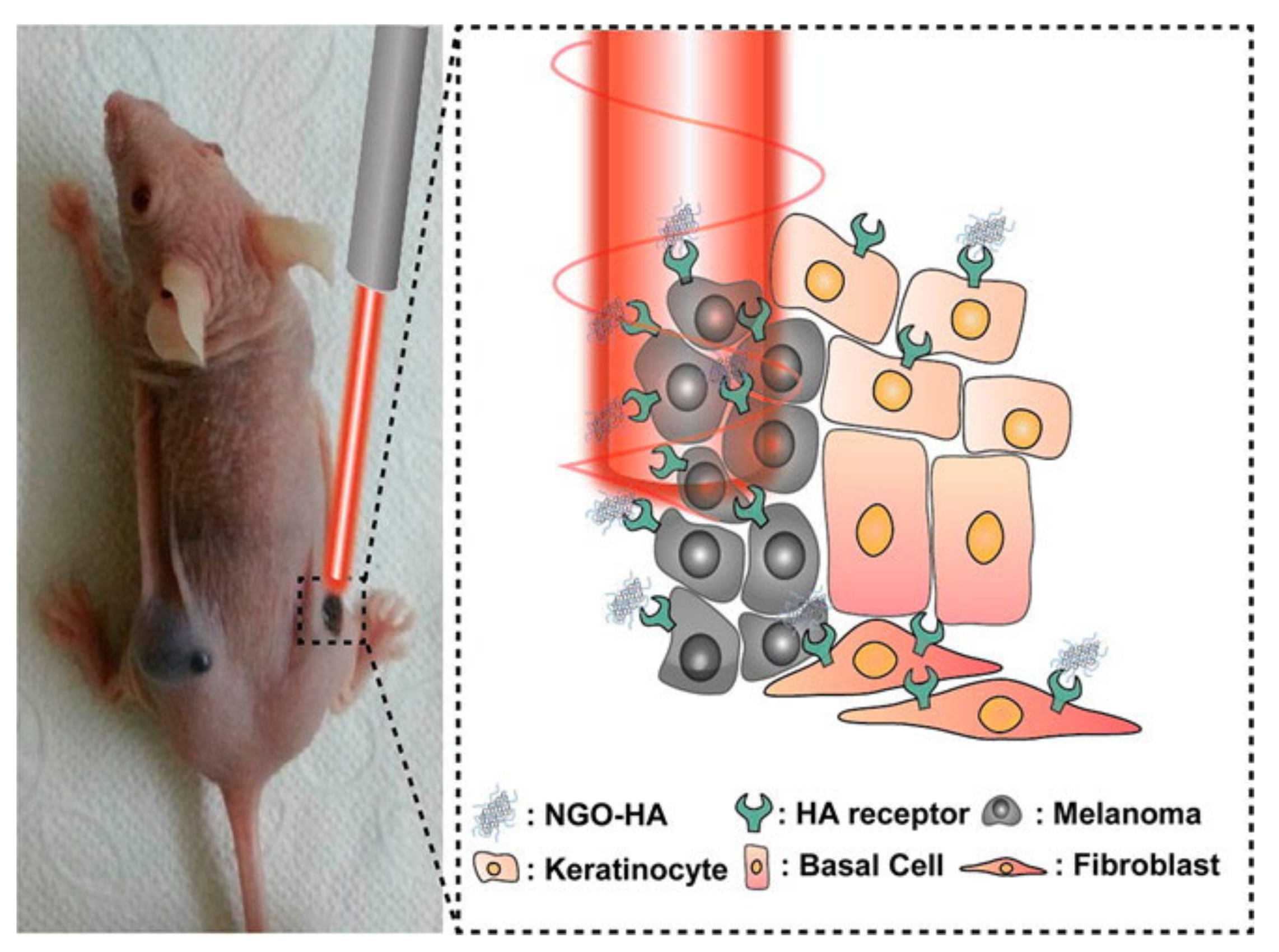
- Jung, H.S.; Kong, W.H.; Sung, D.K.; Lee, M.-Y.; Beack, S.E.; Keum, D.H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Nanographene Oxide–Hyaluronic Acid Conjugate for Photothermal Ablation Therapy of Skin Cancer. ACS Nano 2014, 8, 260–268. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Chapter 1—Antimicrobial Photoinactivation with Functionalized Fullerenes. In Nanobiomaterials in Antimicrobial Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: New York, NY, USA, 2016; pp. 1–27. ISBN 978-0-323-42864-4. [Google Scholar]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In Vitro Comparison of the Photothermal Anticancer Activity of Graphene Nanoparticles and Carbon Nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, Techniques, and Limitations of near Infrared Spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [Green Version]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Shetty, P. The Antibiotic Paradox. Lancet Infect. Dis. 2002, 2, 704. [Google Scholar] [CrossRef]
- D’Amora, M.; Camisasca, A.; Boarino, A.; Arpicco, S.; Giordani, S. Supramolecular Functionalization of Carbon Nano-Onions with Hyaluronic Acid-Phospholipid Conjugates for Selective Targeting of Cancer Cells. Colloids Surf. B Biointerfaces 2020, 188, 110779. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; Quinn, S.J.; Giordani, S. Carbon Nanomaterials: Multi-Functional Agents for Biomedical Fluorescence and Raman Imaging. Chem. Soc. Rev. 2015, 44, 4672–4698. [Google Scholar] [CrossRef]
- Hudson, J.L.; Casavant, M.J.; Tour, J.M. Water-Soluble, Exfoliated, Nonroping Single-Wall Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 11158–11159. [Google Scholar] [CrossRef]
- Bartkowski, M.; Giordani, S. Supramolecular Chemistry of Carbon Nano-Onions. Nanoscale 2020, 12, 9352–9358. [Google Scholar] [CrossRef] [PubMed]
- Camisasca, A.; Giordani, S. Surfactant-Mediated Dispersions of Carbon Nano-Onions in Aqueous Solution. Nano Express 2020, 1, 010018. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Ettorre, V.; Fontana, A. Non-Covalent and Reversible Functionalization of Carbon Nanotubes. Beilstein. J. Nanotechnol. 2014, 5, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.-H.; Luo, J.; Mao, Y.-L.; Lai, S.; Gong, Y.-N.; Zhong, D.-C.; Lu, T.-B. π-π Stacking Interactions: Non-Negligible Forces for Stabilizing Porous Supramolecular Frameworks. Sci. Adv. 2020, 6, eaax9976. [Google Scholar] [CrossRef] [Green Version]
- Rungnim, C.; Arsawang, U.; Rungrotmongkol, T.; Hannongbua, S. Molecular Dynamics Properties of Varying Amounts of the Anticancer Drug Gemcitabine inside an Open-Ended Single-Walled Carbon Nanotube. Chem. Phys. Lett. 2012, 550, 99–103. [Google Scholar] [CrossRef]
- Cao, X.; Tao, L.; Wen, S.; Hou, W.; Shi, X. Hyaluronic Acid-Modified Multiwalled Carbon Nanotubes for Targeted Delivery of Doxorubicin into Cancer Cells. Carbohydr. Res. 2015, 405, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Shrivastava, C.; Jain, A.K. Hyaluronic Acid Conjugated Multi-Walled Carbon Nanotubes for Colon Cancer Targeting. Int. J. Biol. Macromol. 2019, 123, 691–703. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, X.; Li, H.; Lin, Y.; Du, D. Hyaluronic Acid-Modified Multifunctional Q-Graphene for Targeted Killing of Drug-Resistant Lung Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Nahain, A.-A.; Lee, J.-E.; Jeong, J.H.; Park, S.Y. Photoresponsive Fluorescent Reduced Graphene Oxide by Spiropyran Conjugated Hyaluronic Acid for in Vivo Imaging and Target Delivery. Biomacromolecules 2013, 14, 4082–4090. [Google Scholar] [CrossRef]
- Wu, H.; Shi, H.; Wang, Y.; Jia, X.; Tang, C.; Zhang, J.; Yang, S. Hyaluronic Acid Conjugated Graphene Oxide for Targeted Drug Delivery. Carbon 2014, 69, 379–389. [Google Scholar] [CrossRef]
- Song, E.; Han, W.; Li, C.; Cheng, D.; Li, L.; Liu, L.; Zhu, G.; Song, Y.; Tan, W. Hyaluronic Acid-Decorated Graphene Oxide Nanohybrids as Nanocarriers for Targeted and PH-Responsive Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 11882–11890. [Google Scholar] [CrossRef] [PubMed]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Costa, E.C.; Ferreira, P.; Louro, R.O.; Correia, I.J. Hyaluronic Acid Functionalized Green Reduced Graphene Oxide for Targeted Cancer Photothermal Therapy. Carbohydr. Polym. 2018, 200, 93–99. [Google Scholar] [CrossRef]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl Chitosan-Mediated Synthesis of Hyaluronic Acid-Targeted Graphene Oxide for Cancer Drug Delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef]
- Kaya, D.; Küçükada, K.; Alemdar, N. Modeling the Drug Release from Reduced Graphene Oxide-Reinforced Hyaluronic Acid/Gelatin/Poly(Ethylene Oxide) Polymeric Films. Carbohydr. Polym. 2019, 215, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Chu, L.-Y. Controlled Release Systems for Insulin Delivery. Expert Opin. Ther. Pat. 2005, 15, 1147–1155. [Google Scholar] [CrossRef]
- Wang, Q.; She, W.; Lu, X.; Li, P.; Sun, Y.; Liu, X.; Pan, W.; Duan, K. The Interaction of Hyaluronic Acid and Graphene Tuned by Functional Groups: A Density Functional Study. Comput. Theor. Chem. 2019, 1165, 112559. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Lu, X.; Wang, K.; Fang, L.; Ren, F.; Lu, G.; Zhang, H. Interaction Behaviors of Fibrinopeptide-A and Graphene with Different Functional Groups: A Molecular Dynamics Simulation Approach. J. Phys. Chem. B 2017, 121, 7907–7915. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, X.; Weng, J.; Leng, Y. Density Functional Theory Study of the Interaction of Arginine-Glycine-Aspartic Acid with Graphene, Defective Graphene, and Graphene Oxide. J. Phys. Chem. C 2013, 117, 5708–5717. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, M.T.; Gonzalez-Rodriguez, R.; Truly, T.; Lee, B.H.; Green, K.N.; Akkaraju, G.; Naumov, A.V. Graphene Quantum Dot Formulation for Cancer Imaging and Redox-Based Drug Delivery. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102408. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Morantes, C.Y.; Meléndez, E.; Singh, S.P.; Ramírez-Vick, J.E. Cytotoxicity and Reactive Oxygen Species Generated by Ferrocenium and Ferrocene on MCF7 and MCF10A Cell Lines. Cancer Sci. Ther. 2012, 4, 1–5. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Andronescu, E. Chapter 10—Inorganic Nanoarchitectonics Designed for Drug Delivery and Anti-Infective Surfaces. In Surface Chemistry of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: New York, NY, USA, 2016; pp. 301–327. ISBN 978-0-323-42861-3. [Google Scholar]
- Arpicco, S.; Bartkowski, M.; Barge, A.; Zonari, D.; Serpe, L.; Milla, P.; Dosio, F.; Stella, B.; Giordani, S. Effects of the Molecular Weight of Hyaluronic Acid in a Carbon Nanotube Drug Delivery Conjugate. Front. Chem. 2020, 8, 578008. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, P.; Chang, L.; Long, X.; Dong, A.; Liu, J.; Chu, L.; Hu, F.; Liu, J.; Deng, L. Tumor Targeting and PH-Responsive Polyelectrolyte Complex Nanoparticles Based on Hyaluronic Acid-Paclitaxel Conjugates and Chitosan for Oral Delivery of Paclitaxel. Macromol. Res. 2013, 21, 1331–1337. [Google Scholar] [CrossRef]
- Bhirde, A.A.; Chikkaveeraiah, B.V.; Srivatsan, A.; Niu, G.; Jin, A.J.; Kapoor, A.; Wang, Z.; Patel, S.; Patel, V.; Gorbach, A.M.; et al. Targeted Therapeutic Nanotubes Influence the Viscoelasticity of Cancer Cells to Overcome Drug Resistance. ACS Nano 2014, 8, 4177–4189. [Google Scholar] [CrossRef]
- Yao, H.J.; Sun, L.; Liu, Y.; Jiang, S.; Pu, Y.; Li, J.; Zhang, Y. Monodistearoylphosphatidylethanolamine-Hyaluronic Acid Functionalization of Single-Walled Carbon Nanotubes for Targeting Intracellular Drug Delivery to Overcome Multidrug Resistance of Cancer Cells. Carbon 2016, 96, 362–376. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Wang, J.; Fan, L.; Zhu, W.; Cai, D. Hyaluronic Acid-Coated Single-Walled Carbon Nanotubes Loaded with Doxorubicin for the Treatment of Breast Cancer. Die Pharmazie 2019, 74, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Te Boekhorst, V.; Preziosi, L.; Friedl, P. Plasticity of Cell Migration In Vivo and In Silico. Annu. Rev. Cell Dev. Biol. 2016, 32, 491–526. [Google Scholar] [CrossRef] [PubMed]
- Singhai, N.J.; Maheshwari, R.; Ramteke, S. CD44 Receptor Targeted ‘Smart’ Multi-Walled Carbon Nanotubes for Synergistic Therapy of Triple-Negative Breast Cancer. Colloid Interface Sci. Commun. 2020, 35, 100235. [Google Scholar] [CrossRef]
- Yu, B.; Tan, L.; Zheng, R.; Tan, H.; Zheng, L. Targeted Delivery and Controlled Release of Paclitaxel for the Treatment of Lung Cancer Using Single-Walled Carbon Nanotubes. Mater. Sci. Eng. C 2016, 68, 579–584. [Google Scholar] [CrossRef]
- Hou, L.; Yuan, Y.; Ren, J.; Zhang, Y.; Wang, Y.; Shan, X.; Liu, Q.; Zhang, Z. In Vitro and in Vivo Comparative Study of the Phototherapy Anticancer Activity of Hyaluronic Acid-Modified Single-Walled Carbon Nanotubes, Graphene Oxide, and Fullerene. J. Nanopart. Res. 2017, 19, 286. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Pan, Z.; Chen, Y.; Fan, Z.; Tian, H.; Zhou, S.; Zhang, Y.; Shang, J.; Jiang, B.; et al. Multifunctional Nanosystem Based on Graphene Oxide for Synergistic Multistage Tumor-Targeting and Combined Chemo-Photothermal Therapy. Mol. Pharm. 2019, 16, 1982–1998. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Boulos, R.A.; Yasin, F.M.; Lu, H.; Raston, C.; Zhang, H. Pyrene-Conjugated Hyaluronan Facilitated Exfoliation and Stabilisation of Low Dimensional Nanomaterials in Water. Chem. Commun. 2013, 49, 4845–4847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Ma, R.; Wang, L.; Zhang, J.; Liu, R.; Li, L.; Liu, Y.; Hou, L.; Yu, X.; Gao, J.; et al. The Application of Hyaluronic Acid-Derivatized Carbon Nanotubes in Hematoporphyrin Monomethyl Ether-Based Photodynamic Therapy for in Vivo and in Vitro Cancer Treatment. Int. J. Nanomed. 2013, 8, 2361–2373. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Xu, Q.; Liu, F.; Zhou, P.; Gu, Y.; Zeng, J.; An, J.; Dai, W.; Li, X. Hematoporphyrin Monomethyl Ether Photodynamic Damage on HeLa Cells by Means of Reactive Oxygen Species Production and Cytosolic Free Calcium Concentration Elevation. Cancer Lett. 2004, 216, 43–54. [Google Scholar] [CrossRef] [PubMed]
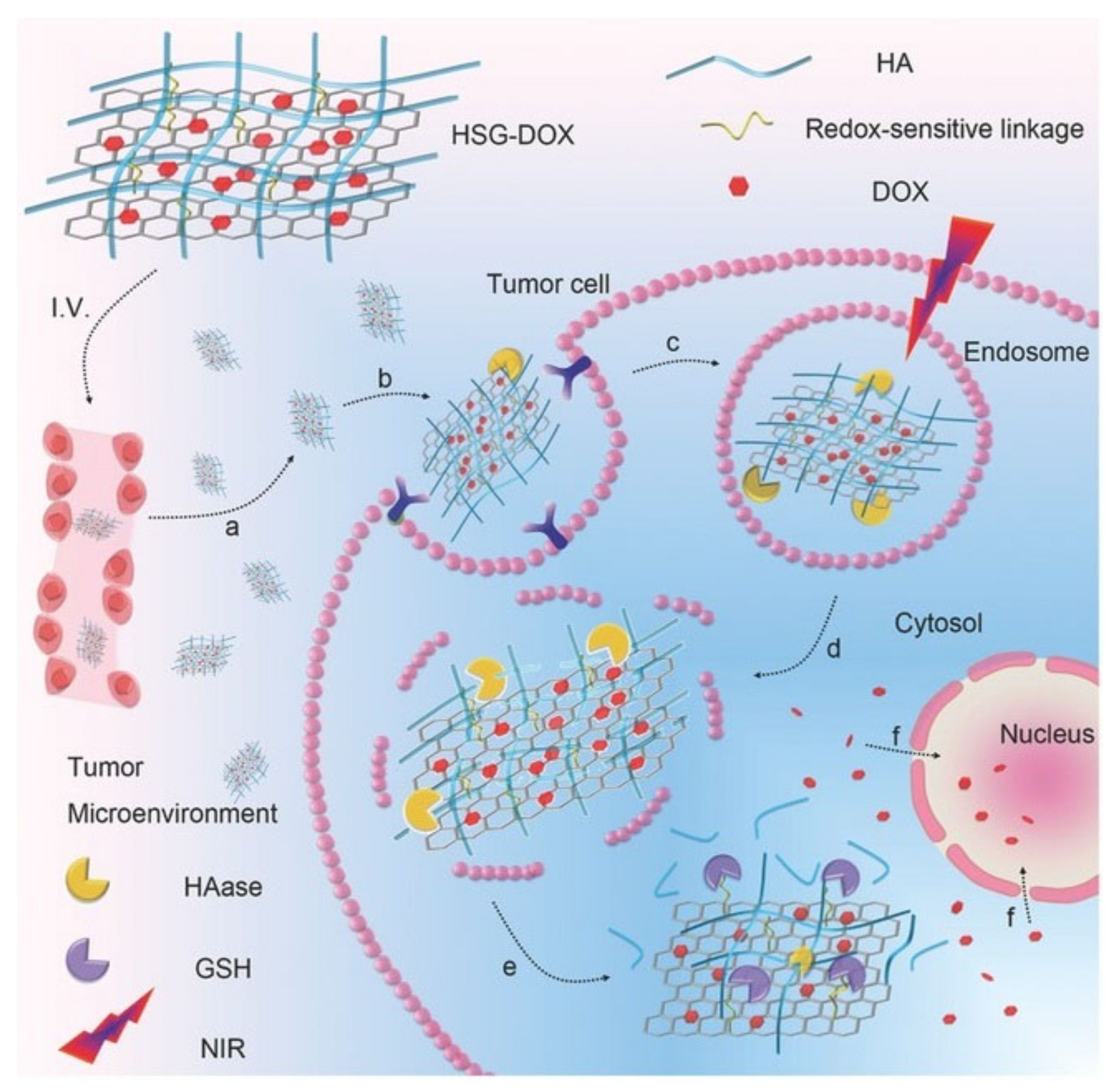
- Hou, L.; Yang, X.; Ren, J.; Wang, Y.; Zhang, H.; Feng, Q.; Shi, Y.; Shan, X.; Yuan, Y.; Zhang, Z. A Novel Redox-Sensitive System Based on Single-Walled Carbon Nanotubes for Chemo-Photothermal Therapy and Magnetic Resonance Imaging. Int. J. Nanomed. 2016, 11, 607–624. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.C. Chapter 2—Application and Uses of Graphene Oxide and Reduced Graphene Oxide. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; Micro and Nano Technologies; Ray, S.C., Ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 39–55. ISBN 978-0-323-37521-4. [Google Scholar]
- Margineanu, A. Chapter 14—Biological Applications of Nanoparticles in Optical Microscopy. In Polymeric Nanomaterials in Nanotherapeutics; Micro and Nano Technologies; Vasile, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–495. ISBN 978-0-12-813932-5. [Google Scholar]
- Ozhukil Valappil, M.; Pillai, V.K.; Alwarappan, S. Spotlighting Graphene Quantum Dots and beyond: Synthesis, Properties and Sensing Applications. Appl. Mater. Today 2017, 9, 350–371. [Google Scholar] [CrossRef]
- Santos, C.M.; Tria, M.C.R.; Vergara, R.A.M.V.; Ahmed, F.; Advincula, R.C.; Rodrigues, D.F. Antimicrobial Graphene Polymer (PVK-GO) Nanocomposite Films. Chem. Commun. 2011, 47, 8892–8894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Huang, X.; Ma, Y.; Huang, Y.; Yang, R.; Duan, H.; Chen, Y. Multi-Functionalized Graphene Oxide Based Anticancer Drug-Carrier with Dual-Targeting Function and PH-Sensitivity. J. Mater. Chem. 2011, 21, 3448–3454. [Google Scholar] [CrossRef]
- Yang, X.; Niu, G.; Cao, X.; Wen, Y.; Xiang, R.; Duan, H.; Chen, Y. The Preparation of Functionalized Graphene Oxide for Targeted Intracellular Delivery of SiRNA. J. Mater. Chem. 2012, 22, 6649–6654. [Google Scholar] [CrossRef]
- Li, F.; Park, S.-J.; Ling, D.; Park, W.; Han, J.Y.; Na, K.; Char, K. Hyaluronic Acid-Conjugated Graphene Oxide/Photosensitizer Nanohybrids for Cancer Targeted Photodynamic Therapy. J. Mater. Chem. B 2013, 1, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Hu, W.; Xiao, L.; Cao, Z.; Li, X.; Zhang, C.; Liao, L.; Liu, L. Enzymatically Cross-Linked Hyaluronic Acid/Graphene Oxide Nanocomposite Hydrogel with PH-Responsive Release. J. Biomater. Sci. Polym. Ed. 2015, 26, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Abdullah-Al, N.; Lee, J.-E.; In, I.; Lee, H.; Lee, K.D.; Jeong, J.H.; Park, S.Y. Target Delivery and Cell Imaging Using Hyaluronic Acid-Functionalized Graphene Quantum Dots. Mol. Pharm. 2013, 10, 3736–3744. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.T.; Chen, C.-H.; Chen, J.-P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Li, H.; Yuan, Y.; Zhang, Z.; Xie, J.; Hwang, D.W.; Zhang, A.; Liu, M.; Zhou, X. Engineered Paramagnetic Graphene Quantum Dots with Enhanced Relaxivity for Tumor Imaging. Nano Lett. 2019, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Waghmode, S.; Louis, M.; Wangnoo, S.; Chavan, P.; Sarkar, D. Graphene Quantum Dots Conjugated Albumin Nanoparticles for Targeted Drug Delivery and Imaging of Pancreatic Cancer. J. Mater. Chem. B 2014, 2, 3190–3195. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Lian, S.; Zheng, J.; Li, B.; Li, T.; Jia, L. Redox-Responsive Hyaluronic Acid-Functionalized Graphene Oxide Nanosheets for Targeted Delivery of Water-Insoluble Cancer Drugs. Int. J. Nanomed. 2018, 13, 7457–7472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafiujjaman, M.; Revuri, V.; Park, H.-K.; Kwon, I.K.; Cho, K.-J.; Lee, Y. Enhanced Photodynamic Properties of Graphene Quantum Dot Conjugated Ce6 Nanoparticles for Targeted Cancer Therapy and Imaging. Chem. Lett. 2016, 45, 997–999. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.E.; Sharker, S.M.; Jeong, J.H.; In, I.; Park, S.Y. In Vitro and In Vivo Tumor Targeted Photothermal Cancer Therapy Using Functionalized Graphene Nanoparticles. Biomacromolecules 2015, 16, 3519–3529. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y. A Hyaluronic Acid Nanogel for Photo–Chemo Theranostics of Lung Cancer with Simultaneous Light-Responsive Controlled Release of Doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, H.; Wang, Y.; Wang, L.; Yang, X.; Zhang, Z. Hyaluronic Acid-Functionalized Single-Walled Carbon Nanotubes as Tumor-Targeting MRI Contrast Agent. Int. J. Nanomed. 2015, 10, 4507–4520. [Google Scholar] [CrossRef]
- Hyaluronic Acid|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/IE/en/search/hyaluronic-acid?focus=products&page=1&perPage=30&sort=relevance&term=Hyaluronic%20Acid&type=product_name (accessed on 25 July 2021).
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation. Processes 2021, 9, 241. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Du, Y.; Bu, H.; Tang, Y.; Huang, Q. Fluorescence Imaging-Guided Cancer Photothermal Therapy Using Polydopamine and Graphene Quantum Dot-Capped Prussian Blue Nanocubes. RSC Adv. 2021, 11, 8420–8429. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, R.; Lu, J.; Zhao, C.; Deng, X.; Wu, Y. Mesoporous Silica Coated Polydopamine Functionalized Reduced Graphene Oxide for Synergistic Targeted Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 1226–1236. [Google Scholar] [CrossRef]
- Deng, L.; Li, Q.; Al-Rehili, S.; Omar, H.; Almalik, A.; Alshamsan, A.; Zhang, J.; Khashab, N.M. Hybrid Iron Oxide–Graphene Oxide–Polysaccharides Microcapsule: A Micro-Matryoshka for On-Demand Drug Release and Antitumor Therapy In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 6859–6868. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Liu, Y.; Chen, Y.; Ouyang, D.; Yang, G.; Yu, T. Graphene Quantum Dots-Gated Hollow Mesoporous Carbon Nanoplatform for Targeting Drug Delivery and Synergistic Chemo-Photothermal Therapy. Int. J. Nanomed. 2018, 13, 5991–6007. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Yang, D.; Mei, L.; Li, Q.; Zhu, H.; Wang, T. Targeting Chemophotothermal Therapy of Hepatoma by Gold Nanorods/Graphene Oxide Core/Shell Nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 12911–12920. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Feng, Q.; Wang, Y.; Zhang, H.; Jiang, G.; Yang, X.; Ren, J.; Zhu, X.; Shi, Y.; Zhang, Z. Multifunctional Nanosheets Based on Hyaluronic Acid Modified Graphene Oxide for Tumor-Targeting Chemo-Photothermal Therapy. J. Nanopart. Res. 2015, 17, 162. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Wang, J.; Ju, E.; Zhou, L.; Ren, J.; Qu, X. A Multi-Synergistic Platform for Sequential Irradiation-Activated High-Performance Apoptotic Cancer Therapy. Adv. Funct. Mater. 2014, 24, 522–529. [Google Scholar] [CrossRef]
- Ferreira, C.G.; Epping, M.; Kruyt, F.A.E.; Giaccone, G. Apoptosis: Target of Cancer Therapy. Clin. Cancer Res. 2002, 8, 2024–2034. [Google Scholar] [PubMed]
- O’Neill, K.L.; Fairbairn, D.W.; Smith, M.J.; Poe, B.S. Critical Parameters Influencing Hyperthermia-Induced Apoptosis in Human Lymphoid Cell Lines. Apoptosis 1998, 3, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, H.; Li, Y.; Wu, F.; Li, Y.; Bao, Y.; Yan, X.; Huang, Z.; Xu, P. Hyaluronic Acid and Arg-Gly-Asp Peptide Modified Graphene Oxide with Dual Receptor-Targeting Function for Cancer Therapy. J. Biomater. Appl. 2017, 32, 54–65. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kearns, O.; Camisasca, A.; Giordani, S. Hyaluronic Acid-Conjugated Carbon Nanomaterials for Enhanced Tumour Targeting Ability. Molecules 2022, 27, 48. https://doi.org/10.3390/molecules27010048
Kearns O, Camisasca A, Giordani S. Hyaluronic Acid-Conjugated Carbon Nanomaterials for Enhanced Tumour Targeting Ability. Molecules. 2022; 27(1):48. https://doi.org/10.3390/molecules27010048
Chicago/Turabian StyleKearns, Oisin, Adalberto Camisasca, and Silvia Giordani. 2022. "Hyaluronic Acid-Conjugated Carbon Nanomaterials for Enhanced Tumour Targeting Ability" Molecules 27, no. 1: 48. https://doi.org/10.3390/molecules27010048






